Abstract
Necrotizing pneumonia (NP) is an emerging complication of community acquired pneumonia (CAP) in children. This study aimed at the evaluation of etiology, clinical features, treatment, and prognosis of NP. The institutional database of children with CAP treated between April 2008 and July 2013 was searched to identify children with NP. Then, data on the NP characteristics were retrospectively reviewed and analyzed. We found that NP constituted 32/882 (3.7 %) of all CAPs. The median age of children with NP was 4 (range 1–10) years. The causative pathogens were identified in 12/32 children (37.5 %) with Streptococcus pneumoniae being the most common (6/32). All but one patient developed complications: parapneumonic effusion (PPE), pleural empyema or bronchopleural fistula (BPF), which required prompt local treatment. The median duration of hospital stay and antibiotic treatment was 26 (IQR 21–30) and 28 (IQR 22.5–32.5) days, respectively. Despite severe course of the disease no deaths occurred. A follow-up visit after 6 months revealed that none of the patients presented with any signs and symptoms which could be related to earlier pneumonia. Chest radiographs showed complete or almost complete resolution of pulmonary and pleural lesions in all patients. We conclude that necrotizing pneumonia is a relatively rare but severe form of CAP that is almost always complicated by PPE/empyema and/or BPF. It can be successfully treated with antibiotics and pleural drainage without major surgical intervention.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Necrotizing pneumonia (NP) is one of the most severe complications of community acquired pneumonia (CAP) in children. Although the number of patients with NP is relatively low, an increasing incidence of this form of pneumonia has been reported in the last decades (Lemaître et al. 2013; Bender et al. 2008; Sawicki et al. 2008; Hsieh et al. 2004). The key pathologic feature of NP, i.e. lung necrosis, is caused by toxins produced by invasive bacterial strains and secondary vascular changes, including vasculitis and intravascular thrombosis (Hsieh et al. 2006). Streptococcus pneumoniae and Staphylococcus aureus are the main etiologic factors of NP in children (Lemaître et al. 2013; Sawicki et al. 2008; Ramphul et al. 2006). Albeit NP can present with highly variable clinical course, the majority of children demonstrate severe disease with high, prolonged fever, dyspnea, and clinical and radiological signs of extensive consolidation of lung parenchyma. In a high percentage of children, NP is complicated by parapneumonic effusion (PPE)/empyema, bronchopleural fistula (BPF), septicemia, and respiratory failure. Thus, the disease requires aggressive, systemic and local treatment.
Since NP is relatively uncommon, our knowledge on the entity is based mainly on case reports and small series (Hasan et al. 2011). The number of publications reporting large series is low (Sawicki et al. 2008) and only one larger European study was published to date (Lemaître et al. 2013). Therefore, we undertook this study to evaluate the etiology, clinical features, treatment strategies, and prognosis of NP in children managed in the largest referral children’s pneumology center in the capital city of Warsaw, Poland.
2 Methods
The study protocol was accepted by the institutional Review Board of Medical University of Warsaw, Poland. The children with NP aged from 1 month to 18 years, treated in a tertiary referral center from April 2008 to July 2013 were included in the study. The cases of NP were selected from a specific electronic database of patients with pneumonia which has been running in our institution since 2003. Numerous clinical, laboratory, and radiological data of all patients have been prospectively collected during patient hospitalization and follow-up. To select a study group, an initial search of database was performed with the identification of all patients suspected to have NP. Then, plain chest radiographs and thorax CT scans of the selected patients were reviewed by two study clinicians (KK, MS). NP was diagnosed in case of typical radiological pattern in a patient with signs and symptoms of pneumonia. Radiographic criteria for NP were as follows: areas of parenchymal consolidation, lucencies within the area of consolidation and multiple thin-walled cavities (Hodina et al. 2002). Data on etiology (based on the results of blood and pleural fluid culture), prior medical history, clinical features (fever, tachypnea, cough, chest pain, and abdominal pain), physical examination, treatment strategies (systemic antibiotic and local treatment), and outcome were collected from electronic databases and completed from medical records, when necessary. The results of the study were summarized by standard descriptive statistics. A nonparametric Mann-Whitney U test or Fisher exact test were used to assess the difference between quantitative variables in different groups. A p-value <0.05 was regarded as significant.
3 Results
Eight hundred eighty two children with CAP were hospitalized in our institution during the study period. One hundred ninety eight (22.4 %) of them had complicated pneumonia (PPE/empyema, lung abscess, or NP). Necrotizing pneumonia was diagnosed in 32 patients and these children were selected as the study group. Thus, the rate of NP among all CAP patients requiring hospital admission was estimated as 3.7 %. The annual number of patients with NP treated in our center ranged between 4 (2010) and 11 (2009).
The study group included 18 girls and 14 boys; median age 4 years (range between 1 and 10 years). Twenty six children with NP were previously treated in other hospitals and transferred to our referral center because of disease progression or its complications. Comorbidities or underlying diseases were found in seven patients, while the remaining children were otherwise healthy. There were no children with primary or secondary immunodeficiency. Two children had viral infection prior to NP. The most common symptoms on admission were fever, tachypnea, and cough. The median duration of fever before hospital admission and during hospital treatment was 6 days (range 1–10 days) and 9 days (range 0–22 days), respectively. Detailed data on medical history and clinical presentation of NP are summarized in Table 1.
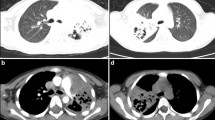
In all children, areas of lung consolidation, lucencies within consolidation areas and multiple thin–walled cavities were identified in plain chest radiograph or CT scans. In 18 and 10 children the abnormal radiographic appearance was limited to the right and left lung, respectively, while 4 patients presented with bilateral lung involvement. Pleural effusion was revealed in 31 patients and pneumothorax in 2 cases (Figs. 1 and 2).
The results of laboratory tests on admission are presented in Table 2.
There were significantly elevated acute phase reactants, with the median CRP value of 18.2 mg/dl (normal range <1 mg/dl) (IQR 15.2–24.1). The median WBC count was 21.3 × 109/L (IQR 17.7–25.2) with the polymorphonuclear cell predominance. Anemia was a common laboratory finding (median Hb concentration – 89.0 g/L, IQR 84.0–99.0 g/L). Fourteen children (43.8 %) required packed red blood cell transfusion. One child had a reduced platelet count, while the remaining children presented with thrombocytosis (median platelet count 818.5 × 109/L, (IQR 557.3–1069.3)). There was a significant percentage of children with declined total serum protein and albumin concentration (56.3 % and 84.4 %, respectively).
All children had blood culture taken on admission. In 20 children, additional blood culture results from regional hospitals were available for analysis. Microbiological studies of pleural fluid were performed in all 31 patients presenting with pleural effusion. Positive results of blood and pleural fluid analysis were found in only 6 (18.8 %) and 7 (22.5 %) children, respectively. Ultimately, blood and pleural fluid cultures enabled identification of the pathogens responsible for NP in 12 patients (1 patient had both blood and pleural fluid cultures positive). The most commonly identified species was Streptococcus pneumoniae (n = 8), followed by Staphylococcus aureus (n = 2), Streptococcus milleri (n = 1), Staphylococcus epidermidis (n = 1) and Stenotrophomonas maltophilia (n = 1). All Streptococcus pneumoniae strains were susceptible to penicillin and Staphylococcus aureus strains were sensitive to methicillin (MSSA).
In all children, antibiotic therapy had been initiated before admission to the referral center. Amoxicillin, ampicillin, amoxicillin/clavulanic acid and cefuroxime were most commonly applied as the first line of treatment. There was no difference between the duration of pre-admission antibiotic therapy in children treated at home (median 3 days; IQR 1.75–4.25) and transferred from other hospitals (median 3 days; IQR 2–6). Due to prolonged symptoms, the initial therapy was switched to the second-line antibiotic treatment in all patients after admission to our center. The second-line therapy included cefotaxime, ceftriaxone, clindamycin, vancomycin, and carbapenems. At least a two drug-regimen was used in all patients. The median duration of antibiotic treatment was 28 days (IQR 22.5–32.5). All but one patient had NP associated complications with PPE/empyema being the most common (Table 3).
Thus, 31 children required additional local treatment (see below). The results of pleural fluid analysis are summarized in Table 4. Two children only required therapeutic thoracentesis, while in 29 (93.5 %) children the chest tube insertion and pleural drainage were necessary. Due to inadequate pleural fluid drainage one patient was subsequently treated with video- assisted thoracoscopic surgery (VATS). Intrapleural fibrynolitic treatment with urokinase was applied in 25 (78.1 %) children. The median duration of pleural drainage was 8.6 days (IQR 6–11.25, range 2–27).
In two children with PPE/pleural empyema small/medium pneumothorax (pyopneumothorax) was found. Neither of them demonstrated an air leak after the chest tube insertion and both were successfully treated with pleural drainage.
Eight children (25 %) with PPE/empyema developed signs of BPF during treatment with pleural drainage. In all these patients, spontaneous healing of BPF was achieved and none of the children required surgical treatment. The median duration of air leak was 10.5 days (IQR 7–17, range 5–20). The duration of pleural drainage in children with BPF was significantly longer than in children without BPF (16 vs. 7 days, p < 0.001).
No deaths occurred in the study group. The duration of hospital stay ranged between 13 and 44 days (median 26). All children had follow-up visits 1 and 6 months after hospital discharge. At the first visit, physical examination revealed asymmetry of the chest and decreased breath sounds in the previously affected areas in 10 (31.3 %) and 14 (45.2 %) children, respectively. Chest radiographs showed residual pulmonary and pleural lesions in all patients. Five months later, none of the children revealed any abnormality on physical examination. Moreover, in all patients chest radiograph showed complete or almost complete resolution of pulmonary and pleural lesions.
4 Discussion
Our study of 32 patients with NP showed that this entity affects mainly immunocompetent children with no underlying disorders and the most common causative organism is Streptococcus pneumoniae. The clinical course of NP is usually complicated by PPE/empyema, BPF, and pneumothorax. Nevertheless, NP can be successfully treated with antibiotics and pleural drainage.
Although pneumonia with associated lung necrosis was reported as early as in the nineteenth century, the first detailed description of four pediatric cases of NP caused by Streptococcus pneumoniae was published only 20 years ago (Kerem et al. 1994). Since then, a significant increase in the incidence of this entity has been reported (Lemaître et al. 2013; Bender et al. 2008; Sawicki et al. 2008; Hsieh et al. 2004). Bender et al. (2008) have found a more than five-fold increase in the incidence of NP caused by Streptococcus pneumoniae between 1997–2000 and 2000–2006. An increasing incidence (two-fold) of NP caused by Streptococcus pneumoniae was also reported in a Taiwan study covering the period from 2001 to 2010 (Hsieh et al. 2011). The incidence of NP shows seasonal variations. The majority of cases in our study were diagnosed during the fall and winter seasons (15 and 12 cases, respectively). Substantial year-to-year variations in the number of new cases were also found with an unexpectedly high number of children with NP (11) in 2009. It might be speculated that this was related to influenza A [H1N1] pandemic occurring in the same period of time. A similar observation was noticed by Lemaître et al. (2013).
Streptococcus pneumoniae is regarded as the most common etiologic factor of NP in children (Tsai and Ku 2012; Ramphul et al. 2006; Hacimustafaoglu et al. 2004). Sawicki et al. (2008) have been able to establish the etiology of NP in 38 (48 %) of 80 children treated in a Children’s Hospital in Boston, MA. Streptococcus pneumoniae was responsible for 18 (22 %) of all cases and for 47.4 % of cases with known etiology. In the present study, the causative organism was identified in 12 children (37.5 %), and pneumococcal predominance was even more significant. Streptococcus pneumoniae was diagnosed as the etiologic factor in more than 58 % of children with NP of known etiology.
On the other hand, the increasing incidence of NP caused by other organisms has also been demonstrated. This was the case in the study by Sawicki et al. (2008), where methicillin resistant Staphylococcus aureus, and Streptococcal spp. such as S. milleri were increasingly identified during the study period (1990–2005). In our study, Staphylococcus aureus was responsible for two cases of NP and both strains were sensitive to methicillin. In a recently published French study which included 41 children the etiology of NP was established in 51 % (21 cases). The most common cause of NP was Staphylococcus aureus (13 cases, 61.9 %). All Staphylococcus aureus strains encoded genes of Panton –Valentine leucocidin and all but one were sensitive to methicillin (Lemaître et al. 2013). Other organisms were also incidentally isolated from patients with NP. Those included Pseudomonas aeruginosa, Fusobacterium spp., Streptococcus pyogenes, Staphylococcus epidermidis (Lemaître et al. 2013; Sawicki et al. 2008). Among atypical bacteria, Mycoplasma pneumoniae seems responsible for rare cases of NP in children (Wang et al. 2004; Wong et al. 2000). Anaerobic bacteria are considered to play a synergistic role in causing NP, but this data come from adult NP cases (Tsai and Ku 2012; Palmacci et al. 2009).
Necrotizing pneumonia should be suspected in all severely ill children with prolonged fever and significantly elevated serum inflammatory markers. Imaging studies, including chest radiograph, and CT scan play a crucial role in the diagnosis of NP. This, in particular, refers to the chest CT scan, which can show necrotizing/cavitary lesions not visible in plain chest radiographs. Thus, CT scan is regarded as the most sensitive diagnostic tool in patients with NP (Tsai and Ku 2012; Sawicki et al. 2008). On the other hand, justification to perform thorax CT scan in all children with cavities in a consolidated lung, a typical appearance of NP seen in the chest radiograph, might by a matter of controversy. It seems that in the era of increasing awareness of radiographic and clinical features of NP, initial chest CT scan is not always a necessary prerequisite for the adequate treatment. This is of particular importance in view of the fact that a significant proportion of children may require subsequent thorax CT scanning due to systemic or local treatment failure. Repeated thorax CT imaging may result in increased radiation exposure.
Despite the undeniable progress in the treatment of NP, controversies regarding the most effective treatment strategies still exist. As to date, no randomized trial comparing different treatment modalities have been performed. Data on treatment efficacy come exclusively from observational studies. Due to a significant variability in the natural course of NP, including its local complications, comparison of the effectiveness of different therapeutic regimens is particularly difficult. Intravenous antibiotics are the cornerstone of the effective treatment. In nearly all cases empirical antibiotic therapy is initially administered. The choice of antibiotics should be based on local epidemiological and microbiological data. Positive results of the microbiological studies identifying etiological factors might be expected only in 11–51 % of patients (Lemaître et al. 2013; Sawicki et al. 2008; Hacimustafaoglu et al. 2004; Wong et al. 2000). In our study, this was the case in 12 (37.5 %) of patients. These results should be used as a guide for further antibiotic treatment. Nevertheless, we did not find differences in the outcome measures in children in whom the causative pathogen was identified and those in whom it was not. Prolonged antibiotic treatment is recommended. In our study, the median duration of antibiotic therapy was 28 days. Similar duration was reported by other authors (Sawicki et al. 2008). Broad-spectrum penicillins, second or third generation of cephalosporins, clindamycin, and vancomycin have been the most commonly used.
Necrotizing pneumonia in children is associated with a very high risk of local complications. In our study, PPE, or pleural empyema were found in as many as 31/32 (97 %) of all patients. The incidence of pleural effusion reported in other studies has been as similar and ranged between 63 and 94 % (Lemaître et al. 2013; Hacimustafaoglu et al. 2004). Thus, it might be stated that pleural complications belong to the typical clinical characteristics of the disease. Treatment of NP associated pleural effusion does not differ from that presented in the guidelines on management of pleural effusion/empyema and include therapeutic thoracentesis, pleural drainage (with or without intrapleural instillation of fibrynolytic agents) and VATS (Balfour-Lynn et al. 2005). The choice between these methods is mainly based on illness severity and local anatomical conditions (pleural fluid volume, its location, the presence of adhesions, etc.). However, personal and hospital experience is an important factor affecting the management strategy. Although an early VATS is certainly justified in some patients’ management, pleural drainage with chest tube (with or without intrapleural fibrinolytics) is probably a sufficient therapeutic option in the vast majority of children. This was shown in our study. Pleural drainage was applied in 28/32 (87.5 %) of our patients and it was shown to be an effective method of treatment in 27 (96.4 %) of them. None of our patients was primarily scheduled for VATS, and only one patient required surgical intervention when local treatment with pleural drainage failed. As NP is a relatively rare condition, no randomized trial comparing the treatment efficacy of pleural drainage or pleural drainage plus intrapleural fibrinolitics vs. VATS has been published to-date. This is not surprising in the context of only several randomized studies comparing the efficacy of these methods in all children with PPE/pleural empyema (Krenke et al. 2010). The second most common local complication of NP is BPF. The incidence of BPF has been reported between 15 and 67 % (Sawicki et al. 2008; Hacimustafaoglu et al. 2004). In our study, BPF was diagnosed in 8/32 (25 %) of children. Interestingly, pneumothorax was not revealed in the chest radiograph taken before chest tube insertion in any of these children, and in all eight patients BPF developed during pleural drainage applied to treat PPE/empyema. It should be noted that the incidence of BPF has been associated with the duration of pleural drainage (Sawicki et al. 2008). Thus, the duration of pleural drainage is a risk factor for the development of BPF and the chest tube inserted to treat PPE/empyema in children with NP should be removed as early as possible and prolonged pleural drainage in these patients should be avoided. Likewise, the present study showed that the duration of pleural drainage in children with BPF was significantly longer than that in children without BPF. The duration of air leak ranged between 5 and 20 days and the hospital stay was significantly longer in children with BPF than without it. In all our patients, BPF healed spontaneously during pleural drainage and none of these children required surgical intervention. This is consistent with the observations of other authors (Sawicki et al. 2008). It might be speculated that adequate systemic (antibiotics) and local treatment (pleural drainage) result in BPF healing and its closure.
Our analysis confirms the observations of other centers that despite severe clinical course of NP in children, the prognosis is good. No deaths occurred in our study group. Likewise, favorable outcome has been demonstrated in three other respective large series which included 80, 41, and 21 patients (Lemaître et al. 2013; Sawicki et al. 2008; Wong et al. 2000). However, as some fatal cases have been also reported, one should be aware of the risk of death due to NP (Al-Saleh et al. 2008; Hacimustafaoglu et al. 2004). Li et al. (2011) have reviewed the factors associated with increased risk of fatal outcome in patients with community-acquired NP caused by Staphylococcus aureus and found that influenza-like symptoms, hemoptysis, and leucopenia are the predictors of unfavorable prognosis. As adolescent patients represented only 10 % of the study group, these findings should not be directly extrapolated to the entire pediatric population.
There are data suggesting that early surgical resection of the affected lung might be associated with less favorable outcome. In a study by Westphal et al. (2010), the mortality rate in 20 children who underwent surgical intervention was as high as 20 %. A high percentage of complications after surgery was also reported with BPF being the most common (4 children – 20 %). Further studies on the relationship between surgical lung resection and outcome in children with NP are warranted. According to some larger series, including the present study group, adequate conservative treatment results in a very low mortality rate; surgical lung resection should thus be limited to a very small number of carefully selected cases.
To conclude, the present study shows that NP affects mainly immunocompetent children with no underlying disorders and the most common causative organism is Streptococcus pneumoniae. Although, the clinical course of NP is usually complicated by PPE/empyema. BPF and pneumothorax, the entity and its complications can be successfully treated with antibiotics and pleural drainage without major surgical intervention.
References
Al-Saleh S, Grasemann H, Cox P (2008) Necrotizing pneumonia complicated by early and late pneumatoceles. Can Respir J 15:129–132
Balfour-Lynn I, Abrahamson E, Cohen G, Hartley J, King S, Parikh D, Spencer D, Thomson AH, Urquhart D (2005) BTS guidelines for the management of pleural infection in children. Thorax 60(Suppl I):i1–i21
Bender JM, Ampofo K, Korgenski K, Daly J, Pavia AT, Mason EO, Byington CL (2008) Pneumococcal necrotizing pneumonia in Utah: dose serotype matter? Clin Infect Dis 46:1346–1352
Hacimustafaoglu M, Celebi S, Sarimehmet H, Gurpinar A, Ercan I (2004) Necrotizing pneumonia in children. Acta Paediatr 93:1172–1177
Hasan RA, Al-Neyadi S, Abuhasna S, Black CP (2011) High-frequency oscillatory ventilation in an infant with necrotizing pneumonia and bronchopleural fistula. Respir Care 56:351–354
Hodina M, Hanquinet S, Cotting J, Schnyder P, Gudinchet F (2002) Imaging of cavitary necrosis in complicated childhood pneumonia. Eur Radiol 12:391–396
Hsieh YC, Hsueh PR, Lu CY, Lee PI, Lee CY, Huang LM (2004) Clinical manifestations and molecular epidemiology of necrotizing pneumonia and empyema caused by Streptococcus pneumoniae in children in Taiwan. Clin Infect Dis 38:830–835
Hsieh YC, Hsiao CH, Tsao PN, Wang JY, Hsueh PR, Chiang BL, Lee WS, Huang LM (2006) Necrotizing pneumococcal pneumonia in children: the role of pulmonary gangrene. Pediatr Pulmonol 41:623–629
Hsieh YC, Wang CW, Lai SH, Lai JY, Wong KS, Huang YC (2011) Necrotizing pneumococcal pneumonia with bronchopleural fistula among children in Taiwan. Pediatr Infect Dis J 30:740–744
Kerem E, Bar Ziv Y, Rudenski B, Katz S, Kleid D, Branski D (1994) Bacteremic necrotizing pneumococcal pneumonia in children. Am J Respir Crit Care Med 149:242–244
Krenke K, Peradzyńska J, Lange J, Ruszczyński M, Kulus M, Szajewska H (2010) Local treatment of empyema in children: a systematic review of randomized controlled trials. Acta Paediatr 99:1449–1453
Lemaître C, Angoulvant F, Gabor F, Makhoul J, Bonacorsi S, Naudin J, Alison M, Faye A, Bingen E, Lorrot M (2013) Necrotizing pneumonia in children: report of 41 cases between 2006 and 2011 in a French Tertiary Care Center. Pediatr Infect Dis J 32:1146–1149
Li HT, Zhang TT, Huang J, Zhou YQ, Zhu JX, Wu BQ (2011) Factors associated with the outcome of life-threatening necrotizing pneumonia due to community-acquired Staphylococcus aureus in adult and adolescent patients. Respiration 81:448–460
Palmacci C, Antocicco M, Bonomo L, Maggi F, Cocchi A, Onder G (2009) Necrotizing pneumonia and sepsis due to Clostridium perfringens: a case report. Cases J 2:50
Ramphul N, Eastham KM, Freeman R, Eltringham G, Kearns AM, Leeming JP, Hasan A, Hamilton LJ, Spencer DA (2006) Cavitatory lung disease complicating empyema in children. Pediatr Pulmonol 41:750–753
Sawicki GS, Lu FL, Valim C, Cleveland RH, Colin AA (2008) Necrotising pneumonia is an increasingly detected complication of pneumonia in children. Eur Respir J 31:1285–1291
Tsai YF, Ku YH (2012) Necrotizing pneumonia: a rare complication of pneumonia requiring special consideration. Curr Opin Pulm Med 18:246–252
Wang RS, Wang SY, Hsieh KS, Chiou YH, Huang IF, Cheng MF, Chiou CC (2004) Necrotizing pneumonitis caused by Mycoplasma pneumoniae in pediatric patients: report of five cases and review of literature. Pediatr Infect Dis J 23:564–567
Westphal FL, Lima LC, Netto JC, Tavares E, Andrade Ede O, Silva Mdos S (2010) Surgical treatment of children with necrotizing pneumonia. J Bras Pneumol 36:716–723
Wong KS, Chiu CH, Yeow KM, Huang YC, Liu HP, Lin TY (2000) Necrotising pneumonitis in children. Eur J Pediatr 159:684–688
Conflicts of Interest
The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Krenke, K. et al. (2014). Necrotizing Pneumonia and Its Complications in Children. In: Pokorski, M. (eds) Pulmonary Infection. Advances in Experimental Medicine and Biology(), vol 857. Springer, Cham. https://doi.org/10.1007/5584_2014_99
Download citation
DOI: https://doi.org/10.1007/5584_2014_99
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17457-0
Online ISBN: 978-3-319-17458-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)