Abstract
Our previous studies show that provinol, a polyphenolic compound, has anti-inflammatory activity during allergic inflammation. In the present study we investigated the effects of provinol and its combinations with clinically used antiasthmatics: budesonide or theophylline on airway defense mechanisms during experimental allergic asthma. Separate groups of guinea pigs were treated during the course of 21-day ovalbumin sensitization with provinol (20 mg/kg/day, p.o.), or budesonide (1 mM by inhalation), or theophylline (10 mg/kg/day, i.p.), and with a half-dose combination of provinol+budesonide or provinol+theophylline. Airways defense mechanisms: cough reflex and specific airway resistance (sRaw) were evaluated in vivo. Tracheal smooth muscle reactivity and mucociliary clearance were examined in vitro. The findings were that provinol caused significant decreases in sRaw and in tracheal smooth muscle contractility, a suppression of cough reflex, and positively modulated ciliary beat frequency. The bronchodilatory and antitussive effects of provinol were comparable with those of budesonide and theophylline. Provinol given as add-on treatment significantly potentiated the effects of budesonide or theophylline, although the doses of each were halved. We conclude that provinol not only has bronchodilatory and antitussive effects, but also potentiates similar effects exerted by budesonide and theophylline.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Asthma is a chronic inflammatory disease of lower airways that affects 300 million people worldwide (Bossé 2012; Umetsu and DeKruyff 2006). Currently, treatment of asthma includes β2-adrenergic antagonists, anticholinergics, corticosteroids, leukotriene antagonists, theophylline, and antiIgE medicines. These therapies provide relief only to a fraction of patients and the side effect profiles limit their use (Kandhare et al. 2013). Therefore, other substances with and anti-inflammatory efficiency are searched for. One such group is polyphenolic substances contained in fruits, vegetables, nuts, herbs, cocoa, tea, and red wine (Tangney and Rasmussen 2013). Polyphenols are recognized to possess antioxidant properties and to positively affect pathophysiological processes in the cardiovascular system (Boyer and Liu 2004). Antiallergic, anti-inflammatory and bronchodilatory properties of polyphenols have also been recorded in the respiratory tract (Homma et al. 2000).
Red wine is a rich source of a variety of polyphenols with multiple biological activities (Šeruga et al. 2011). In previous studies, we monitored the effects of provinol (dry powder, mixture of polyphenolic compounds from red wine) on experimental model of allergic asthma. Franova et al. (2011) confirmed that oral administration of provinol positively influenced the airway inflammation by reducing the level of inflammatory cytokines. Furthermore, provinol inhibited histamine-induced airway smooth muscle hyperreactivity in guinea pigs sensitized with ovalbumine (Franova et al. 2007).
The aim of the present study was to evaluate the long-term effect of provinol and its combinations with clinically used antiasthmatics: budesonide and theophylline on allergen-induced experimental asthma in guinea pigs. The outcome measures in this study were the airway defense mechanisms: cough reflex and specific airway resistance in vivo and tracheal smooth muscle reactivity and mucociliary clearance in vitro.
2 Methods
The study was approved by the Ethics Committee of Jessenius Faculty of Medicine in Martin, Slovakia (permit No. EK 1178/2012). Provinol (dry powder of red wine polyphenolic compounds) was provided by D. Ageron (Société Francaise de Distillerie, Vallont Pont d’ Arc, France). Ovalbumin (OVA, egg albumin, grade III), histamine (histamine- 2HCl), citric acid, and other chemicals were purchased from Sigma-Aldrich Chemicals (St. Louis, MO).
2.1 Animals
Male guinea pigs (TRIK, 200–350 g) were randomly divided into seven experimental groups consisting of ten animals each:
-
Group 1: control – treated for 21 days with saline
-
Group 2: sensitized 21 days with OVA
-
Group 3: sensitized 21 day with OVA and treated with provinol (20 mg/kg, daily, p.o.)
-
Group 4: sensitized 21 day with OVA and treated with budesonide (1 mM during 5 min nebulization)
-
Group 5: sensitized 21 day with OVA and treated with theophylline (10 mg/kg, daily, i.p.)
-
Group 6: sensitized 21 day with OVA and treated daily with provinol plus budesonide, with half-doses of the above outlined both drugs
-
Group 7: sensitized 21 day with OVA and treated daily with provinol plus theophylline, with half-doses of the above outlined both drugs.
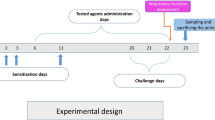
2.2 Sensitization of Guinea Pigs
Guinea pigs were sensitized with 5 mg ovalbumin (OVA) and 1 mg aluminium hydroxide, administered in 1 ml of physiological saline. The allergen was injected both s.c. and i.p. on Day 1; and then i.p. only on Days 4, 11, and 15 and s.c. only on Days 9 and 14. The guinea pigs were daily exposed to nebulized OVA (1 % in 0.9 % NaCl) in an aerosol chamber for the last 6 days of sensitization. The animals were used for in vivo experiments and were sacrificed 24 h after the last day of sensitization.
2.3 Airway Reactivity In Vitro
Reactivity of tracheal smooth muscles was estimated 21 days after sensitization. Tracheal strips were placed in a 20 ml organ baths containing Krebs-Henseleit buffer at 36.5 ± 0.5 °C, pH of 7.5 ± 0.1, and being continuously aerated with a mixture of 95 % O2 and 5 % CO2. The amplitude of contraction (mN) of muscle strips in response to the cumulative doses of histamine (10−8–10−3 mol/l) was used as a measure of smooth muscle reactivity.
2.4 Airway Reactivity In Vivo
Airway resistance was evaluated on Days 7, 14, and 21 of sensitization. The guinea pigs were placed individually in a double-chamber whole-body plethysmograph box (type 855 with Pulmodyn Pennock software; Hugo Sachs Elektronik-Hardvard, Hugstetten, Germany) and specific airway resistance (sRaw) was measured in response to inhalation of the bronchoconstricting mediator histamine (10−6 mol/l) aerosolized in physiological saline. Reactivity after nebulization of saline alone was used as control. There was an interval of 5 min between exposures, during which fresh air was insufflated into the nasal chamber.
2.5 Chemically Induced Cough
Cough was induced by inhalation of 0.3 M citric acid in conscious guinea pigs placed in the double chamber of a body plethysmograph as described above. Cough was monitored by two independent observers and the number of cough efforts was recorded during 3 min of citric acid inhalation. Cough was detected from typical changes in the airflow curve, and cough movements and sounds. Codeine was used as an antitussive standard for comparisons with the effects of the substances tested.
2.6 Evaluation of Mucociliary Clearance
Ciliary beat frequency (CBF) was evaluated by a “brushing” method in vitro. Samples were taken from trachea by a cytology brush and placed on the slides kept at 36.5 ± 0.5 °C. Subsequently, the movement of ciliated epithelium was recorded by BASLER A504KC camera (Interflex Camera Link, Germany) using 256–512 framers per second. The recordings acquired were analyzed with LabVIEW software to generate ciliary regions of interest. The median CBF for the each region of interest in a preparation was selected, followed by the calculation of an arithmetic mean for the preparation.
2.7 Statistical Analysis
All results were expressed as means ± SE. Differences between mean data were assessed with one-way analysis of variance ANOVA. Significant differences were defined as p < 0.05.
3 Results
Provinol alone and in combinations with clinically antiasthmatics decreased sRaw in response to 10−6 mol/l histamine nebulization during the in vivo allergic condition. In all tested groups, a significant decrease of sRaw values, enhanced due to sensitization, was already observed on Day 7 (Fig. 1A). The effects were similar on Day 14 (data not shown), but the strongest sRaw reduction took place on Day 21 of therapy. The bronchodilatory effect of provinol was comparable to those of theophylline and budesonide. However, the add-on provinol potentiated the effects of theophylline or budesonide, although the doses of each were halved (Fig. 1B).
Specific airway resistance (sRAV) after inhalation of histamine (10-6 mol/l) in control healthy guinea pigs; ovalbumin-sensitized (OVA); OVA-sensitized treated with provinol (POVA); OVA-sensitized treated with theophylline (TOVA); OVA-sensitized treated with budesonide (BOVA); OVA-sensitized treated with a combination of provinol+theophylline (PTOVA); and OVA-sensitized treated with provinol+budesonide (PBOVA). Changes were evaluated on Day 7 (Panel A) and Day 21 (Panel B) of sensitization. Data are means ± SE; n = 10 in each group; #p < 0.05; #p < 0.001 for OVA vs. control and *p < 0.05; **p < 0.01; ***p < 0.001 for test substances vs. OVA
Provinol suppressed the number of cough efforts induced in guinea pigs by citric acid aerosol (10−3 mol/l) on Day 7 of OVA-sensitization (Fig. 2A). Provinol’s cough suppressive effect, although significant, was less than that of the clinical antitussive codeine, but it was comparable with budesonide and theophylline. Furthermore, the half-dose combination of provinol+budesonide exceeded the antitussive effect of either substance used in monotherapy on Day 21 of sensitization (Fig. 2B).
Cough reflex after inhalation of citric acid (10−3 mol/l) in control healthy guinea pigs); ovalbumin-sensitized (OVA); OVA-sensitized treated with codeine; OVA-sensitized treated with provinol (POVA); OVA-sensitized treated with theophylline (TOVA); OVA-sensitized treated with budesonide (BOVA); OVA-sensitized treated with a combination of provinol + theophylline (PTOVA); OVA-sensitized treated with provinol + budesonide (PBOVA). Changes were evaluated on Day 7 (Panel A) and Day 21 (Panel B) of sensitization. Data are means ± SE; n = 10 in each group; #p < 0.05; #p < 0.001 for OVA vs. control and *p < 0.05; **p < 0.01; ***p < 0.001 for test substances vs. OVA
The effects of provinol, budesonide, and theophylline on tracheal smooth muscle reactivity, after their administration over the 21-day OVA-sensitization period were evaluated in response to cumulative doses of histamine (10−8–10−3 mol/l). Provinol inhibited the OVA-enhanced contractile airway response to histamine. The combination therapy provinol+budesonide and provinol+theophylline failed, however, to show a further smooth muscle contraction easing compared with monotherapy (Fig. 3).
Tracheal smooth muscle contractile response to cumulative doses of histamine (10−8–10−3 mol/l) in control healthy guinea pigs; OVA-sensitized (OVA); OVA-sensitized treated with provinol (POVA); OVA-sensitized treated with theophylline (TOVA); OVA-sensitized treated with budesonide (BOVA); OVA-sensitized treated with a combination of provinol+theophylline (PTOVA); and OVA-sensitized treated with provinol+budesonide (PBOVA). Data are means ± SE; n = 10 for each group; **p < 0.01 for OVA vs. control
Mucociliary clearance was assessed 24 h after the last challenge with OVA. Provinol as well as its combination with budesonide led to a significant reduction in ciliary beating frequency. The other substances tested failed to significantly affect the mucociliary clearance; except for theophylline after which ciliary beating frequency was increased (Fig. 4).
Ciliary beat frequency in control healthy guinea pigs; OVA-sensitized (OVA); OVA-sensitized treated with provinol (POVA); OVA-sensitized treated with theophylline (TOVA); OVA-sensitized treated with budesonide (BOVA); OVA-sensitized treated with a combination of provinol+theophylline (PTOVA); and OVA-sensitized treated with provinol+budesonide (PBOVA). Data are means ± SE; n = 10 for each group; *p < 0.05; **p < 0.01 for test substances vs. OVA
4 Discussion
Activation of inflammatory cells in allergen-induced bronchial asthma leads to the release of mediators which, cause bronchial smooth muscle contraction, inflammatory cell infiltration, mucus hypersecretion, airway hyperresponsiveness and, ultimately, airway remodeling (Jung et al. 2010; Bousquet et al. 2000). In the present study, we evaluated the effects of long-term administration of provinol, a polyphenolic compound mixture present in red wine, on experimental allergic asthma in guinea pigs. Provinol contains more than 95 % polyphenols consisting of 480 mg proantocyanins, 61 mg anthocyanins, 38 mg catechins, 18 mg hydroxycinnamic acid, 14 mg flavonols, and 370 mg polymeric tannins per g of dry powder. We found that provinol exerted a bronchodilating effect, as judged from its being a reducer of bronchial smooth muscle reactivity in vitro, an antitussive effect, consisting of decreased cough efforts, and it positively modulated mucociliary clearance in the bronchial tree. Provinol also mitigated bronchial hyperreactivity assessed from the response to histamine. These actions were grossly comparable with those of budesonide and theophylline used as reference drugs. Moreover, a mixture of provinol with either budesonide or theophylline appreciably enhanced the antitussive and lessening bronchial hyperactivity effects, compared with the effects of monotherapy, which in addition was observed at a half-dose of each compound enabling at the same time to lower the dose of each drug in half. The possibility to lower the dose of drugs used in allergic asthma, with an apparent gain in beneficial efficacy, by the add-on treatment with provinol, or, by inference, by the long-term use of dietary supplements containing provinol or related compounds, seems the most worthwhile conclusion drawn from the present study.
Wine phenolics have been shown to posses several health promoting activities (Ali et al. 2013). These protective effects could be due to one or many components of the complex mixture of bioactive compounds present in red wine including resveratrol, flavonols, anthocyanins, phenolic acids, as well as their metabolites (Rodrigo et al. 2011). Cruz et al. (2012) showed that quercetin reduces airway hyperreactivity and inflammation by suppressing mast cell degranulation. Chlorogenic acid present in wine may help reduce asthmatic symptoms and incidence asthma (Kim et al. 2010). Provinol has a notable anti-inflammatory and antioxidant activity that may protect against asthma (Franova et al. 2011). It decreases IL-4 and IL-5 in bronchoalveolar lavage fluid. IL-4 is a cytokine directing B lymphocytes to synthesize IgE. In an allergic disease, IgE activates mast cells through interactions with receptors (FcɛRI, FcɛRII) on the cell surface, which leads to the release of the bronchoconstricting mediators histamine and leukotrienes. IL-5 is a critical cytokine in regulating the function and recruitment of eosinophils, which underlies the progression inflammatory processes of allergic asthma (Mauad et al. 2011).
There are other possible ways to affect bronchodilator activity in allergic asthma. Nitric oxide appears to play a key role in airway muscle tone regulation. Inflammatory mediators reduce the function of constitutive NO synthesis (cNOS); thereby negatively affecting the bronchodiling effects of NO. Provinol activates cNOS and inhibits inducible NOS in experimental allergic asthma (Franova et al. 2007). Meeyoung et al. (2013) demonstrated that resveratrol inhibits OVA-induced airway hyperreactivity. The present findings showed that long-term administration of provinol, better yet together with clinically used antiasthmatics, caused a decline in both airway smooth muscle reactivity in vitro and in specific airway resistance after nebulization of histamine in vivo. These findings are of applicable importance considering that enhanced contractility or hypertrophy of airway smooth muscles resulting from inflammation leads to decreased lung function (Wenzel 2006). Bronchodilatators play a central role in asthma treatment. They provide relief of symptoms, but evidence of their adverse effects underlines the need for caution in use. Research develops that involves different combinations of clinical antiasthmatics with substances that may contribute to a dose reduction and to alleviation of adverse effects (Bateman and Boulet 2011). Provinol, a mixture of polyphenolics of red wine, significantly enhanced the antitussive and bronchidilatory effects of theophylline and budesonide in the present study. The antitussive effect of provinol in a dose of 20 mg/kg was comparable with the efficacy of codeine. Cough is a hallmark of respiratory diseases and is an airway defense mechanism closely associated with bronchoconstriction. Although there is no direct evidence that polyphenols act on the cough reflex, Widdicombe (2003) showed that rapidly adapting lung receptors are fully enabled by bronchospasm in asthma patients. Therefore, antitussive effects of provinol might plausibly be attributed to its bronchodilatatory activity.
Mucociliary clearance is yet another defense mechanisms in allergic asthma acting to clear the lungs of bacteria and foreign particulate matter. It is a well-coordinated system consisting of airway secretory cells that produce a mucus layer on the airway surface and ciliated cells that propel the mucus up and out of the lungs (Bennett 2002). In the present study we found that ovalbumine induced a significant increase in ciliary beat frequency. Likewise, theophylline increased the frequency of cilia beating during the allergic inflammation. The frequency of ciliary beating is a phenomenon not yet fully elucidated. Therefore, we cannot evaluate the importance of this effect. However, provinol alone and in combination with budesonide reversed the frequency of cilia beating to the baseline level, which is presumed to be related to the anti-inflammatory activity of these substances. Goh et al. (2012) reported that ovalbumin-challenged mice developed goblet cell hyperplasia and mucus hypersecretion in the bronchi; the latter being suppressed by fisetin, a flavonol belonging to the group of polyphenols. That supports the notion of that polyphenolic substances are at play in mucociliary clearance.
In conclusion, we found that a polyphenolic compound mixture of red wine affected the airway defense mechanisms in a beneficial way, mostly consisting of bronchodilatory and antitussive effects. Further more, provinol as add-on to the standard drugs budesonide and theophylline allowed slashing the dosage of individual substances in half, with a better therapeutic efficacy achieved than that resulting from monotherapy. The study shows that provinol could be an adjunct treatment of allergic asthma.
References
Ali K, Iqbal M, Fortes AM, Pais MS, Korthout AAJ, Verpoorte R, Choi YH (2013) Red wines attenuate TNFα production in human histiocytic lymphoma cell line: an NMR spectroscopy and chemometrics based study. Food Chem 14:3124–3130
Bateman ED, Boulet LP (2011) Bronchodilator therapies for severe asthma. Eur Respir Monogr 51:253–257
Bennett WD (2002) Effect of β-adrenergic agonists on mucociliary clearance. J Allergy Clin Immunol 110:291–297
Bossé Y (2012) Asthmatic airway hyperresponsiveness: the ants in the tree. Trends Mol Med 18:627–633
Bousquet J, Jeffery P, Busse WW, Johnson M, Vignola AM (2000) Asthma. From bronchoconstriction to airway inflammation and remodeling. Am J Respir Crit Care Med 161:1720–1745
Boyer J, Liu RH (2004) Apple phytochemicals and their health benefits. Nutr J 3:5
Cruz EA, Reuter S, Martin H, Dehzad N, Muzitano MF, Costa SS, Rossi-Bergman B, Buhl R, Stassen M, Taube C (2012) Kalanchoe pinnata inhibits mast cell activation and prevents allergic airway disease. Phytomedicine 19:115–121
Franova S, Nosalova G, Pechanova O, Sutovska M (2007) Red wine polyphenolic compounds inhibit tracheal smooth muscle contraction during allergen-induced hyperreactivity of the airways. J Pharm Pharmacol 59:727–732
Franova S, Joskova M, Sutovska M, Novakova E, Adamicova K, Pechanova O, Nosalova G (2011) The efficiency of polyphenolic compounds on allergen induced hyperreactivity of the airways. Biomed Prev Nutr 1:232–235
Goh FY, Upton N, Guan S, Cheng C, Shanmugam MK, Sethi G, Leung BP, Wong WS (2012) Fisetin, a bioactive flavonol, attenuates allergic airway inflammation trough negative regulation of NF-κB. Eur J Pharmacol 679:109–116
Homma M, Minam M, Taniguchi C, Oka K, Morita S, Nitsuma T, Hayashi T (2000) Inhibitory effects of lignans and flavonoids in saiboku-to. A herbal medicine for bronchial asthma, on the release of leukotrienes from human polymorphonuclear leukocytes. Planta Med 66:88–91
Jung CH, Lee JY, Park JH, Cho BJ, Sim SS, Kim CJ (2010) Flavonols attenuate the immediate and late-phase asthmatic response to aerosolized-ovalbumin exposure in the conscious guinea pig. Fitoterapia 81:803–812
Kandhare AD, Bodhankar SL, Singh V, Mohan V, Thakurdesai PA (2013) Anti-asthmatic effects of type-A procyanidine polyphenols from cinnamon bark in ovalbumin-induced airway hyperresponsiveness in laboratory animals. Biomed Aging Pathol 3:23–30
Kim HR, Lee DM, Lee SH, Seong AR, Gin DW, Hwang JA, Park JH (2010) Chlorogenic acid suppresses pulmonary eosinophilia, IgE production, and Th2-type cytokine production in an ovalbumin-induced allergic asthma: activation of STAT-6 and JNK is inhibited by chlorogenic acid. Int Immunopharmacol 10:1242–1248
Mauad T, Poon AH, Hamid Q (2011) Pathology, inflammation and cytokines of severe asthma. Eur Respir Monogr 51:97–106
Meeyoung L, Soyoung K, Ok-Kyoung K, Sei-Ryang O, Hyeong-Kyu L, Kyungseop A (2013) Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic njgf from cinnamon bark in ovalbumin-induced airway hyperresponsiveness in laboratory animals. Biomed Aging Pathol 3:23–30
Rodrigo R, Miranda A, Vergara L (2011) Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin Chim Acta 412:410–424
Šeruga M, Novak I, Jakobek L (2011) Determination of polyphenols content and antioxidant activity of some red wines by differential pulse voltammetry, HPLC and spectrophotometric methods. Food Chem 124:1208–1216
Tangney CC, Rasmussen HE (2013) Polyphenols, inflammation and cardiovascular disease. Curr Atheroscler Rep 15:324. doi:10.1007/s11883-013-0324-x
Umetsu DT, DeKruyff RH (2006) The regulation of allergy and asthma. Immunol Rev 212:238–255
Wenzel SE (2006) Asthma: defining of the persistent adult phenotypes. Lancet 368:804–813
Widdicombe JG (2003) Functional morphology and physiology of pulmonary rapidly adapting receptors (RARs). Anat Rec A: Discov Mol Cell Evol Biol 270:2–10
Acknowledgments
This work was supported by the Slovak Research and Development Agency contract no. APVV-0305-12; CEKR II and by grants VEGA 1/0020/11 and MZ 2012/35-UKMA-12. The project was co-financed from the EU sources for increasing the opportunities for career growth in research and development in medical sciences.
Conflicts of Interest
The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kazimierová, I., Jošková, M., Pecháňová, O., Šutovská, M., Fraňová, S. (2014). Effects of Provinol and Its Combinations with Clinically Used Antiasthmatics on Airway Defense Mechanisms in Experimental Allergic Asthma. In: Pokorski, M. (eds) Allergens and Airway Hyperreactivity. Advances in Experimental Medicine and Biology(), vol 838. Springer, Cham. https://doi.org/10.1007/5584_2014_75
Download citation
DOI: https://doi.org/10.1007/5584_2014_75
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-10008-1
Online ISBN: 978-3-319-10009-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)