Abstract
Dopamine (DA) is a putative neurotransmitter in the carotid body engaged in the generation of the hypoxic ventilatory response (HVR). However, the action of endogenous DA is unsettled. This study seeks to determine the ventilatory effects of increased availability of endogenous DA caused by inhibition of DA enzymatic breakdown. The peripheral inhibitor of MAO – debrisoquine, or COMT – entacapone, or both combined were injected to conscious rats. Ventilation and its responses to acute 8 % O2 in N2 were investigated in a whole body plethysmograph. We found that inhibition of MAO augmented the hyperventilatory response to hypoxia. Inhibition of COMT failed to influence the hypoxic response. However, simultaneous inhibition of both enzymes, the case in which endogenous availability of DA should increase the most, reversed the hypoxic augmentation of ventilation induced by MAO-inhibition. The inference is that when MAO alone is blocked, COMT takes over DA degradation in a compensatory way, which lowers the availability of DA, resulting in a higher intensity of the HVR. We conclude that MAO is the enzyme predominantly engaged in the chemoventilatory effects of DA. Furthermore, the findings imply that endogenous DA is inhibitory, rather than stimulatory, for hypoxic ventilation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Carotid body
- Catechol-O-methyltransferase
- Dopaminergic system
- Endogenous dopamine
- Hypoxic ventilatory response
- Minute ventilation
- Monoaminooxidase
1 Introduction
Dopamine (DA) is present in a substantial amount and is considered a putative neurotransmitter in the carotid body (Hellström 1977), a paired sensory organ of neural crest origin whose chemoreceptor cells generate the excitatory response to chemical stimuli, most notably to reductions in partial oxygen pressure in arterial blood (PaO2). DA is released from carotid chemoreceptor cells in proportion to the strength of the hypoxic stimulus and binds to D2 receptors on the plasma membrane to trigger the cellular transduction cascade, ending up in increased discharge rate in the sinus nerve endings apposing chemoreceptors (Gonzalez et al. 1994). The carotid body discharge is then relayed to the brain stem respiratory areas to evoke a hyperventilatory response (Faff et al. 1999).
Exogenous DA and D2 receptor antagonists have been extensively used as pharmacological tools, taking advantage of the incapability of the hydrophilic DA to cross the blood-brain barrier, which enables to study the peripheral DA-mediated effects without vagueness. Nevertheless, studies failed to determine the exact role of DA in ventilatory regulation and the issue remains contentious. DA seems to have a well established inhibitory role for ventilation and its responses to acute hypoxia in the majority of species, such as the cat (Llados and Zapata 1978), the rabbit (Matsumoto et al. 1980), or the rat (Bee and Pallot 1995; Monteiro et al. 2011), but not in the dog where it has a stimulatory effect (Black et al. 1972). However, the notion of DA-mediated ventilatory inhibition has found support in the action of domperidone, a peripheral D2 antagonist, which increases ventilation (Bee and Pallot 1995), although in some reports the increase has only been found after birth and was lost with maturation (Tomares et al. 1994). The issue is further confounded by the postulate of the existence of the low affinity excitatory post-synaptic carotid body dopamine D2 receptor responding to high doses of DA, as opposed to the inhibitory effects on the hypoxic ventilator responses exerted by low doses of DA through high affinity D2 receptors in animals and man (Gonzales et al. 1994; Ward and Bellville 1982). The probable presence of two subtypes of D2 receptors, differing in affinity to DA, makes an understanding of the action of endogenous DA, released in a small concentration, unclear.
On the premise that DA is basically inhibitory for ventilation as mentioned above we made the hypothesis that the stimulatory ventilatory response to hypoxia might be attenuated by the inhibition of DA breakdown, and thus enhancement of its endogenous availability for the D2 receptors within the carotid body. There are reports showing that blockade of MAO and COMT effectively increases the dopamine content in a tissue (Wang et al. 2001). Therefore, we addressed this issue in conscious rats by using specific peripheral antagonists of the two enzymes responsible for DA degradation: monoaminooxidase (MAO) and catechol-O-methyltransferase (COMT). We established that MAO is the predominating enzyme engaged in the chemosensory effects of DA degradation in the carotid body. Although inhibition of MAO augmented the hyperventilatory response to hypoxia, the augmentation was abrogated by the simultaneous addition of COMT; the situation when the accumulation of endogenous DA should be the most. The inference is that endogenous DA is inhibitory for ventilation. The abrogation of MAO-induced ventilatory stimulation, when both COMT and MAO inhibitors were used, could be due to COMT taking over DA degradation when MAO alone is blocked; a phenomenon reported in the literature (Trendelenburg 1984).
2 Methods
2.1 Animals and Instrumentation
The study was approved by the IV Local Ethics Committee for Animal Experiments in Warsaw, Poland (Permit Number: 29/2010) and was conducted in accord with the guiding principles for the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Council of Europe No 123, Strasbourg 1985). A total of 26 adult male, conscious Wistar rats, weighing 299.9 ± 3.0(SE) g were used. All rats were kept on a 12 h light-dark cycle, temperature of 21 ± 2 °C, humidity of 50–60 %, and were fed with a standard animal chow and had water ad libitum. The animals were divided into the following 4 groups: control group (n = 5) that received the vehicle – 0.3 ml dimethylsulfoxide (DMSO), the debrisoquine group (n = 7) that received 40 mg/kg of the peripheral MAO inhibitor debrisoquine, the entacapone group (n = 7) that received 30 mg · kg−1 of the peripheral COMT inhibitor entacapone, and finally the last group (n = 7) received both inhibitors in the above-mentioned doses. All drugs were administered intraperitoneally in a 0.3 ml volume; entacapone being dissolved in DMSO and debrisoquine in physiological saline, and the injections of both inhibitors were made 2 min apart symmetrically into either side of the peritoneal cavity. Entacapone was generously provided by Orion Corporation – Orion Pharma (Espoo, Finland) and debrisoquine was purchased from Sigma-Aldrich (St. Louis, MO).
Lung ventilation and its responses to acute hypoxia were measured in a whole body rodent plethysmograph (model PLY3223; Buxco Electronix Inc., Wilmington, NC). Each unrestricted rat was placed in the recording chamber. Chamber temperature was maintained constant at 21 °C throughout the experiment. Bias flow at a rate of 2.5 l · min−1 between the hypoxic tests was used, via a flow pump reservoir system (PLY1020, Buxco Electronics), for removing CO2 build-up from the chamber. Pressure difference between the experimental and reference chamber was measured with a differential pressure transducer. The pressure signal was amplified and then integrated by data analysis software (Biosystem XA for Windows SFT3410 v. 2.9; Buxco Electronics).
2.2 Ventilatory Measurements
Volume (V T ) and frequency (f) components of lung ventilation were measured breath-by-breath were processed to yield instantaneous minute ventilation (VE, ml · min−1, BTPS). Data were further processed off-line to show the 30 s time points of the 180 s course of the hypoxic ventilatory response. Each point constituted an average of a 10 s bin of a given variable preceding the 30 s time mark.
2.3 Study Protocol
Each animal was used once. At the beginning of the experiment, the animal was allowed to accustom to the chamber in ambient air for about 15 min. Then, the gas in the chamber was switched to 8 % O2 in N2. The equilibrium of a gas mixture in the chamber was achieved within 40 s, after which a 3-min hypoxic poikilocapnic recording started. During the recovery period in room air, the DMSO vehicle or the enzymes’ inhibitors were injected according to the scheme and doses above outlined. The hypoxic tests were repeated after 30 and 60 min from the injection in like manner.
2.4 Blood Pressure
Blood pressure was measured noninvasively with a CODA tail-cuff blood pressure system using a volume pressure recording (VPR) sensor technology and software that enables to continuously monitor data in real-time (Kent Scientific, Torrington, CT, USA).
2.5 Data Evaluation
Data were presented as means ± SE. Ventilatory variables were normalized to weight in kg. The data turned out to be normally distributed; checked with the Shapiro-Wilk test. Statistical elaboration took into account three main points of interest characterizing VE changes along the hypoxic time course: prehypoxic baseline, peak hypoxic increase, and hypoxic depressant nadir. One-way ANOVA was applied to compare VE at these time points across each hypoxic profile and also across the corresponding time points of the three experimental conditions: untreated control, 30 min, and 60 min after a given pharmacological intervention. If significant, the source of differences was further evaluated with the post-hoc Scheffe test. Statistical significance was defined as P < 0.05.
3 Results
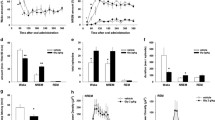
All hypoxic responses recorded in the present study had a classical biphasic stimulatory/inhibitory character. VE peaked at about 30 s from the start of hypoxia, which was followed by a gradual roll-off reaching nadir within 150–180 s. In this depressant phase, VE decreased by about 15–20 % of the peak increase, remaining significantly above the baseline prehypoxic level.
The results show that debrisoquine, 30 min after the injection, significantly enhanced both the resting baseline ventilation and the ventilatory response to 8 % hypoxia along its recorded course. The enhancement was most pronounced at peak response where VE increased from 1.52 ± 0.82 before to 2.33 ± 0.30 l·min−1·kg−1 after debrisoquine; P < 0.05 (Fig. 1A). However, this effect failed to be long-lived. The ventilatory response to hypoxia repeated 60 min after debrisoquine injection showed that the peak VE regressed to 1.66 ± 0.12 l · min−1 · kg−1; a downfall also seen at the following time marks of the hypoxic profile, nearly to the level present in the control untreated condition. The increases in hypoxic ventilation after debrisoquine were achieved due to contributions of both frequency and tidal ventilatory components. Peak breathing frequency amounted to 139 ± 10 breaths·min−1 in the untreated condition and changed to 168 ± 12 and 145 ± 10 breaths · min−1 at 30 and 60 min post-debrisoquine. The corresponding values for tidal component were 12.0 ± 1.6, 14.0 ± 1.6, and 11.0 ± 0.6 ml · kg−1. Although the joint action of both components led to significant changes in VE, changes in either component turned out to be insignificant (P > 0.05, one-way ANOVA).
Hypoxic ventilatory responses in the untreated control condition, and then 30 and 60 min after i.p. administration of debrisoquine (A) and entacapone (B). *p < 0.05 for the differences at the corresponding time marks (vertical) as follows: at 0 s mark – between 30 and 60 min post-debrisoquine VE vs. untreated prehypoxic baseline VE; at 30 s mark – between 30 min post-debrisoquine peak hypoxic VE vs. untreated peak VE, and at 180 s mark – between post-debrisoquine hypoxic VE nadir vs. untreated VE nadir; †p < 0.05 for the differences along the sequential time marks (horizontal) of the hypoxic courses as follows: prehypoxic untreated VE vs. the peak and nadir VE in each experimental condition in both panels. The insets show the augmentation of VE from the normoxic baseline to hypoxic peak 30 and 60 min after debrisoquine (Panel A; *significantly higher 30 min after debrisoquine, P < 0.05) (Panel B – insignificant differences)
Entacapone, on the other hand, failed to appreciably affect the hypoxic ventilatory response either 30 or 60 min after injection (Fig. 1B). Likewise, entacapone combined with debrisoquine failed to affect the course of the hypoxic ventilatory response in a significant way, although there was a tendency remaining for a higher peak hypoxic VE compared with the untreated condition, which was gone 60 min after injection (P > 0.05) (Fig. 2). Thus, the ventilatory augmentation evoked by MAO inhibition alone was gone when the COMT inhibitor was simultaneously used.
Hypoxic ventilatory responses in the untreated control condition, and then 30 and 60 min after debrisoquine and entacapone administered together. There were no significant differences noted at the corresponding time marks (vertical) of the three experimental conditions. †Prehypoxic untreated VE (0 s time mark) significantly lower than peak (30 s time mark) and nadir (180 s time mark) VE in each of the three experimental conditions; p < 0.05. The inset shows the augmentation of VE from normoxic baseline to hypoxic peak 30 and 60 min post-debrisoquine+entacapone; the differences being insignificant
Hypoxic ventilatory responsiveness remained grossly unchanged after DMSO-vehicle injection, compared with that before DMSO, during the 60 min time span recorded, although DMSO showed a slight tendency to dampen both baseline and hypoxic ventilatory levels (Table 1).
Neither debrisoquine nor entacapone caused any meaningful changes in arterial blood pressure recorded up to 90 min after the injection (Table 2).
4 Discussion
This investigation demonstrates that MAO-mediated oxidation is the major pathway of dopamine degradation in the carotid body as judged by changes in the hypoxic chemoreflex in response to pharmacological blockade of the enzyme. The predominant role of MAO in DA metabolism is in line with the prevailing presence of MAO in many a cell type, such as peripheral neurons, glial cells, and others (Weyler et al. 1990; Hovevey-Sion et al. 1989). Separate inhibition of the COMT pathway remained without any ventilatory effects, showing no basic role of COMT in DA degradation.
The augmentation of the hyperventilatory response to hypoxia after the peripheral MAO inhibition seemingly ran counter to our working presumption that MAO inhibition, by slowing down DA metabolism and increasing the availability of DA at its functional receptor sites in carotid body chemoreceptors, ought to bring up the inhibitory character of DA regarding the ventilatory regulation. However, simultaneous inhibition of both COMT and MAO, the condition in which endogenous DA should accumulate the most, reversed the hypoxic augmentation of ventilation induced by MAO-inhibition. These results imply the biological plausibility that when MAO alone is blocked, COMT takes over DA degradation in a compensatory way, which lowers the availability of DA, resulting in a higher intensity of the hypoxic response. The corollary is endogenous DA is in fact inhibitory for ventilation. The ability of COMT to compensate for the lost function of MAO, but not the other way around, has been reported in the rat heart (Trendelenburg 1984).
The role of DA in carotid body function is a highly contentious issue. DA is present in the carotid body in high amounts in chemoreceptor cells and shapes their sensory responses, being released in proportion to the hypoxic stimulus strength (Gonzalez et al. 1994). DA infusion inhibits carotid body responses to hypoxia and DA D2 receptor blockade increases these responses in most species (Monteiro et al. 2009; Chow et al. 1986; Eyzaguirre and Zapata 1984). The issue is further confounded by the apparent discrepancy in translation of the sensory carotid body discharge into ventilatory outcome. Smatresk et al. (1983) have reported that a non-specific D2 antagonist, haloperidol, injected intravenously in a dose of 1 mg/kg in anesthetized cats, increases the carotid sensory discharge in the sinus nerve, but attenuates the ventilatory response to hypoxia. The investigators concluded that haloperidol, which penetrates into the brain, blocks the central integration of peripheral chemoreceptor input. There is some supportive evidence to this end showing no effect of haloperidol on ventilation during normoxia or hypoxia (Bainbridge and Heistad 1980), or a depressant effect on the response to hypercapnia (Lundberg et al. 1979).
DA infusion in some species, such as goats or dogs, causes initially a burst of excitatory carotid sensory discharge, particularly observed after high doses of DA, later followed by depression (Bisgard et al. 1979). Since ventilation follows carotid body discharge rate, DA can cause stimulation or inhibition of the responses to natural chemical stimuli. To reconcile these divergent effects of DA on hypoxic ventilation, Gonzalez et al. (1994) have proposed the existence of a dichotomous D2 receptor population located at the carotid chemoreceptor cell/sinus nerve ending synapses consisting of two opposed classes: low affinity post-synaptic receptors at which DA would act as an excitatory neurotransmitter and high affinity D2 autoreceptors on chemoreceptor cells at which DA would be inhibitory, and which would regulate DA release by these cells. It follows that high doses of DA would stimulate sinus nerve activity, and consequently ventilation, through low affinity receptors and vice versa low doses of DA would inhibit sinus nerve activity through high affinity autoreceptors. Although we did not measure DA content in the carotid body, which would require alternative study design, the possibility of a direct facilitatory effect on ventilation of DA seems unlikely in face of the disappearance of ventilatory augmentation when both MAO and COMT were blocked simultaneously, the condition which favors DA accumulation. Our findings are in rapport with the concept that low concentrations of DA at the carotid body would act to dampen ventilation through high affinity D2 receptors.
The reversion of MAO-mediated ventilatory augmentation when both MAO and COMT were blocked also makes a plausible role of noradrenaline unlikely in the effects observed. A slowdown of DA metabolism could increase the content of noradrenaline that is formed by dopamine β-hydroxylase, and then is metabolized by both MAO and COMT. Noradrenaline, in contrast to DA, is present mostly in sympathetic nerve fibers reaching the carotid body from the superior cervical ganglion and participates in vascular and blood flow regulation (Hanbauer and Hellström 1978) rather than in the inherent chemotransduction mechanisms. There is less noradrenaline than DA in the carotid body; the ratio being about 1:5 (Vicario et al. 2000). Nonetheless, hypoxia stimulates sympathetic nerve activity and release of noradrenaline in the carotid body and noradrenaline increases the chemosensory discharge rate and ventilation (Somers et al. 1989; Joels and White 1968). Chemoreceptor cells function also is sensitive to arterial blood pressure changes (Lahiri et al. 1980) that could be induced by the accumulation of catecholamine. Lack of blood pressure changes in the present study, when both enzymes were blocked, makes the involvement of sympathetic background in the results obtained unlikely. Others studies have also reported that simultaneous blockade of MAO and COMT does not influence hemodynamics or concentrations of unconjugated norepinephrine in plasma (Illi et al. 1996).
In conclusion, the present findings demonstrate an augmentation of hypoxic ventilatory reactivity at the carotid body level after peripheral pharmacological blockade of MAO, likely mediated by DA degradation through a switched-on COMT pathway. The inference would be that less endogenous DA augments ventilation, and vice versa, increased availability of DA dampens ventilation. Although the study design could not settle the exact role of DA in ventilatory chemoregulation, we believe the results may help understand the niceties of DA action. In addition, the findings of increased hypoxic ventilatory reactivity after the MAO inhibitor debrisoquine and no effect on ventilation of the COMT inhibitor entacapone, both clinically used drugs, may be of therapeutic consequence.
References
Bainbridge CW, Heistad D (1980) Effect of haloperidol on ventilatory responses to dopamine in man. J Pharmacol Exp Ther 213:13–17
Bee D, Pallot DJ (1995) Acute hypoxic ventilation, carotid body cell division, and dopamine content during early hypoxia in rats. J Appl Physiol 79:1504–1511
Bisgard GE, Mitchell RA, Herbert DA (1979) Effects of dopamine, norepinephrine and 5-hydroxytryptamine on the carotid body of the dog. Respir Physiol 37:61–80
Black AMS, Comroe JH, Jacobs L (1972) Species difference in carotid body response of cat and dog to dopamine and serotonin. Am J Physiol 223:1097–1102
Chow CM, Winder C, Read DJC (1986) Influence of endogenous dopamine on carotid body discharge and ventilation. J Appl Physiol 60:370–375
Eyzaguirre C, Zapata P (1984) Perspectives in carotid body research. J Appl Physiol 57:931–957
Faff L, Kowalewski C, Pokorski M (1999) Protein kinase C – a potential modifier of carotid body function. Monaldi Arch Chest Dis 54:172–177
Gonzalez C, Almaraz L, Obeso A, Rigual R (1994) Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74:829–898
Hanbauer I, Hellström S (1978) The regulation of dopamine and noradrenaline in the rat carotid body and its modification by denervation and by hypoxia. J Physiol Lond 282:21–31
Hellström S (1977) Putative neurotransmitters in the carotid body. Mass fragmentographic studies. Adv Biochem Psychopharmacol 16:257–263
Hovevey-Sion D, Kopin IJ, Stull RW, Goldstein DS (1989) Effects of monoamine oxidase inhibitors on levels of catechols and homovanillic acid in striatum and plasma. Neuropharmacology 28:791–797
Illi A, Sundberg S, Ojala-Karlsson P, Scheinin M, Gordin A (1996) Simultaneous inhibition of catechol-O-methyltransferase and monoamine oxidase A: effects on hemodynamics and catecholamine metabolism in healthy volunteers. Clin Pharmacol Ther 59:450–457
Joels N, White H (1968) The contribution of the arterial chemoreceptors to the stimulation of respiration by adrenaline in the cat. J Physiol Lond 197:1–23
Lahiri S, Nishino T, Mokashi A, Mulligan E (1980) Relative responses of aortic and carotid body chemoreceptors to hypotension. J Appl Physiol 48:781–788
Llados F, Zapata P (1978) Effects of dopamine analogues and antagonists on carotid body chemosensors in situ. J Physiol Lond 274:487–499
Lundberg DG, Breese G, Mueller R (1979) Dopaminergic interaction with the respiratory control system in the rat. Eur J Pharmacol 54:153–159
Matsumoto S, Nishimura Y, Kohno M, Nakajima T (1980) Effects of haloperidol on chemoreceptor reflex ventilatory response in the rabbit. Arch Int Pharmacodyn Ther 247:234–242
Monteiro TC, Obeso A, Gonzalez C, Monteiro EC (2009) Does ageing modify ventilatory responses to dopamine in anaesthetised rats breathing spontaneously? Adv Exp Med Biol 648:265–271
Monteiro TC, Batuca JR, Obeso A, Gonzalez K, Monteiro EC (2011) Carotid body function in aged rats: responses to hypoxia, ischemia, dopamine, and adenosine. Age 33:337–350
Smatresk NJ, Pokorski M, Lahiri S (1983) Opposing effects of dopamine receptor blockade on ventilation and carotid chemosensory activity. J Appl Physiol 54:1567–1573
Somers VK, Mark AL, Zavala DC, Abboud FM (1989) Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol 67:2101–2106
Tomares SM, Bamford OS, Sterni LM, Fitzgerald RS, Carroll JL (1994) Effects of domperidone on neonatal and adult carotid chemoreceptors in the cat. J Appl Physiol 77:1274–1280
Trendelenburg U (1984) The influence of inhibition of catechol-O-methyl transferase or of monoamine oxidase on the extraneuronal metabolism of 3H-(-)-noradrenaline in the rat heart. Naunyn Schmiedebergs Arch Pharmacol 327:285–292
Vicario I, Rigual R, Obeso A, Gonzalez C (2000) Characterization of the synthesis and release of catecholamine in the rat carotid body in vitro. Am J Physiol Cell Physiol 278:C490–C499
Wang Y, Berndt TJ, Gross JM, Peterson MA, So MJ, Knox FG (2001) Effect of inhibition of MAO and COMT on intrarenal dopamine and serotonin and on renal function. Am J Physiol Regul Integr Comp Physiol 280:R248–R254
Ward DS, Bellville JW (1982) Reduction of hypoxic ventilator drive by dopamine. Anesth Analg 61:333–337
Weyler W, Hsu YP, Breakefield XO (1990) Biochemistry and genetics of monoamine oxidase. Pharmacol Ther 47:391–417
Conflicts of Interest
The authors declare no conflicts of interest regarding this publication.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bialkowska, M., Zajac, D., Mazzatenta, A., Di Giulio, C., Pokorski, M. (2014). Inhibition of Peripheral Dopamine Metabolism and the Ventilatory Response to Hypoxia in the Rat. In: Pokorski, M. (eds) Neurotransmitter Interactions and Cognitive Function. Advances in Experimental Medicine and Biology(), vol 837. Springer, Cham. https://doi.org/10.1007/5584_2014_72
Download citation
DOI: https://doi.org/10.1007/5584_2014_72
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-10005-0
Online ISBN: 978-3-319-10006-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)