Abstract
The photochemical behaviors of polyoxometalates (POMs) have been extensively studied and widely used in various photo-redox reactions. Incorporating photo-sensitive organic molecules onto the POM platform not only allows the rational design and fine-controlled synthesis of novel organic–inorganic hybrids but also gives the possibility to explore their potential applications in diverse areas such as photochromics, optical switching, and smart self-assemblies. Herein, we summarize the latest progress of photo-sensitive POM hybrids with a particular focus on the photo-induced reversible coloration, polymerization, and supramolecular self-assembly. The mechanisms and the crucial roles of POM clusters in such reversible processes are also considered.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Polyoxometalates (POMs) represent a large family of versatile and discrete polyanions, which are usually formed by condensation of early transition metal oxides in acid solution [1,2,3,4]. Such oxygen clusters exhibit attractive properties such as dynamic structure complexity, multi-electron redox, and Brønsted acidity, thus leading to a diverse range of applications ranging from catalysis, medicine, to materials science [5,6,7,8,9,10].
Perhaps one of the most intriguing properties of POMs lies in the photo-redox reactions of such clusters that can be easily processed in the presence of sacrifice and reoxidation agents. The reduced POMs, or the so-called heteropoly blues, have been intensively studied and used for colorimetric analysis and catalysis. The photochemistry of POMs both in solution and in solid state has been well-documented in several excellent reviews [11, 12]. To put it simply, electrons from the low-energy oxygen 2p orbitals (HOMO) will be promoted to the high-energy metal d orbitals (LUMO) upon irradiation due to the O → M ligand-to-metal charge transfer (LMCT), thus leading to a photoexcited state of highly oxidized POM clusters which can be readily reduced into heteropoly blues in the presence of sacrifice agents. The reduced form is often possible to be reoxidized into the ground state by using molecular oxygen or hydrogen peroxide. Another advantage of the photoexcited O → M LMCT bands is the photoluminescence and intramolecular energy transfer in POM solids, especially in the lanthanide-containing POMs. For example, the photoexcitation of the O → W LMCT bands in the Weakley-type POM, Na9[EuW10O36]∙32H2O, leads to a strong Eu3+ emission which arises from the 5D0 excited state of Eu3+ and terminates at the 7Fj ground state [11]. Once the intramolecular energy transfer is interrupted, fluorescence quenching of Eu3+ will be observed. Such “on/off” state of lanthanide-containing POMs has been used as an effective tool in sensoring [13].
Apart from the extraordinary chemical and physical properties of POM anions, one common problem faced during practical applications is the poor processibility of the highly crystallized POM clusters. Large efforts such as the immobilization of POMs onto supporters have been allocated to solve this problem. However, in most of the cases weak non-covalent interactions between POM anions and the counterparts are used, which inevitably results in another tricky issue of leaking. Covalent modification of POMs seems to be promising since it offers several advantages that non-covalent interactions can hardly compete [14,15,16]. Moreover, the covalent strategy gives rise to products with well-defined anchoring modes and stoichiometry, thus leaving the resulting hybrids with predictable architectures and fine-tuned properties. In this article, we focus on the recent development of covalently modified POM hybrids that exhibit photo-sensitive features and their applications in self-assembly formulation and photochromics will also be discussed. In accordance with previous definition, the term “hybrid” will refer to covalently modified species [16].
2 Covalent Modification Methods
Organically functionalized POM hybrids give rise to materials that possess physical properties fundamentally different from their parent two parts. The resulting synergistic effects between the organic and inorganic moieties often offer extraordinary enhanced performances in practical applications. Moreover, the covalently modified POMs provide building blocks that are capable to be further functionalized into giant self-assembled architectures [17, 18]. However, currently the POM hybrids are still synthetically challenging and only a few types of clusters could be modified. The methodologies of modification of POMs (direct functionalization and post-functionalization) have been summarized in several outstanding reviews [14,15,16, 19,20,21], so here in this section we will give a very brief introduction.
Lindqvist POMs
The hexamolybdate anion, [Mo6O19]2−, which is also known as Lindqvist POM, has six reactive terminal oxygen atoms that are apt to be partially [20] or completely [22] substituted with organoimido ligands (Fig. 1). The resulting organoimido hybrids with strong d–pπ covalent bonds dramatically modify the electronic and redox properties of the Lindqvist cluster. Another type of organically modified Lindqvist cluster is the {V6O13((OCH2)3CR)2}2− (R = CH3, CH2CH3, CH2OH, NO2, and NH2), which can be prepared from the {H3V10O28}3− cluster in the presence of tripodal alcohols [23, 24].
Schematic view of the organoimido functionalized Lindqvist cluster [22]. {Mo6} stands for {Mo6O19}2−
Anderson POMs
The functionalization of Anderson polyanions is first reported by Hasenknopf et al. [25] and then fully developed by Song et al. [26,27,28,29,30] This well-established method is mainly based on the {MnIIIMo6O18((OCH2)3CNH2)2}3− cluster, which can be easily generated from the {α-Mo8O26}4− cluster. Latest researches by authors’ group and other groups reveal a more facile route for the synthesis of such tripodal alcohol modified hybrids by using the B-type Anderson cluster as parent POM [31,32,33]. A detailed survey of the functionalization methods of Anderson cluster and the applications of the resulting hybrids have been reviewed recently [21].
Dawson and Keggin POMs
Except the V-substituted Dawson cluster [34,35,36] which can be modified by tripodal alcohols (Fig. 2), the rest of the Dawson and Keggin hybrids are prepared by grafting addenda organometallic compounds onto the lacunary POM clusters. Organosilyl, organostannyl, and organophosphoryl are the most common organometallic reagents used. Readers can refer to those brilliant reviews and the references thereafter for more detailed discussions [14,15,16].
3 Photo-Sensitive Polyoxometalate Hybrids
Based on the covalent modification methods, various organic photo-sensitive and photochromic molecules have been attached onto POM clusters to investigate the fascinating intramolecular electron transfer and charge separation between the two counterparts under irradiation. Such POM hybrids are considered to be potentially suitable as optical switches, photosensors, and even smart windows. In this section, we will review the latest progresses made in this field.
3.1 Spiropyran-Containing Polyoxometalate Hybrids
Spiropyrans (SPs) are dynamic molecules that can switch reversibly between the two isomers of the closed SP form and the opened merocyanine (MC) form in response to stimuli such as light, temperature, and mechanical stress [37]. SPs have been widely investigated and employed for the construction of light-responsive systems due to their high performance in optical coloration. Oms et al. [38] first studied the photo-/electrochromic properties of SP-containing POM hybrids by tethering SP-Tris derivatives on to Anderson-type cluster (Fig. 3). It was demonstrated that the strong covalent bonds developed a remarkable photochromic effect and a fast coloration speed when compared to the electrostatically associated ones [39]. Moreover, the free amino group in the asymmetrically modified SP-Anderson cluster 1 allows the further functionalization, thus offering the opportunity to elaborate more sophisticated assemblies.
Schematic representation of asymmetrically modified SP-Anderson cluster (1) and symmetrically modified SP-Anderson cluster (2). (a) Color changes of 1 at different UV 365 nm irradiation times; (b) Kubelka–Munk transformed reflectivity spectra of 1 under 365 nm irradiation; and (c) the absorption/time plots of 1, 2, and SP-Tris [38]
Bearing in this in mind, Chu et al. [40] developed a new type of amphiphiles by combining the SP fragment and a long alkyl chain onto a hydrophilic Anderson cluster (Fig. 4). Due to the amphiphilic nature, the hybrids self-assembled into nanosized vesicles and reverse vesicles in polar solvents and nonpolar solvents, respectively. More importantly, the self-assembly process of the vesicles could be triggered by UV irradiation because of the structural isomerization of SP moieties. The obtained reverse vesicles, on the other hand, remained unaffected when irradiated either with UV or visible light.
Top: Schematic view of the asymmetrical SP-Anderson amphiphile; bottom: (a) the self-assembled model of the asymmetrical SP-Anderson hybrid in polar solvents, and (b) TEM image of the asymmetrical SP-Anderson hybrid in 2.5 v/v% water/acetonitrile solution [40]
Besides the photo-sensitive SP molecules, the same catalogue spironaphthoxazines (SNs) which exhibit more fatigue resistance have also been incorporated into POM hybrids. For example, symmetrically modified SN-Anderson-SN and asymmetrically modified SN-Anderson-SP hybrids have been successfully fabricated (Fig. 5). It was evidenced that the SN-Anderson-SP hybrids were highly effective solid-state photochromic materials. Controlled experiments of the SN-Tris derivatives showed that such pure organic molecules in solid states were hardly photochromic, which demonstrated the crucial role of POM clusters on the optical properties of such materials. In addition, the SN-Anderson-SN hybrids showed a relatively faster thermal fading process and a better anti-fatigue performance when compared to the SP groups modified ones [41]. Aside from the aforementioned SP modified Anderson hybrids, the Keggin-type hybrids bearing one or two SP fragments and highly fluorescent boron-dipyrromethene (BODIPY) functionalized Anderson hybrids were also reported [42, 43].
Top: Representation of the SN-Anderson-SN and SN-Anderson-SP hybrids; bottom: color changes of: (a) SN-Anderson-SP powders and (b) SN-Anderson-SN powders upon UV 365 nm irradiation (coloration) and without irradiation (fading). Note: the pink color of SN-Anderson-SP is attributed to the initially photo-activated SP group [41]
3.2 Coumarin-Containing Polyoxometalate Hybrids
Due to the strong UV absorption and high radiative quantum yield, coumarins have been widely used as laser dyes, fluorescent probes, and photo-sensitizers. Another fascinating property of coumarins lies in the photo-triggered cycloaddition reactions that will lead to photodimerization processes. Recently, authors’ group synthesized a series of coumarin-containing POM hybrids and the photochemistry of such materials had been systematically investigated. The first example would be the coumarin-Anderson cluster, which undergoes a remarkable optical behavior when irradiated at UV 365 nm (Fig. 6). [2 + 2] cycloadditions between the coumarin moieties were clearly observed in the UV absorption spectra and had been unambiguously demonstrated in 1H NMR and 2D COSY NMR. Dynamic light scanning analysis before and after UV 365 nm irradiation showed dramatic increase in particle size. Obvious color changes upon irradiation could be also observed from light orange to deep brown. To strengthen the potential applications of such photo-switchable process, the coumarin-Anderson hybrids were embedded into polymer matrix giving rise to a solid-state film sample. Similar to the performance in solution, the film sample exhibited reversible coloration and fading process over five consecutive cycles of UV 365 and 254 nm irradiation [44].
Left: schematic illustration of the reversible photopolymerization process of coumarin-Anderson hybrids; right: size distributions of coumarin-Anderson hybrids before and after irradiation. Inset: photographs of the coloration process of coumarin-Anderson upon 365 nm irradiation [44]
To broaden our understanding of the coumarin-containing POM hybrids during such photo-triggered coloration phenomenon, we chose to modify the V-substituted Dawson cluster with coumarin-Tris derivative (Fig. 7). The obtained hybrids were somehow different from the coumarin-Anderson ones since only one coumarin moiety was attached onto the POM platform and photodimerization was implemented instead. The photo-controlled reversible dimerization had been proved by UV-Vis, 2D COSY NMR, and ESI-MS analysis. Moreover, a second photo-assistant process was observed in the Dawson cluster as the reduction of VV to VIV was clearly confirmed by X-ray photoelectron spectroscopy and electron paramagnetic resonance. On the contrary, no clear evidence was obtained in the unmodified Dawson cluster, which verified the importance of the covalent modification and the corresponding synergistic effects between the organic moieties and the inorganic clusters [45].
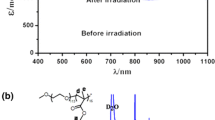
Similar to coumarins, chalcone molecules also possess photo-sensitive α,β-unsaturated carbonyl groups, which are capable to carry out fast [2 + 2] cycloaddition reaction under UV initiation. Thus, chalcone-containing POM hybrid was also prepared and fully investigated in our group (Fig. 8). For example, when irradiated at UV 365 nm the hybrid underwent a [2 + 2] cycloaddition reaction, leading to an absorbance decrease at 332 nm and a new absorbance peak at 245 nm attributed to the generated cyclobutane structure. This process gave a total photopolymerization conversion of 37.45% and was found able to restore when exposed to UV light of 254 nm. After five cycles of alternative irradiation at 365 and 254 nm, the amount of the hybrid monomer still maintained at 85%. 1H NMR spectrum of the hybrid also exhibited significant changes when irradiated at 365 nm. Signals of the unsaturated carbonyl group of the chalcone moieties at δ = 7.83 and 7.73 ppm shifted to high field after irradiation and gave corresponding cyclobutane signal at δ = 4.80 ppm. These results were in good agreement with the UV-Vis analysis. The weight-average molecular weight of the hybrid polymer was examined to be 9.78 × 105 g/mol as demonstrated by the gel permeation chromatography analysis [46].
Left: representation of photo-controlled polymerization of chalcone-Anderson hybrids; right: (a) UV-Vis spectra of chalcone-Anderson under UV 365 nm irradiation, (b) the photopolymerization conversion ratio of chalcone-Anderson hybrid, (c) UV-Vis spectra of the photo-disassociation under UV 254 nm irradiation, and (d) cycle experiment of alternative irradiation at 365 and 254 nm [46]
3.3 Azobenzene-Containing Polyoxometalate Hybrids
Azobenzene derivatives constitute a large family of photo-sensitive molecules and have been intensively investigated as promising candidates in various applications such as photo-switches, optical memory materials, and protein probes. Azobenzene derivatives can undergo reversible trans/cis isomerizations when irradiated with appropriate light sources and exhibit remarkable photo-stability [47].
We recently prepared a category of azobenzene-Anderson hybrids and carefully examined their polymolecular aggregation processes with ion-mobility mass spectrometry (IMS-MS) [48]. IMS-MS emerged as an efficient tool in monitoring the structure and conformational dynamics of proteins and coordination compounds. By using this powerful technique, we were able to investigate the conformational changes of the photo-sensitive azobenzene-Anderson hybrids in solution (Fig. 9). After 5 min consecutive irradiation under UV 365 nm light, the azobenzene-Anderson hybrids were then quickly transferred into the IMS-MS device for measurements. To our surprise, only quaternary structures of the hybrids with four or more TBA counter cations were observed, which strongly indicated the existence of high π–π interactions between azobenzene moieties. The conformational changes resulted in a significant difference in the drift time spectra where the equilibria for the formation of quaternary structures clearly shifted. The much higher intensity for the peak of dimeric assembly centered on a drift time of 14.22 ms after 365 nm irradiation clearly indicated a higher stability of the azobenzene-Anderson hybrids in the cis conformation when compared to that in the trans conformation. As demonstrated above, π–π stacking of the azobenzene molecules plays a crucial role during the self-assembly or aggregation process of azobenzene-containing POM hybrids. More convincing evidences could be obtained by investigating the assembly behaviors of such amphiphilic hybrids in mixed polar solvents. To this end, photo-responsive azobenzene moieties had been grafted onto the lacunary Keggin cluster [49]. The hydrophilic/hydrophobic interactions of the azobenzene-containing Keggin POMs drove the hybrid assembled into 0D nanospheres in acetonitrile-dominating acetonitrile/water mixture. Gradually increasing the water contents in mixed solvents would induce the hydrophobic packing of azobenzene moieties and thus enhance the π–π interactions among them. Such variations allowed the switching of assembled structures from 0D nanospheres into 1D nanorods.
Left: switching of the photo-sensitive azobenzene-containing Anderson cluster; right: 2D IMS/MS spectrum of azobenzene-containing Anderson cluster and the comparison of drift time before and after UV irradiation [48]
Liquid crystals (LCs) represent a diverse class of soft matter materials that exhibit anisotropic and switching properties. The inherent rigidity of azobenzene groups renders them as mesogens in the construction of LCs. As such, we adopted a straightforward procedure to prepare liquid crystalline azobenzene-containing POM hybrids by anchoring dendritic gallic acid derivatives on to lacunary Keggin clusters [50]. As demonstrated by the differential scanning calorimetry (DSC), optical polarized microscopy (OPM), and small-angle X-ray scattering (SAXS) analyses, all the POM hybrids exhibited a lamellar smectic A phase with interlayer distances independent of temperature and a neighboring POM cluster distance of 12.4 Å. It should be underlined that the pure organic azobenzene-embedded gallic acid derivatives were found to display no liquid crystalline properties at all, which manifested the importance of POM clusters in the construction of LCs.
Besides the advantages of azobenzene fragments in the preparation of liquid crystalline soft materials, the trans/cis isomerization of such functional groups also leaves the possibility to finely control the transformation of mesogenic phases and optical switching of LCs. To this end, a similar type of dendritic azobenzene-containing POM hybrid was fabricated, and its applications in stabilizing the blue phase (BP) LCs as well as in optically switching the Bragg reflection band of BPs were also investigated (Fig. 10). BPs are 3D ordered phases which can be observed in cholesteric LCs with strong chirality strength. In despite of their promising efficient LC display and emerging photonic applications, the fact that BP LCs are limited to only one or two degrees temperature range due to their high free energies of the disclination lines drastically restricts the application of BP LCs. When POM hybrids were physically doped into the BP samples, the stabilization temperature range of BP was expanded to 7.5 °C. Moreover, when the POM hybrids doped BP samples were exposed to UV 365 nm light, a clear red shift from 539 to 617 nm of the Bragg reflection bands was observed because of the trans to cis isomerization of azobenzene moieties. Various BP platelet colors from green to red were also observed with UV irradiation under polarized microcopy. Such stabilization and optical switching of BP LCs could be explained as follows: firstly, the azobenzene-containing POM hybrids induced the increase of bending K 33 elastic constant, which was beneficial in stabilizing BP samples; secondly, the transformation from trans to cis isomer of the azobenzene groups in POM hybrids resulted in the extension of the double twisted cylinder structure of BPs, thus leading to an increase in the lattice constant [51].
Top: schematic presentation of the azobenzene-containing Keggin cluster; bottom: (a) the reflectance spectrum of POM-doped blue phase liquid crystals under UV irradiation, and (b) the reflective color of POM-doped blue phase liquid crystals in polarized microscopy under UV irradiation [50]
Self-assemblies that can be modulated by external stimuli and gave dynamic morphological transitions are of great interests in smart functional materials. In 2010, Yan et al. [52] reported an example of azobenzene-functionalized Anderson cluster, which exhibited reversible aggregation changes as a result of photo-controlled structure alternation. As shown in Fig. 11, surfactant dimethyldioctadecylammonium encapsulated azobenzene-Anderson hybrids gave a fiber-like morphology in the trans state, while upon UV irradiation such fiber-like structures gradually transferred into spheres. Leaving the self-assembled spherical structure either in visible light or in the dark, it could switch back to fibers. Such reversible photo-controlled morphological changes were revealed to be manipulated by the destruction and rebuilding of the hydrogen bonds between azobenzene moieties and the Anderson cluster through photo-irradiation. By using the same azobenzene-containing Anderson hybrid and a pyridine-modified cyclodextrin cation, the same group constructed a self-crosslinking complex system which showed a photo-controllable chiral assembly process [53].
Left: the structural isomerization of azobenzene-Anderson hybrid under UV and visible light; right: SEM images of surfactant encapsulated azobenzene-Anderson hybrids in CHCl3/CH3OH solution after: (a) 0, (b) 1, (c) 5, (d) 15, (e) 30, and (f) 120 min of UV 365 nm irradiation [52]
3.4 Other π-Conjugated Polyoxometalate Hybrids
Thanks to the versatile covalent modification methods, a large amount of π-conjugated organic molecules have been tethered onto the skeletons of POM clusters up to date. These functional molecules include pyrene [54], porphyrin [55,56,57,58], perylene [59], fullerene [60, 61], and polypyridinyl complexes [62,63,64,65]. Since the photochemistry and photophysics of these POM hybrids have been well-documented in several remarkable reviews [66,67,68], we will not give further statements here.
4 Conclusion
In summary, we have discussed the synthetic methodologies and the recent developments of photo-sensitive POM-based hybrid materials in photochromics, optical switching, and smart self-assembly. Interestingly, the choice of methodology and the type of POM clusters have a profound influence on the functional performance of such POM hybrids. In addition, the close interactions between POM clusters and the covalently attached organic fragments favor the electron transfer process between the two subunits, which might be of great potential in photocatalysis, water oxidation, photovoltaics, and even solar cells.
References
Long D-L, Burkholder E, Cronin L (2007) Chem Soc Rev 36:105–121
Long D-L, Tsunashima R, Cronin L (2010) Angew Chem Int Ed Engl 49:1736–1758
Hill CL (1998) Chem Rev 98:1–2
Cronin L, Müller A (2012) Chem Soc Rev 41:7333–7334
Wang S-S, Yang G-Y (2015) Chem Rev 115:4893–4962
Omwoma S, Chen W, Tsunashima R, Song Y-F (2014) Coord Chem Rev 258–259:58–71
Rhule JT, Hill CL, Judd DA (1998) Chem Rev 98:327–357
Bijelic A, Rompel A (2015) Coord Chem Rev 299:22–38
Song Y-F, Tsunashima R (2012) Chem Soc Rev 41:7384–7402
Ji Y, Huang L, Hu J, Streb C, Song Y-F (2015) Energy Environ Sci 8:776–789
Yamase T (1998) Chem Rev 98:307–325
Streb C (2012) Dalton Trans 41:1651–1659
Wang X, Wang J, Tsunashima R, Pan K, Cao B, Song Y-F (2013) Ind Eng Chem Res 52:2598–2603
Proust A, Thouvenot R, Gouzerh P (2008) Chem Commun 1837–1852
Dolbecq A, Dumas E, Mayer CR, Mialane P (2010) Chem Rev 110:6009–6048
Proust A, Matt B, Villanneau R, Guillemot G, Gouzerh P, Izzet G (2012) Chem Soc Rev 41:7605–7622
Macdonell A, Johnson NAB, Surman AJ, Cronin L (2015) J Am Chem Soc 137:5662–5665
Izzet G, Abécassis B, Brouri D, Piot M, Matt B, Serapian SA, Bo C, Proust A (2016) J Am Chem Soc 138:5093–5099
Peng Z (2004) Angew Chem Int Ed Engl 43:930–935
Zhang J, Xiao F, Hao J, Wei Y (2012) Dalton Trans 41:3599–3615
Blazevic A, Romel A (2016) Coord Chem Rev 307:42–64
Strong JB, Haggerty BS, Rheingold AL, Maatta EA (1997) Chem Commun 1137–1138
Chen Q, Goshorn DP, Scholes CP, Tan X-L, Zubieta J (1992) J Am Chem Soc 114:4667–4681
Li D, Song J, Yin P, Simotwo S, Bassler AJ, Aung Y, Roberts JE, Hardcastle KI, Hill CL, Liu T (2011) J Am Chem Soc 133:14010–14016
Hasenknopf B, Delmont R, Herson P, Gouzerh P (2002) Eur J Inorg Chem 1081–1087
Song Y-F, Long D-L, Cronin L (2007) Angew Chem Int Ed Engl 46:3900–3904
Song Y-F, Long D-L, Kelly SE, Cronin L (2008) Inorg Chem 47:9137–9139
Song Y-F, McMillan N, Long D-L, Kane S, Malm J, Riehle MO, Pradeep CP, Gadegaard N, Cronin L (2009) J Am Chem Soc 131:1340–1341
Song Y-F, Long D-L, Cronin L (2010) CrystEngComm 12:109–115
Yvon C, Macdonell A, Buchwald S, Surman AJ, Follet N, Alex J, Long D-L, Cronin L (2013) Chem Sci 4:3810–3817
Wu P, Yin P, Zhang J, Hao J, Xiao Z, Wei Y (2011) Chem Eur J 17:12002–12005
Lin C-G, Chen W, Long D-L, Cronin L, Song Y-F (2014) Dalton Trans 43:8587–8590
Ai H, Wang Y, Li B, Wu L (2014) Eur J Inorg Chem 2766–2772
Zeng H, Newkome GR, Hill CL (2000) Angew Chem Int Ed Engl 39:1772–1774
Pradeep CP, Long D-L, Newton GN, Song Y-F, Cronin L (2008) Angew Chem Int Ed Engl 47:4388–4391
Pradeep CP, Misdrahi MF, Li F-Y, Zhang J, Xu L, Long D-L, Liu T, Cronin L (2009) Angew Chem Int Ed Engl 48:8309–8313
Klajn R (2014) Chem Soc Rev 43:148–184
Oms O, Hakouk K, Dessapt R, Deniard P, Jobic S, Dolbecq A, Palacin T, Nadjo L, Keita B, Marrot J, Mialane P (2012) Chem Commun 48:12103–12105
Mialane P, Zhang G, Mbomekalle IM, Yu P, Compain J-D, Dolbecq A, Marrot J, Sécheresse F, Keita B, Nadjo L (2010) Chem Eur J 16:5572–5576
Chu Y, Saad A, Yin P, Wu J, Oms O, Dolbecq A, Mialane P, Liu T (2016) Chem Eur J 22:11756–11762
Saad A, Oms O, Marrot J, Dolbecq A, Hakouk K, El Bekkachi H, Jobic S, Deniard P, Dessapt R, Garrot D, Boukheddaden K, Liu R, Zhang G, Keita B, Mialane P (2014) J Mater Chem C 2:4748–4758
Parrot A, Izzet G, Chamoreau L-M, Proust A, Oms O, Dolbecq A, Hakouk K, El Bekkachi H, Deniard P, Dessapt R, Mialane P (2013) Inorg Chem 52:11156–11163
Saad A, Oms O, Dolbecq A, Menet C, Dessapt R, Serier-Brault H, Allard E, Baczko K, Mialane P (2015) Chem Commun 51:16088–16091
Tong U, Chen W, Ritchie C, Wang X, Song Y-F (2014) Chem Eur J 20:1500–1504
Chen W, Tong U, Zeng T, Streb C, Song Y-F (2015) J Mater Chem C 3:4388–4393
Zhang J, Long Y, Xuan W, Lin C-G, Song Y-F (2017) Chin Sci Bull 62:685–692
Bandara HMD, Burdette SC (2012) Chem Soc Rev 41:1809–1825
Thiel J, Yang D, Rosnes MH, Liu X, Yvon C, Kelly SE, Song Y-F, Long D-L, Cronin L (2011) Angew Chem Int Ed Engl 50:8871–8875
Chen W, Ma D, Yan J, Boyd T, Cronin L, Long D-L, Song Y-F (2013) ChemPlusChem 78:1226–1229
Lin C-G, Chen W, Omwoma S, Song Y-F (2015) J Mater Chem C 3:15–18
Wang J, Lin C-G, Zhang J, Wei J, Song Y-F, Guo J (2015) J Mater Chem C 3:4179–4187
Yan Y, Wang H, Li B, Hou G, Yin Z, Wu L, Yam VWW (2010) Angew Chem Int Ed Engl 49:9233–9236
Yue L, Ai H, Yang Y, Lu W, Wu L (2013) Chem Commun 49:9770–9772
Matt B, Renaudineau S, Chamoreau L-M, Afonso C, Izzet G, Proust A (2011) J Org Chem 76:3107–3112
Harriman A, Elliott KJ, Alamiry MAH, Le Pleux L, Séverac M, Pellegrin Y, Blart E, Fosse C, Cannizzo C, Mayer CR, Odobel F (2009) J Phys Chem C 113:5834–5842
Elliott KJ, Harriman A, Le Pleux L, Pellegrin Y, Blart E, Mayer CR, Odobel F (2009) Phys Chem Chem Phys 11:8767–8773
Allain C, Schaming D, Karakostas N, Erard M, Gisselbrecht J-P, Sorgues S, Lampre I, Ruhlmann L, Hasenknopf B (2013) Dalton Trans 42:2745–2754
Ahmed I, Farha R, Huo Z, Allain C, Wang X, Xu H, Goldmann M, Hasenknopf B, Ruhlmann L (2013) Electrochim Acta 110:726–734
Odobel F, Séverac M, Pellegrin Y, Blart E, Fosse C, Cannizzo C, Mayer CR, Elliott KJ, Harriman A (2009) Chem Eur J 15:3130–3138
Bonchio M, Carraro M, Scorrano G, Bagno A (2004) Adv Synth Catal 346:648–654
Zhou S, Feng Y, Chen M, Li Q, Liu B, Cao J, Sun X, Li H, Hao J (2016) Chem Commun 52:12171–12174
Duffort V, Thouvenot R, Afonso C, Izzet G, Proust A (2009) Chem Commun 6062–6064
Matt B, Coudret C, Viala C, Jouvenot D, Loiseau F, Izzet G, Proust A (2011) Inorg Chem 50:7761–7768
Matt B, Moussa J, Chamoreau L-M, Afonso C, Proust A, Amouri H, Izzet G (2012) Organometallics 31:35–38
Santoni M-P, Pal AK, Hanan GS, Tang M-C, Furtos A, Hasenknopf B (2014) Dalton Trans 43:6990–6993
Santoni M-P, Hanan GS, Hasenknopf B (2014) Coord Chem Rev 281:64–85
Walsh JJ, Bond AM, Forster RJ, Keyes TE (2016) Coord Chem Rev 306:217–234
Izzet G, Volatron F, Proust A (2017) Chem Rec 17:250–266
Acknowledgements
This research was supported by the National Basic Research Program of China (973 program, 2014CB932104), the National Nature Science Foundation of China (U1407127, U1507102, 21521005, 21625101), and Fundamental Research Funds for the Central Universities. C.-G.L. appreciates financial support from the China Postdoctoral Science Foundation (2016M591048).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Lin, CG., Chen, W., Song, YF. (2017). Polyoxometalate-Based Photo-Sensitive Functional Hybrid Materials. In: Song, YF. (eds) Polyoxometalate-Based Assemblies and Functional Materials. Structure and Bonding, vol 176. Springer, Cham. https://doi.org/10.1007/430_2017_1
Download citation
DOI: https://doi.org/10.1007/430_2017_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75903-6
Online ISBN: 978-3-319-75904-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)