Abstract
Glyphosate-resistant (GR) crops, commercially referred to as glyphosate-tolerant (GT), started the revolution in crop biotechnology in 1996. Growers rapidly accepted GR crops whenever they became available and made them the most rapidly adopted technology in agriculture history. Adoption usually meant sole reliance on glyphosate [N-(phosphonomethyl)glycine, CAS No. 1071-83-6] for weed control. Not surprisingly, weeds eventually evolved resistance and are forcing growers to change their weed management practices. Today, the widespread dissemination of GR weeds that are also resistant to other herbicide modes-of-action (MoA) has greatly reduced the value of the GR crop weed management systems. However, growers continue to use the technology widely in six major crops throughout North and South America. Integrated chemistry and seed providers seek to sustain glyphosate efficacy by promoting glyphosate combinations with other herbicides and stacking the traits necessary to enable the use of partner herbicides. These include glufosinate {4-[hydroxy(methyl)phosphinoyl]-DL-homoalanine, CAS No. 51276-47-2}, dicamba (3,6-dichloro-2-methoxybenzoic acid, CAS No. 1918-00-9), 2,4-D [2-(2,4-dichlorophenoxy)acetic acid, CAS No. 94-75-7], 4-hydroxyphenyl pyruvate dioxygenase inhibitors, acetyl coenzyme A carboxylase (ACCase) inhibitors, and other herbicides. Unfortunately, herbicide companies have not commercialized a new MoA for over 30 years and have nearly exhausted the useful herbicide trait possibilities. Today, glyphosate-based crop systems are still mainstays of weed management, but they cannot keep up with the capacity of weeds to evolve resistance. Growers desperately need new technologies, but no technology with the impact of glyphosate and GR crops is on the horizon. Although the expansion of GR crop traits is possible into new geographic areas and crops such as wheat and sugarcane and could have high value, the Roundup Ready® revolution is over. Its future is at a nexus and dependent on a variety of issues.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biotechnology
- Formulation
- Genetically modified crops
- Herbicide-resistant
- Herbicide-tolerant
- Resistance
- Tolerance
- Traits
- Weed
- Weed management
1 Introduction
Enabling the use of glyphosate as a selective crop herbicide in 1996 was one of the most important innovations of the twentieth century. It started the plant biotech crop revolution. Growers made glyphosate-resistant (GR) crops, generally known commercially as glyphosate-tolerant (GT), the most rapidly adopted technology in the history of agriculture because it was cheaper, more effective, and more convenient than the selective herbicides they were using. Today, six main crops have transgenes that confer glyphosate resistance: soybeans [Glycine max (L.) Merr.], corn (Zea mays L.), cotton (Gossypium hirsutum L.), canola (Brassica napa L.), alfalfa (Medicago sativa L.), and sugarbeets (Beta vulgaris L.). In 2018, 26 countries (21 developing and 5 industrialized countries) planted 191.7 million hectares of biotech crops, which added 1.9 million hectares to the 2017 record. Most genetically modified (GM) crops are resistant to glyphosate (ISAAA 2020).
Glyphosate was the ideal herbicide for developing herbicide-resistant (HR) crops. Its low-cost, high efficacy on nearly all weeds, low environmental impact, and low toxicity made it a “Once-in-a-Century Herbicide” (Duke and Powles 2008). Glyphosate is readily absorbed and translocated throughout weeds, where it inhibits 5-enolpyruvyl-shikimate-3-phosphate synthase (EPSPS; EC 2.5.1.19), an enzyme of the aromatic biosynthesis pathway in autotrophic organisms (Siehl 1997). When the pathway is blocked, the plant cannot synthesize essential metabolites such as aromatic amino acids, auxin hormones, and quinones including tocochromanols and plastoquinones.
Synthetic chemical herbicides are still the first option for weed control even after 70 years of widespread use. We discuss the impact of GR crops and the resulting evolution of GR weeds on chemical weed control. Despite the prevalence of GR weeds, glyphosate and GR crop systems will continue to have value when used in combination with herbicides with different modes-of-action (MoA) and other weed management tactics. New GR crops could have value through expansion into new geographies and crops, depending on public and regulatory acceptance and the success with weed management practices that sustain glyphosate utility (Bøhn and Millstone 2019; Green 2018).
2 Development of Glyphosate-Resistant Crops
Glyphosate was already widely used for nonselective vegetation control when Monsanto introduced GR crops in 1996. Monsanto began the long process of developing GR crops in 1983 when plant biotechnology was in its infancy. It saw the potential for GR crops when few others did (Kishore et al. 1992). However, achieving commercially acceptable tolerance to glyphosate was more difficult than expected due to the difficulty in finding a form of EPSPS with sufficient insensitivity to glyphosate and the requisite catalytic performance. Eventually, Monsanto scientists discovered an EPSPS with a high degree of insensitivity (Ki = 1970 μM) in an Agrobacterium strain called CP4, surviving in the manufacturing waste stream at Luling, LA (Barry et al. 1992).
Scientists generally consider two options for creating a herbicide trait; a target enzyme desensitized to inhibition by the herbicide or an enzyme that metabolizes the herbicide into an inactive molecule. For glyphosate, metabolic inactivation is feasible, but desensitization of the target has been the commercially successful approach.
Search for desensitized EPSPS. EPSPS catalyzes the transfer of a carboxyvinyl group from phosphoenolpyruvate (PEP) to shikimate-3-phosphate (S3P) (Fig. 1). The crystal structure of the E. coli enzyme shows glyphosate bound adjacent to S3P in the PEP binding site (Schönbrunn et al. 2001), accounting for the consistent observation that inhibition is competitive with PEP (Boocock and Coggins 1983; Steinrucken and Amrhein 1984; Dong et al. 2019). The reaction proceeds through an oxocarbenium ion of PEP, generated by the enzyme. Glyphosate has a charge distribution and steric configuration like that of the carbenium resonance structure of PEP (Fig. 1). Tight binding (values for Ki for plant EPSPS in the range of 50 nM (Baerson et al. 2002) to 70 nM (Dong et al. 2019)) and observably slow release of glyphosate from an E:S3P:glyph complex (Dong et al. 2019) support the concept that glyphosate is a reaction intermediate analog.
EPSPS reaction. The reaction is an addition/elimination in which an enzymic base deprotonates the 5-hydroxyl of S3P, allowing the electron pair to attack the oxocarbenium ion of PEP (shown to suggest the species mimicked by glyphosate), generated by the enzyme. Originally published in the Journal of Biological Chemistry. Dong et al. (2019), Desensitizing plant EPSP synthase to glyphosate: Optimized global sequence context accommodates a glycine-to-alanine change in the active site. J. Biol. Chem. 2019; 294: 716–725 © the Author(s)
The following is an evaluation of naturally occurring and mutant variants of EPSPS for their ability to confer commercial-level glyphosate resistance in crop plants, either by cis- or transgenic expression. The key parameters are kcat (reactions per unit time at Vmax), Km PEP (enzyme-substrate binding affinity; lower value = higher affinity), and Ki (binding affinity for glyphosate; lower value = higher affinity). Derivatives of those parameters provide an expression of catalytic efficiency (kcat/Km), selectivity for PEP vs glyphosate (Ki/Km), and an expression, [(kcat/Km)*Ki] that captures both catalytic efficiency and selectivity. The values in Table 1 are useful for comparing the variants described because they were all obtained with nearly pure enzymes with mutations constructed in the same backbone (maize EPSPS) and analyzed in the same lab by the same procedure (Dong et al. 2019). All constructs had an N-terminal 10x-Histidine tag which, coupled with very high expression levels in E. coli, facilitate the purification of larger numbers of purified EPSPS variants for kinetic analysis. We calculated the concentration of each variant using a custom extinction coefficient calculated by vNTI, based on its amino acid sequence. We used a highly sensitive continuous spectrophotometric assay wherein the phosphate released from PEP was detected by reacting it with 2-amino-6-mercapto-7-methylpurine ribonucleoside (MESG, CAS No: 55727-10-1), catalyzed by purine nucleoside phosphorylase (EC 2.4.2.1), yielding the highly absorbent 2-amino-6-mercapto-7-methylpurine. Our kinetic parameters are similar to those of Baerson et al. (2002), who use highly sensitive detection of 14C-EPSP produced from 14C-PEP, and Yu et al. (2015), who detect phosphate with the MESG reagent.
Singly mutagenized EPSPS from plants, E. coli, or Salmonella yielded no variant with properties adequate for conferring commercial tolerance to glyphosate. The known plant mutations and close homologs, e.g., E. coli (termed Class I EPSPS), exert their effect by modulating the position of Gly101 (numbering according to mature maize EPSPS [CAA44974.1]; 96 in E. coli) in a way that creates interference with the binding of glyphosate through one of its phosphonate oxygens (Schönbrunn et al. 2001). The longer length of glyphosate relative to PEP allows for fine-tuning the differential affinity for the two ligands. In a crystallographic study of the E. coli enzyme, changing proline 101 (E. coli numbering) to serine or leucine had the effect of moving the alpha carbon of Gly96 closer to glyphosate, consequently reducing affinity for glyphosate (Ki with P101S, 14-fold increase; with P101L, 165-fold increase; Healy-Fried et al. 2007). The P101S substitution did not significantly affect affinity for PEP, while the P101L substitution reduced affinity (increased Km PEP) 2.5-fold. The P101S (E. coli numbering) mutation was discovered by mutagenesis of bacterial genes (Stalker et al. 1985). Since then, eight species of weeds have emerged as resistant to glyphosate by virtue of substitutions at the equivalent position (proline 106, mature maize EPSPS numbering; Baerson et al. 2002; Huffman et al. 2016; Ngo et al. 2018; Sammons and Gaines, 2014). Depending on the substitution and where no other resistance mechanisms are suspected, the dose required for 50% mortality is twofold to sevenfold greater in resistant plants relative to sensitive ones. The available kinetic data reflect a similar degree of desensitization (increased Ki) to glyphosate. In the Zea mays backbone, P106S elevated Ki by fivefold, but Km PEP by only 20% (Table 1). The same mutation in GR goosegrass (Eleusine indica) raised Km PEP by 2.3-fold (8.9 μM for P106S vs 3.8 μM in the native EPSPS), but the effect on Ki was much greater (0.048 vs 1.04 μM, Baerson et al. 2002).
Just as the proline to leucine substitution perturbed affinity for PEP while very significantly desensitizing the E. coli enzyme to glyphosate (Healy-Fried et al. 2007, vide supra), the same mutation had similar effects in maize EPSPS, where Km PEP was elevated fivefold and Ki, 60-fold (Table 1). P106L has been identified in at least three GR weed species (Kaundun et al. 2011; Chen et al. 2015; Ngo et al. 2018). Ngo and colleagues isolated populations of GR Rhodes grass (Chloris virgata) with either the P106S or P106L mutation and noted that the lines containing P106L were 2.9-fold to 4.9-fold more resistant than the lines with P106S. Despite greater desensitization to glyphosate compared with P106S, the fivefold elevated Km PEP with P106L limits the fitness of the enzyme, perhaps accounting for its lower occurrence in GR weeds relative to P106S.
A second glyphosate-desensitized EPSPS variant is a double mutant maize enzyme in which threonine at position 102 is changed to isoleucine in concert with the P106S mutation. The enzyme, termed TIPS, is highly desensitized to glyphosate (10,000-fold increased Ki), whereas its Km for phosphoenolpyruvate is nearly normal (16.2 μM vs 9.5 for the native; Table 1). However, it has only 6% of the kcat of the native enzyme (Table 1; also, Funke et al. 2009; Yu et al. 2015). Like the Pro106 mutations, the TIPS mutations exert their effect by shifting Gly101 closer to the glyphosate binding site (Funke et al. 2009). The catalytic efficiency (kcat/Km PEP) for TIPS EPSPS is only 4% of that for native maize EPSPS (6.5 min−1 μM−1 for TIPS vs 172 for native, Table 1), insufficient if a tolerance trait is to be created by natural mutagenesis or gene editing. However, given its excellent discrimination between PEP and glyphosate (Ki/Km PEP = 45 vs 0.0069 for native maize, calculated from Table 1), it can perform well given sufficiently high transgenic expression, as in GA21 maize (Spencer et al. 2000). While P106X mutations in resistant weeds have been known for years, it was recently shown in a tropical weed that a mutation at the TIPS partner position, 102, can also confer resistance (Li et al. 2018). In this case, the T102 change was serine, not isoleucine. Kinetic characterization of the variant showed that it was no fitter than P106S and thus unsuitable as a commercial tolerance trait (Table 1).
Stepwise acquisition of both T102I and P106S mutations was documented in Eleusine indica (Yu et al. 2015). However, out of a population of 193 individuals, only 1.6% were homozygous for TIPS. The highest frequency allelic combination was TIPS/P106S, suggesting that the normal catalytic efficiency contributed from the P106S allele was more important for fitness than having the second allele encode a highly insensitive but catalytically deficient enzyme.
Moehs et al. (2020) recently used chemical mutagenesis, DNA-based screening, and conventional crossing to create the TIPS mutations in two of the three subgenome homoeologous copies of the EPSPS gene in wheat. The third homoeologous copy had either wild type EPSPS or was homozygous for the T101L mutation. The impaired catalytic capacity of the TIPS mutations in two of the three EPSPS isozymes appeared to impair growth in the absence of glyphosate treatment, despite the presence of a third, wild type, enzyme. The presence of other chemically induced mutations throughout the genome could also have contributed to impaired growth. The plants exhibited a “substantial” tolerance to glyphosate at spray rates of 630 and 870 g/ha.
The most direct way to influence the binding affinity of glyphosate through position 101 (maize numbering) is to substitute alanine for Gly101, which places an additional methyl group in the active site near the phosphate end of PEP or phosphonate end of glyphosate. This mutation was first reported with the enzyme from a GR strain of Klebsiella (Sost and Amrhein 1990). The first naturally occurring EPSPS known to have alanine in place of glycine at position 101 was that from Agrobacterium strain CP4, the organism found surviving at the glyphosate manufacturing plant and used to develop Roundup Ready® crops (Padgette et al. 1995). It is highly insensitive to glyphosate (Ki = 1970 μM) while maintaining a high affinity for PEP (Km = 15 μM). However, it has only 15% of the catalytic efficiency (kcat/Km) of the plant enzyme due mainly to a much lower kcat (Table 1), necessitating tissue-specific, high expression transformation cassettes. Plasmid pPV-GMGT04, for example, has one copy of the CP4 EPSPS gene driven by the Cauliflower Mosaic Virus 35S promoter and a second copy driven by the Figwort Mosaic Virus 35S promoter. Both copies were fused to the petunia EPSPS chloroplast transit peptide for targeting to the organelle with the entire aromatic biosynthesis pathway. An improved expression cassette was used for introducing CP4 EPSPS into Roundup Ready2 Yield soybeans and Roundup Ready Flex cotton (Meyer 2006). The plasmid, designated PV-GMGOX20, contains a chimeric promoter consisting of enhancer sequences from the 35S promoter of the Figwort Mosaic virus and the promoter from the Tsf1 gene of Arabidopsis thaliana encoding elongation factor EF-1 alpha. Grain yield from commercial lines derived from the initial transformation event is 5% greater than that obtained from the original Roundup Ready soybeans (Meyer 2006).
Schönbrunn’s group investigated the molecular basis for CP4’s exquisite discrimination between glyphosate and PEP using X-ray crystallography. Though CP4 and E. coli EPSPS share only 26% amino acid sequence identity, they share the same fold and topology (Pollegioni et al. 2011; Duke 2021), allowing direct comparisons of CP4 with a representative Class I EPSPS. Funke et al. (2006) compared the crystal structure of CP4 ligated with S3P and glyphosate (PDB 2GGA) with a structural model of E. coli EPSPS where the contextually equivalent glycine (position 96, E. coli numbering) was changed to alanine, also ligated with S3P and glyphosate (Eschenburg et al. 2002). Funke et al. observed that the alanine methyl group in CP4 is 0.3 Å further away from the phosphonate group of glyphosate than the same alanine in the E. coli modeled structure. Presumably, the sequence context of CP4 places the methyl group of alanine 96 in an ideal position to interfere with glyphosate binding but not PEP.
Plant EPSPS with the G101A mutation has similar insensitivity to glyphosate as CP4 but only 1.4% of the catalytic efficiency of the native plant enzyme, mainly due to the 40-fold increase in Km for PEP imposed by the additional methyl group (Table 1; also, Padgette et al. 1991). Its low affinity for PEP precludes the G101A mutation from being found in a GR weed as a single mutation. The divergent amino acid sequence of CP4 versus Class I EPSPSs was thought to provide the structural context for an optimal spatial location of the alanine methyl group. However, scientists at Corteva Agriscience showed that with 17 or more additional mutations (discovered by an iterative process of random mutagenesis, combinatorial gene shuffling, and selection), the enzyme from maize could be adapted to accommodate the G101A mutation, resulting in kinetic parameters equal to or better than those of CP4 (Dong et al. 2019). The maize variants are no closer in homology to CP4 than is the native maize enzyme, showing that the amino acid sequence context provided by CP4 that positions alanine for optimal discrimination between glyphosate and PEP is not unique but can be arrived at by modern methods of protein engineering. In theory, the substitutions defined by in vitro optimization could be created by CRISPR/Cas9-enabled gene editing.
Questions that emerge from the preceding review are (1) which kinetic parameters are most important for enabling glyphosate resistance in crops and weeds, (2) what are the ideal values for them, and (3) do they differ for crops and weeds? The ideal EPSPS for either crops or weeds would exhibit the normal ability to maintain flux through the EPSPS reaction in the presence of glyphosate concentrations up to 1 mM, a concentration attainable in tissues, especially meristems, receiving metabolite flow from treated leaves (Kirkwood et al. 2000). The term (kcat/Km)*Ki combines an expression of catalytic efficiency (kcat/Km) with one of affinity for inhibitor compared to the substrate (Ki/Km) (Lu et al. 2017). However, while the term is useful for assessing the intrinsic capacity for activity in the presence of a competitive inhibitor, it can be misleading for predicting in vivo fitness (reaction velocity under application conditions, i.e., plants sprayed with glyphosate). It omits concentrations of substrates and inhibitor, factors that are not intrinsic to the enzyme, but on which the reaction rate depends, as seen in the Michaelis–Menten equation for reaction velocity (v) in the presence of a competitive inhibitor (I):
Reaction velocity is directly proportional to kcat and nearly so to 1/Km. A very low value for Ki will greatly increase the denominator, thereby reducing v. Higher values for Ki effectively improve fitness, but only until Ki reaches the approximate inhibitor concentration, after which further increases will proportionately increase (kcat/Km)*Ki, but can only effect an additional twofold increase in v. The ideal gauge of enzyme fitness would be a single rate measurement made under the conditions of the application (pH, ionic strength, substrate, and inhibitor concentrations) if known. For optimizing maize EPSPS-G101A, we used a rate measurement under conditions designed to mimic intracellular conditions (pH 7, 100 mM KCl, 5% ethylene glycol; Dong et al. 2019). Ideally, concentrations of PEP and S3P would have been set at 10 or 15 μM, which we assume approximate in vivo concentrations based on their values for Km, but the sensitivity of our assay limited us to 30 μM each. Glyphosate was set at 1 mM. The reaction velocity (μM min−1) expressed as a function of enzyme concentration (μM) yields units of min−1, which we termed “kgly”. Figure 2 is a graphic comparison of (kcat/Km)*Ki with kgly. The two measures of fitness correlated rather well except for CP4. With its very high Ki, CP4 displayed a disproportionately high (kcat/Km)*Ki. (Note: Values of (kcat/Km)*Ki for CP4 and G101A-optimized are much farther apart than they appear on the log scale (See Table 1). This is due to the greater impact of Ki on that parameter compared with its impact on the velocity equation for competitive inhibition, which our kgly parameter seeks to represent. The relatively low kgly for CP4 given its outstanding selectivity (Ki/Km) is attributable to its low value for kcat. We conclude that the answer to question 1 is that all three raw kinetic parameters contribute to fitness for glyphosate resistance, and the composite term (kcat/Km)*Ki is a good surrogate for fitness except when Ki is very much higher than [I]. The ideal GR-enabling EPSPS (Question 2) would have the insensitivity of CP4 (Ki > ~1,500 μM) and a catalytic efficiency approaching that of the native plant enzyme (kcat/Km > 150 min−1μM−1). It would be instructive to learn whether such an enzyme would meet commercial requirements for glyphosate resistance if endowed by gene editing.
Fitness (kgly1) of EPSPS variants as a function of values (kcat/Km)*Ki 1kgly; reaction velocity (min−1) of the EPSPS variant in the presence of 30 μM each of PEP and S3P, 1 mM glyphosate. For rational as a fitness parameter, see Text, Sect. 2
Regarding Question 3, from the data presented here, weeds appear to require a far less fit EPSPS variant than crops. The P106S mutation was shown conclusively to solely account for the resistance seen in a Tennessee isolate of GR Eleusine indica (Huffman et al. 2016). Yet the mutation has not been exploited as a GR trait in a crop, probably due to its modest insensitivity to glyphosate. In contrast, the TIPS mutations, while shown to carry a severe fitness penalty in weeds (Han et al. 2017), doubtless due to the impaired kcat (Table 1), can enable glyphosate resistance in maize, given sufficiently high transgenic expression (GA21 maize; Spencer et al. 2000). Crop resistance must be sufficient to withstand a double dose of herbicide due to overlapping spray. Further, crops place a high demand for the products of biosynthetic pathways, and any impaired flux will cause yield loss. In contrast, weeds need only to be fit enough to produce viable seeds. Also, a desensitized EPSPS variant encoded on the native gene may have an advantage over a transgene in that it is optimized by nature for appropriate expression in all tissues and growth stages.
Glyphosate Resistance Through Derivatization or Degradation of Glyphosate
An alternative way to confer herbicide resistance in crops is to express an enzyme that degrades or derivatizes the herbicide. In one such approach, N-acetylation of glyphosate was discovered in a soil bacterium, Bacillus licheniformis (Castle et al. 2004). The activity was far too weak to confer tolerance but was increased 9,000-fold by gene shuffling. Although the native substrate is not known, robust activity (kcat/Km = 1,500 min−1 mM−1 versus 4 min−1 mM−1 for glyphosate) was found with D-2-amino-3-phosphonopropionate (D-AP3) an isomer of glyphosate (Siehl et al. 2007). Though no antibiotic activity has been ascribed to D-AP3, the existence of an N-acetyltransferase with activity toward it is reminiscent of the mechanism for detoxifying glufosinate.
In microorganisms, glyphosate is degraded by two distinct pathways, as shown in Fig. 3. Glyphosate is not metabolically degraded in most plant species. However, appreciable oxidation to glyoxylate and aminomethylphosphonate (AMPA) was observed in soybean (Komossa et al. 1992; Duke 2011). The enzyme responsible was not identified, but recently, an isolate of Echinochloa colona with low-level resistance to glyphosate showed elevated expression of an aldol-keto reductase capable of cleaving glyphosate to AMPA and glyoxylate (Pan et al. 2019). Elsewhere, a bacterial glycine oxidase (GO) was engineered to accept glyphosate as a substrate, yielding glyoxylate and AMPA (Pedotti et al. 2009). They engineered a 15,000-fold shift in the ratio of kcat/Km glyph/kcat/Km glycine in their improved variants relative to the native enzyme, mainly by raising Km for glycine 150-fold (0.7–105 mM) while reducing Km for glyphosate by a similar magnitude (87–0.5 mM). Values for kcat were about 1 s−1 for wild type and improved variant with either substrate. Alfalfa plants expressing the improved glyphosate oxidase linked to a chloroplast targeting sequence exhibited “moderate” resistance (Nicolia et al. 2014).
Another glyphosate oxidase termed GOX was identified in Ochrobactrum anthropi strain LBAA (Barry and Kishore 1995). Like GO, GOX is a flavoenzyme but catalyzes oxidative cleavage of the C2-N bond of glyphosate, yielding glyoxylate and AMPA, by a different mechanism. GOX acts in concert with CP4 EPSPS to confer glyphosate resistance in the first GR canola.
In addition to cleavage of the C2-N bond catalyzed by glyphosate oxidases, many soil microbes cleave the C-P bond, yielding N-methylglycine (sarcosine) and phosphate (Hove-Jensen et al. 2014). N-methylglycine occurs naturally and can be further metabolized to glycine by several routes. Thus, a tolerance mechanism by which the C-P lyase pathway metabolizes glyphosate would reduce if not eliminate the synthetic pesticide residues. Hove-Jensen and colleagues also elucidated the genetic and mechanistic details of the C-P lyase pathway. The multiple enzymes and transporters required for the pathway are encoded by 14 genes (more or less), usually on a single operon. Seven are considered the “core complex”, with the phnJ gene product known to catalyze the key reaction, the S-adenosyl-l-methionine-dependent radical cleavage of 5-phosphoribosyl-1-phosphonate to produce 5-phosphoribosyl 1,2-cyclic phosphate and the corresponding alkane. Though tantalizing as a mechanism for glyphosate tolerance, it is a daunting prospect to express a multigenic trait coding for a multi-enzyme complex that normally functions anaerobically.
There are microbial enzymes that cleave C-P bonds by a hydrolytic mechanism, each specific for a particular phosphonate compound (Villareal-Chiu et al. 2012). It is tempting to use directed evolution to make these single-gene hydrolases accept glyphosate as a substrate. However, all the native substrates have a carbonyl group at the position beta to the phosphorus atom. The carbonyl oxygen can accommodate the electron pair that must be displaced from the phosphorus atom, precluding the C-P bond of glyphosate from being hydrolyzed by this mechanism.
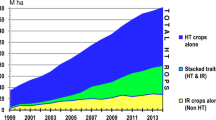
3 Rapid Adoption of Glyphosate-Resistant Crops
Most of the economic impact of GR crops has been due to one gene, CP4 EPSPS (Fig. 4). Today, GR traits are widely available in breeding germplasm. Breeders can easily maintain glyphosate resistance as a background trait in their germplasm and satisfy the expectations of many growers that it is in the seed they purchase (Green 2014). GR soybeans sales started in 1996 with cotton, canola, and corn ensuing soon after. Sales of GR alfalfa and sugarbeets began a decade later. GR crops made the widely used nonselective glyphosate into a selective herbicide. Growers urgently needed the technology when it became available. Weeds were evolving resistance to selective herbicides such as triazines and inhibitors of acetolactate synthase and acetyl-CoA carboxylase, which required growers to use complicated and expensive mixtures to manage. Managing resistant weeds was taking too much time as farms were getting bigger and employing fewer people. Glyphosate was initially the ideal solution to control resistant weeds.
GR crops gave the seed industry a new way to create intellectual property (IP) capture value. The first GR crop systems were not perfect (Elmore et al. 2001; Green 2009). Crop yields were low, safety margins narrow, and some application timings were tightly restricted. Monsanto also required growers to pay a technology fee and sign a contract. The contract required growers to agree to not replant seed, which was essential to maintain the trait value in soybeans. Growers strongly objected to the contract but still signed. Control of the glyphosate trait through this agreement was more valuable than a patent because it gave control of the technology indefinitely.
GR crops gave a range of benefits to growers. In addition to enabling the cost savings of using glyphosate instead of more expensive selective herbicides and realizing increased yields due to more effective weed control, GR crops enabled growers to reduce or even eliminate tilling. A pre-plant spray of glyphosate requires less fossil fuel than turning the soil, reducing fuel costs. Incidental but very welcome benefits to both growers and the environment were less soil erosion, reduced carbon dioxide emissions from tractors, and increased carbon sequestration in the soil. The net result of these benefits is that from 1996 to 2015 in North and South America was a cost-saving totaling $70 billion (Brookes et al. 2017).
The success of GR crops had an unfortunate unintended consequence. Although profits and research budgets generally increased, companies shifted funding away from herbicide discovery, which they perceived to have been largely rendered obsolete, to biotechnology and crop genetics (Charles 2001). Furthermore, generic manufacturers took advantage of the expiration of key glyphosate patents, which occurred not long after GR crops became available, and sold glyphosate at low prices. The continued decline in the cost of glyphosate reduced the demand for selective herbicides despite price reductions. In 2002, after 6 years of glyphosate sales in GR crops, the number of herbicides used on 10% or more of the US soybeans had decreased from 11 to just one, glyphosate (Duke and Powles 2009). Thus, GR crops were causing a problem (focusing selection pressure for weed resistance on one herbicide) and inhibiting the solution (discovering new herbicides with new MoA to partner with glyphosate).
4 Evolution and Consequences of Resistant Weeds
When growers adopted GR crops, they usually adopted the practice of using only glyphosate to control weeds (Baek et al. 2021). The crucial question always was whether a glyphosate-only system would be sustainable. Before GR crops, glyphosate was a widely used nonselective herbicide with very few suspected cases of resistance. Some believed that weeds would not evolve resistance because mutations in plant EPSPS were only modestly insensitivity to glyphosate or caused catalytic impairment (see above). Further, plants seemed to have minimal ability to degrade glyphosate to nontoxic metabolites (Duke 2011). However, applying glyphosate alone over vast areas of GR crops put tremendous selection pressure on weeds to evolve resistance, and they eventually did (Powles 2008). GR weeds are common now and reached the tipping point where many growers can no longer rely on glyphosate alone to provide commercially acceptable weed control. The epidemic of GR weeds has significantly reduced the value of the GR crop weed management system. Still, many growers continue to use GR crops throughout North and South America because competing systems are not any better, more expensive, or difficult to use.
In retrospect, growers should have used glyphosate in combination with existing selective herbicides to diversify their weed management practices. Photosystem II (PSII) inhibitors such as triazine and urea herbicides, lipid synthesis inhibitors such as S-metolachlor (2-amino-4-(hydroxymethylphosphinyl)butanoic acid, CAS No. 87392-12-9), and inhibitors of phytoene desaturase (PDS) or protoporphyrinogen oxidase (PPO) could have provided soil residual to control and delayed the evolution and spread of GR weeds (Green and Owen 2011). However, growers en masse used only glyphosate year after year. Today, 48 weed species have evolved resistance to glyphosate with at least ten different mechanisms (Sammons et al. 2016, Heap 2020). Unfortunately, many GR weeds in GR crop systems are also resistant to other herbicides. Currently, 20 weed species are known to be resistant to glyphosate and at least one other herbicide type (Heap 2020). These multiple HR weeds complicate weed management and threaten current crop production practices. Many growers are now almost out of options and must use large volumes of old and partially effective selective herbicide mixtures (Perry et al. 2016). The use of diverse herbicide systems in GR crops is now imperative.
The most difficult to control GR weeds in GR crops include Amaranthus palmeri S. Wats., A. tuberculatus (Moquin-Tandon) J. D. Sauer, Ambrosia artemisiifolia L., A. trifida L., and Conyza canadensis (L.) (Heap 2020). The first response of many growers when they see weed escapes is to reapply glyphosate at higher rates and then use glyphosate mixtures with other herbicides. Using a plethora of combinations of old, imperfect herbicides that growers had stopped using is a temporary solution and not a technological step forward (Green and Owen 2011).
The recent mergers of some of the largest pesticide companies, such as Bayer and Monsanto, Dow and DuPont, and Syngenta with ChemChina and Sinochem, ensure the continuance of herbicide discovery programs with critical mass (Mulvany and Decker 2019). Ironically, the slowdown in herbicide discovery has not meant a decline in the chemical herbicide business. Growers now must spend more money on more herbicides to combat HR weeds, creating a resurgence of the crop protection herbicide business (Sfiligoj 2014). In the same way, seed companies also benefit when growers buy higher-priced seeds with more herbicide traits that enable new options to control HR weeds.
5 Next Generation of Glyphosate-Resistant Crops
When the patents for the “first generation” GR crops were about to expire (Shah et al. 1990), there was no process established on how to handle generic GM crops as there was for generic pesticides. Improved GR crops overcame some of the deficiencies of the first GR crops and created new intellectual property protection. The improved crops made retaining total control of the first GR crops less important for Monsanto. The new GR soybeans claimed a yield advantage over the original; the new GR cotton claimed improved crop safety with a wider application window, and the new GR canola claimed improved crop safety with a wider application window even without the glyphosate oxidase (gox) gene. New GR crops are still being introduced. In February 2019, Argentina approved a GM soybean coded DBN-09004-6 with the CP4 EPSPS and pat genes developed by Beijing Dabeinong Biotechnology Co., Ltd., becoming the first GM crop developed by a Chinese company approved for planting outside of China.
The days when crops are only resistant to glyphosate have ended (Green and Castle 2010; Que et al. 2010, Nandula 2019). A new generation of GR crops is well underway with combinations of glyphosate and other herbicide traits (Table 2). Today, most new HR crops are stacks with resistance to glyphosate, glufosinate, and one of four different herbicide types. In 2020, US soybean growers can now choose new varieties with various combinations of five HR traits (Ungelesbee 2019). These varieties have glyphosate and dicamba traits; glyphosate, dicamba, and glufosinate traits; glyphosate, glufosinate, and HPPD-inhibitor traits; and glyphosate, 2,4-D, and glufosinate traits, so growers can apply mixtures of glyphosate with other herbicides. Unfortunately, herbicide companies have not commercialized a new MoA for over 30 years and have nearly exhausted the useful herbicide trait combination. Growers desperately need new herbicide technology (Han et al. 2016; Dayan 2019), but the chance of finding another herbicide with a similar impact to glyphosate is small.
The newest HR crop technologies have resistance to a synthetic auxin herbicide, ACCase-inhibitor, or one of two HPPD-inhibitors (Behrens et al. 2007; Wright et al. 2010). Glyphosate resistance stacked with dicamba resistance is getting the most attention. The dicamba trait is conferred by a monooxygenase from Pseudomonas maltophilia (strain DI-6) that converts dicamba to 3,6-dichlorosalicylic acid (DCSA) and formaldehyde (Behrens et al. 2007). The oxygenase reaction requires two electrons and two protons, which in the bacterium originate from NADH and are shuttled via a reductase to ferredoxin. Interestingly, robust tolerance is conferred in plants by transformation only with the oxygenase. The plant has orthologs of the reductase and ferredoxin that are fully adequate to complete the electron transfer.
After three years of strong growth, experts expect plantings of dicamba-resistant crops to plateau this year at about 20 million hectares. Four companies are promoting the technology and hold registrations for foliarly applied dicamba. Dicamba-resistant soybeans and cotton enable new uses of an old herbicide with a long history of off-target drift problems. The first seasons of dicamba use in dicamba-resistant soybeans caused millions of hectares of damage to nontarget sensitive soybeans and other plants (Bradley 2018; Hager 2019). Opinions differ sharply on what caused the problem (Li et al. 2013; Egan et al. 2014). Despite the difficulties, the EPA extended dicamba product registration for two and five more years (EPA 2018, 2020).
The analogous use of 2,4-D in resistant crops is expanding greatly this year, making it about 2 years behind dicamba use and similarly depends on new directions for use and a new salt and formulation. Corteva expects its 2,4-D resistant seed to capture about 20% of the US crop in 2020, the first year it has been widely available. The 2,4-D trait is conferred in soybeans by AAD-12, an Fe(II)/α-ketoglutarate-dependent dioxygenases from Delftia acidovorans that degrades the acetic acid side chain of 2,4-D, yielding non-phytotoxic dichlorophenol and glyoxylate (Wright et al. 2010).
Crops with resistance to HPPD-inhibiting herbicides with some soil residual could also help control key GR weeds. The evolution of HPPD-resistant Palmer amaranth and waterhemp before market introduction reduces the value of this technology and requires its use with other herbicides (Green 2012). Two HPPD traits with different characteristics are under development, both of which involve an HPPD with reduced sensitivity (Allen et al. 2012; Miller et al. 2013). As with auxin herbicides, corn generally has natural tolerance to most HPPD herbicides, so the technology has more utility in soybeans and cotton.
BASF and Bayer, in cooperation with Sumitomo, are developing crops resistant to PPO-inhibiting herbicides (Green 2018). BASF and Sumitomo Chemical may have a new generation of broad-spectrum PPO herbicides that could be commercially available early next decade. The concept of matching broad-spectrum resistance-busting PPO-inhibiting herbicides with PPO-resistant crops could be very beneficial if researchers can identify the right herbicides and traits. However, a PPO-resistant crop system is not a new idea. Syngenta had a similar effort with the trade name of Accuron™ more than a decade ago (Li and Nicholl 2005).
Transgenic and non-transgenic crops are also commercially available with resistance to ACCase- and acetolactate synthase (ALS)-inhibiting herbicides (Green and Owen 2011). To get broad-spectrum crop resistance to a range of herbicides, researchers are investigating metabolic degradation by cytochrome P450 monooxygenase and glutathione-S-transferase (GST). Such metabolic mechanisms giving crop safety to a wide range of herbicides would be highly valuable until weeds evolve similar resistance mechanisms (Han et al. 2016; Délye 2013).
Growers need the new HR crop technologies to use with new nonselective and selective herbicides. Unfortunately, the future pipeline of herbicide options with commercial utility for the HR crop stacking is mostly exhausted (Green 2018). Current options already have resistant weed problems and other limitations. Most do not meet the standard of overlapping weed spectrum with an effective and different MoA (Vencill et al. 2012; Young 2015). Currently, three-way herbicide stacks are commercially available in cotton and will soon be in soybeans. Plans are for four-way herbicide stacks in the mid-2020s and a five-way by 2028. These multiple HR crops will help growers manage resistant weeds.
6 Outlook for Glyphosate-Resistant Crops
The agrochemical industry has encountered a downturn in the agricultural economy, as US farm income declined by 40% between 2013 and 2016 (https://www.cobank.com/knowledge-exchange/general/recessions-us-agriculture). Simultaneously, increasing regulations made the introduction of new herbicides and herbicide traits more expensive (Phillips 2020). Together, these trends slowed the introduction of new technology. Some politicians and regulators want to ban these technologies, so growers must contemplate a future without them. Weed scientists in Australia recently did just that, modeling five agronomic settings where glyphosate use would be restricted or banned (Beckie et al. 2020). The participants outlined alternative methods of weed control using nonchemical weed management practices combined with preemergence herbicides. The study is a model for formulating appropriate strategies in regions currently relying on glyphosate and GR crops.
Human safety of GR crops – Glyphosate has been widely used for more than five decades and GR crops for more than two decades. Still, GR crop systems remain controversial and the target of activists. Today, questions about safety dominate the news (Kabat 2019; WSSA 2019). In considering the potential toxicity of the proteins introduced into GR crops, we first point out that there have been no reports of direct carcinogenic, teratogenic, or mutagenic effects associated with the ingestion of proteins in general (Hammond et al. 2013). That is not surprising given that proteins are not taken up intact by the intestine, but are denatured by low pH in the stomach, then hydrolyzed into amino acids and di- and tri-peptides by intestinal proteases. Many pseudo-scientific reports claim adverse effects from consuming food derived from crops containing GR and other traits created by genetic technology. A report claimed that Cry insect control proteins from Bacillus thuringiensis caused hematotoxicity in mice when Bt spores containing various Cry proteins were administered by stomach tube (Mezzomo et al. 2013). Besides the irrelevant mode of administration, the control was water instead of spores lacking the Cry genes, thereby failing to account for the many substances in the spores that may have caused the observed effects. Other studies use physiologically unattainable doses of up to 1,000-fold typical exposure levels, attempting to demonstrate a hazard, as opposed to an actual risk (Hammond et al. 2013).
In GR crop-derived food, the protein introduced is either CP4 EPSPS or TIPS doubly mutated maize EPSPS. In the latter case, the same mutations are present in naturally occurring GR weeds (see above). Regarding CP4, its crystal structure overlaid with those of EPSPS of crop plants show that in addition to having the same function, they share the same structural fold and topology (Hammond et al. 2013). Homologous EPSPS proteins are ubiquitous in plant, yeast, and microbial food sources and have widely ranging degrees of amino acid sequence identity. All have a long history of safe use. No form of EPSPS, including CP4, has been reported to be toxic or allergenic.
The US EPA and other regulatory agencies support the use of glyphosate in GR crops and assure the public that it is safe when used according to label directions. Still, there is strong opposition that prevents their deployment in many crops. Businesses that own approved HR crop regulatory packages have a significant competitive advantage as the process of getting new approvals is too costly and too slow for most investors. For example, the commercialization of a single transgenic herbicide tolerance trait typically costs ~$136 million and takes over 13 years. Codeveloping a broad-spectrum herbicide in conjunction with a transgenic tolerance trait increases the risk and the cost, explaining why companies are shifting resources to less regulated methodologies such as gene editing (Crop Life America 2012, 2016). At the current rate that weeds evolve resistance, one new HR crop trait would not be enough to ensure sustainability.
Forecasts predicting that glyphosate would soon be the first pesticide to reach $10 billion annual sales have disappeared, but nobody predicts zero sales. Glyphosate is still the most broadly effective herbicide for most growers on most weeds (Abnewswire 2016). However, the spread of GR weeds is raising the cost of weed control, which creates more incentive for the industry to renew herbicide discovery efforts. Although the payoff for the simultaneous paired discovery of highly effective herbicides with a new MOA and associated trait would be very high, so are the risks associated with the high cost of discovery and development, long timelines, and the threat that non-target site resistance that could confer cross-resistance to a herbicide before it reaches the market. Growers and scientists agree that no weed management system used alone is sustainable for very long as weeds eventually evolve resistance to any single management tactic. This imperative is driving the industry to discover multiple new herbicide MoA and tolerance traits.
Today, scientists are exploring alternative ways to create glyphosate tolerance, such as wide-crossing from related resistant weed species, gene editing, gene shuffling, and new transgene options. One effort to displace CP4 EPSPS gene technology was to shuffle a gene encoding the acetyltransferase enzyme (EC 2.3.1.13). The gene shuffling methodology resulted in very high crop tolerance to glyphosate (Castle et al. 2004; Green et al. 2009). Recently, an isolate of Echinochloa colona with low-level resistance to glyphosate showed elevated expression of an aldol-keto reductase capable of cleaving glyphosate to AMPA and glyoxylate (Pan et al. 2019). Though the resistance factor was modest, well-established enzyme optimization methods could identify amino acid substitutions that could greatly improve activity and be introduced into the native gene through CRISPR/Cas9-facilitated gene editing. The other known pathway for glyphosate degradation is through cleavage of the C-P bond, yielding phosphate and N-methylglycine (sarcosine), catalyzed by C-P lyase, described in Sect. 2. Because the pathway minimally requires seven gene products, it would be difficult to express in plants through transformation and impossible by gene editing. However, such a crop would have significantly reduced glyphosate residue with no nonnatural metabolites. A new metabolic trait that eliminates glyphosate residues in food crops such as wheat and sugarcane could be highly valuable, especially if it is non-transgenic.
New geographies for the introduction of GR crops should include developing countries, where they could satisfy a huge unmet need. In sub-Saharan Africa, for example, cassava, a staple crop relied upon by 500 million people, is normally hand-weeded by women and children. This primitive method often fails to optimize yield, is enormously time-consuming, and can result in spinal deformation (Gianessi 2013). GR and many other traits could be a great benefit to African farmers.
In the Americas, the “Roundup Ready Revolution” is over. Still, the use of glyphosate traits in combination with other traits could expand into some new regions and crops if public concerns about glyphosate and GR crops lessen. Growers that do not have GR crops yet can learn from the American experience and use glyphosate to expand the diversity of weed management practices to sustain the utility of the GR crop system. The new multiple HR crops enable more diverse and improved stewardship practices if growers follow advice from experts and label directions (Kaskey and Mulvany 2016; Heacox 2015).
7 Conclusion
No technology with the impact of glyphosate and GR crops is on the horizon (Westwood et al. 2018). Glyphosate-based crop systems will continue to be the mainstays of weed management in many areas, but they have lost value because they cannot keep up with the capacity of weeds to evolve resistance. Crops resistant only to glyphosate are not an acceptable options in most situations because of the evolution and spread of GR weeds. Relying on one weed management solution does not work anymore. Growers need multi-HR crops to combine glyphosate with other potent HR-enabled herbicides to control GR weeds. Traditional approaches by companies, sometimes called the pesticide and transgenic treadmills, cannot provide new solutions fast enough to match the speed that weeds evolve resistance (PAN 2016; Binimelis et al. 2009). Managing weeds using all currently available tactics in a systems approach is working for most growers, but nobody knows for how much longer. Hopefully, long enough to develop new weed management technologies.
In many market segments, it is challenging to buy seeds without a glyphosate trait. Many growers expect the glyphosate trait to be in the seed. The cost and time to introduce new HR crops is a high hurdle that slowed research for glyphosate trait combinations. Opposition to GR crops and the associated use of glyphosate and transgenic methods is still strong even after a quarter of a century of widespread use. The outlook for GR crops is at a nexus and depends on the following issues:
-
Economic, e.g., input costs, farm income, and demands to increase production;
-
Social, e.g., public opposition to glyphosate and GM crops;
-
Environmental, e.g., regulations requiring minimum and no-tillage practices, drift control, and other label mandates;
-
Biological, e.g., the continued evolution and spread of GR weeds;
-
Technological, e.g., the effectiveness of new chemical and nonchemical weed management technologies to combat GR weeds;
-
Sustainable, e.g., the continued utility of current technologies such as glufosinate, dicamba, 2,4-D, as well as HPPD- and PPO-inhibiting herbicides and their trait technologies;
-
Regulatory, e.g., any removal or approval of GR crops or other technologies;
-
Legal, e.g., resolution of the current and future environmental and human safety litigation; and
-
Political, e.g., how public officials respond to activist pressure to restrict and even ban glyphosate and GM crops.
Abbreviations
- ACCase:
-
Acetyl coenzyme A carboxylase
- ALS:
-
Acetolactate synthase
- EFSA:
-
European Food Safety Authority
- EPA:
-
Environmental Protection Agency
- GM:
-
Genetically modified
- GST:
-
Glutathione-S-transferase
- HPPD:
-
4-Hydroxyphenyl pyruvate dioxygenase
- HR:
-
Herbicide-resistant
- HT:
-
Herbicide-tolerant
- IP:
-
Intellectual property
- ISAAA:
-
International Service for the Acquisition of Agri-Biotech Applications
- NTO:
-
Nontarget organism
- NTSR:
-
Non-target site resistance
- PDS:
-
Phytoene desaturase
- PPO:
-
Protoporphyrinogen oxidase
- PSII:
-
Photosystem II
References
Abnewswire (2016) Global glyphosate market is expected to cross US$ 10.0 Billion by 2021. Abnewswire http://www.abnewswire.com/pressreleases/global-glyphosate-market-is-expected-to-cross-us-100-billion-by-2021-by-market-research-engine_52900.html
Allen J, Hinz J, Essner FJ, Van Wert S (2012) Introducing a new soybean event with glyphosate and HPPD tolerance. Abstr Weed Sci Soc Am 52:190
Baek Y, Bobadilla LK, Giacomini DA, Montgomery JS, Murphy BP, Tranel PJ (2021) Evolution of glyphosate-resistant weeds. Rev Environ Contam Toxicol. https://doi.org/10.1007/398_2020_55
Baerson SR, Rodriguez DJ, Tran M, Feng Y, Biest NA, Dill GM (2002) Glyphosate-resistant goosegrass: identification of a mutation in the target enzyme 5-enolpyruvylshikimate-3-phosphate synthase. Plant Physiol 129:1265–1275
Barry GF, Kishore GA (1995) Glyphosate tolerant plants. 5,463,175. US Patent
Barry G, Kishore G, Padgette S, Taylor M, Kolacz K, Weldon M, Re D, Eichholtz D, Fincher D, Hallas L (1992) Inhibitors of amino acid biosynthesis: strategies for imparting glyphosate tolerance to crop plants. In: Singh BK, Flores HE, Shannon JC (eds) Biosynthesis and molecular regulation of amino acids in plants. American Society of Plant Physiologists, Rockville, pp 139–145
Beckie HJ, Flower KC, Ashworth MB (2020) Farming without glyphosate. Plan Theory 9:96. https://doi.org/10.3390/plants9010096
Behrens MR, Mutlu N, Cjalrabprtu S, Dumitru R, Jiang WZ, LaVallee BJ, Herman PL, Clemente TE, Weeks DP (2007) Dicamba resistance: enlarging and preserving biotechnology-based weed management strategies. Science 316:1185–1188
Binimelis R, Pengue W, Monterroso I (2009) “Transgenic treadmill”: responses to the emergence and spread of glyphosate-resistant johnsongrass in Argentina. Geoforum 40:623–633
Bøhn T, Millstone E (2019) The introduction of thousands of tonnes of glyphosate in the food chain – an evaluation of glyphosate tolerant soybeans. Foods 8:669. https://doi.org/10.3390/foods8120669
Boocock MR, Coggins JR (1983) Kinetics of 5-enolpyruvylshikimate-3-phosphate synthase inhibition by glyphosate. FEBS Lett 154:127–133
Bradley K (2018) July 15 dicamba injury update. Different year, same questions. University of Missouri Integrated Pest Management, Columbia. https://ipm.missouri.edu/ipcm/2018/7/July-15-Dicamba-injury-update-different-year-same-questions/
Brookes G, Taheripour F, Tyner W (2017) The contribution of glyphosate to agriculture and potential impact of restrictions on use at the global level. GM Crops Food 8:216–228
Castle LA, Siehl DL, Gorton DL, Patten PA, Chen YH, Certain S, Cho H-J, Duck N, Wong J, Liu D, Lassner MW (2004) Discovery and directed evolution of glyphosate tolerance gene. Science 304:1151–1154
Charles D (2001) Lords of the harvest: biotech, big money, and the future of food. Perseus Publishing, Cambridge, 348 pp
Chen J, Huang H, Zhang C, Wei S, Huang Z, Chen J, Wang X (2015) Mutations and amplification of EPSPS gene confer resistance to glyphosate in goosegrass (Eleusine indica). Planta 242:859–868
Crop Life America (2012) Getting a biotech crop to the market. Crop Life America, Washington. http://croplife.org/wp-content/uploads/2014/04/Fact-Sheet-Getting-a-Biotech-Crop-to-Market.pdf
Crop Life America (2016) A consultancy study for CropLife America. Crop Life America, Washington. http://croplifeamerica.org/wpcontent/uploads/2016/04/Phillips-McDougall-Final-Report_4.6.16.pdf
Dayan FE (2019) Current status and future prospects in herbicide discovery. Plan Theory 8(9):341. https://doi.org/10.3390/plants8090341
Délye C (2013) Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: a major challenge for weed science in the forthcoming decade. Pest Manag Sci 69:176–187
Dong Y, Ng E, Lu J, Fenwick T, Tao Y, Bertain S, Sandoval M, Bermudez E, Hou Z, Patten P, Lassner M, Siehl D (2019) Desensitizing plant EPSP synthase to glyphosate: optimized global sequence context accommodates a glycine-to-alanine change in the active site. J Biol Chem 294:716–725
Duke SO (2011) Glyphosate degradation in glyphosate-resistant and -susceptible crops and weeds. J Agric Food Chem 59:5835–5841
Duke SO (2021) Glyphosate: uses other than in glyphosate-resistant crops, mode of action, degradation in plants, and effects on non-target plants and agricultural microbe. Rev Environ Contam Toxicol. https://doi.org/10.1007/398_2020_53
Duke SO, Powles SB (2008) Glyphosate: A once-in-a-century herbicide. Pest Manag Sci 64:319–325
Duke SO, Powles SB (2009) Glyphosate-resistant crops and weeds: now and in the future. AgBioforum 12:346–357
Eschenburg S, Healy ML, Priestman MA, Lushington GH, Schönbrunn E (2002) How the mutation glycine96 to alanine confers glyphosate insensitivity to 5-enolpyruvyl shikimate-3-phosphate synthase from Escherichia coli. Planta 216:129–135
Egan JF, Barlow KM, Mortensen DA (2014) A meta-analysis on the effects of 2,4-D and dicamba drift on soybean and cotton. Weed Sci 62:193–206
Elmore GA, Roeth FW, Nelson LA, Shapiro CA, Klein RN, Knezevic SZ et al (2001) Glyphosate-resistant soybean cultivar yields compared with sister lines. Agron J 93:408–412
EPA (2018) EPA announces changes to dicamba registration. https://www.epa.gov/newsreleases/epa-announces-changes-dicamba-registration
EPA (2020) EPA announces 2020 dicamba registration decision. https://www.epa.gov/newsreleases/epa-announces-2020-dicamba-registration-decision
Funke T, Han H, Healy-Fried ML, Fischer M, Schönbrunn E (2006) Molecular basis for the herbicide resistance of roundup ready crops. Proc Natl Acad Sci U S A 103:13010–13015
Funke T, Yang Y, Han H, Healy-Fried M, Olesen S, Becker A, Schönbrunn E (2009) Structural basis of glyphosate resistance resulting from the double mutation Thr-97 3 Ile and Pro-101 3 Ser in 5-enolpyruvylshikimate-3-phosphate synthase from Escherichia coli. J Biol Chem 284:9854–9860
Gianessi LP (2013) The increasing importance of herbicides in worldwide crop production. Pest Manag Sci 69:1099–1105
Green JM (2009) Evolution of glyphosate-resistant crop technology. Weed Sci 57:108–117
Green JM (2012) The benefits of herbicide-resistant crops. Pest Manag Sci 68:1323–1331
Green JM (2014) Current state of herbicides in herbicide-resistant crops. Pest Manag Sci 70:1351–1367
Green JM (2018) The rise and future of glyphosate and glyphosate-resistant crops. Pest Manag Sci 74:1035–1039
Green JM, Castle LA (2010) Transitioning from single to multiple herbicide-resistant crops. In: Nandula VK (ed) Glyphosate resistance in crops and weeds: history, development, and management. Wiley, Hoboken, pp 67–91
Green JM, Owen MDK (2011) Herbicide-resistant crops: utilities and limitations for herbicide-resistant weed management. J Agric Food Chem 59:5819–5829
Green JM, Hale T, Pagano MA, Andreassi JL II, Gutteridge SA (2009) Response of 98140 corn with gat4621 and hra transgenes to glyphosate and ALS-inhibiting herbicides. Weed Sci 57:142–148
Hager A (2019) Observations from the field: dicamba. The bulletin. University of Illinois Extension, Urbana. http://bulletin.ipm.illinois.edu/?page_id=1196&pf=4834
Hammond B, Kough J, Herouet-Guicheney H, Jez JM (2013) Toxicological evaluation of proteins introduced into food crops. Crit Rev Toxicol 43:25–42. https://doi.org/10.3109/10408444.2013.842956
Han H, Yu Q, Owen MJ, Cawthray GR, Powles SB (2016) Widespread occurrence of both metabolic and target-site herbicide resistance mechanisms in Lolium rigidum populations. Pest Manag Sci 72:255–263
Han H, Vila-Aiub MM, Jalaludin A, Yu Q, Powles SB (2017) A double EPSPS gene mutation endowing glyphosate resistance shows a remarkably high resistance cost. Plant Cell Environ 40:3031–3042
Heacox L (2015) Spray drift enters more complex era. CropLife. http://www.croplife.com/crop-inputs/adjuvants/spray-drift-enters-more-complex-era/
Healy-Fried ML, Funke T, Priestman MA, Han H, Schönbrunn E (2007) Structural basis of glyphosate tolerance resulting from mutations of Pro-101 in Escherichia coli 5-enolpyruvylshikimate-3-phosphate synthase. J Biol Chem 282:32949–32955
Heap I (2020) International survey of herbicide resistant weeds. http://www.weedscience.org/summary/Species.aspx. Accessed 14 May 2020
Hove-Jensen B, Zechel DL, Jochimsen B (2014) Utilization of glyphosate as phosphate source: biochemistry and genetics of bacterial carbon-phosphorus lyase. Microbiol Mol Biol Rev 78:176–197
Huffman JL, Riggins CW, Steckel LE, Tranel PJ (2016) The EPSPS Pro106Ser substitution solely accounts for glyphosate resistance in a goosegrass (Eleusine indica) population from Tennessee, United States. J Integr Agric 15:1304–1312
ISAAA (2020) GM approval database http://www.isaaa.org/gmapprovaldatabase/
Kabat B (2019) Who’s afraid of roundup. Iss Sci Technol 36:64–73
Kaskey J, Mulvany L (2016) Monsanto seeds unleash unintended consequences across US farms. Bloomberg. http://www.bloomberg.com/news/articles/2016-09-01/a-soybean-killing-pesticide-spreads-across-america-s-farm-belt
Kaundun SS, Dale RP, Zelaya IA, Dinelli G, Marotti I, McIndoe E, Cairns A (2011) A novel P106L mutation in EPSPS and an unknown mechanism(s) act additively to confer resistance to glyphosate in a south African Lolium rigidum population. J Agric Food Chem 59:3227–3233
Kirkwood R, Hetherington R, Reynolds TL, Marshall G (2000) Absorption, localisation, translocation and activity of glyphosate in barnyard grass (Echinochloa crus-galli (L) Beauv): influence of herbicide and surfactant concentration. Pest Manag Sci 56:359–367
Kishore G, Padgette S, Fraley R (1992) History of herbicide-tolerant crops, methods of development and current state of the art – emphasis on glyphosate tolerance. Weed Technol 6:626–634
Komossa D, Gennity I, Sanderman H (1992) Plant metabolism of herbicides with C-P bonds: Glyphosate. Pestic Biochem Physiol 43:85–94
Li X, Nicholl D (2005) Development of PPO inhibitor-resistant cultures and crops. Pest Manag Sci 61:277–285
Li M, Tank H, Kennedy A, Zhang H, Downer B, Ouse D, Liu L (2013) Enlist duo herbicide: a novel 2,4-D plus glyphosate premix formulation with low potential for off-target movement. In: Bernards ML (ed) Pesticide formulations and delivery systems: 32nd volume, innovating legacy products for new uses. American Society for Testing Materials International, West Conshohocken, pp 125–161
Li J, Peng Q, Han H, Nyporko A, Kulynych T, Yu Q, Powles S (2018) Glyphosate resistance in Tridax procumbens via a novel EPSPS Thr-102-Ser substitution. J Agric Food Chem 66:7880–7888
Lu J, Dong Y, Ng EC, Siehl DL (2017) Novel form of the Michaelis-Menten equation that enables accurate estimation of (Kcat/Km)*Ki with just two rate measurements: utility in directed evolution. Protein Eng Des Sel 30:295–299
Meyer JJ (2006) Petition for the determination of nonregulated status for roundup RReady2Yield™ Soybean MON 89788. https://www.aphis.usda.gov/brs/aphisdocs/06_17801p.pdf
Mezzomo BP, Miranda-Vilela A-L, de Souza FI, Barbosa LCP, Portilho FA, Lacava ZGM, Grisolia CK (2013) Hematotoxicity of Bacillus thuringiensis as spore-crystal strains Cry1Aa, Cry1Ab, Cry1Ac or Cry2Aa in Swiss Albino Mice. J Hematol Thromb Dis 1:104
Miller B, Manley BA, Terpstra K, Vail G, Silverstone A, Allen J, Fischer J, Hinz J, Bloomberg J (2013) Development of next generation herbicide tolerant traits to enable enhanced weed management. Abstr Weed Sci Soc Am 53:279
Moehs CP, Austill WJ, Facciotti D, Holm A, Loeffler D, Lu Z, Mullenberg JC, Slade AJ, Steine MN, van Boxtel J, McGuire C (2020) Development of non-transgenic glyphosate tolerant wheat by TILLING. bioRxiv. https://doi.org/10.1101/2020.07.23.218883
Mulvany L, Decker S (2019) Post-chemical world takes shape as agribusiness goes green. Bloomberg. https://www.bloombergquint.com/business/agribusiness-goes-green-with-eco-friendly-fertilizer-pesticides
Nandula VK (2019) Herbicide resistance traits in maize and soybean: current status and future outlook. Plan Theory 8(9):337. https://doi.org/10.3390/plants8090337
Ngo TD, Krishnan M, Boutsalis P, Gill G, Preston C (2018) Target-site mutations conferring resistance to glyphosate in feathertop Rhodes grass (Chloris virgata) populations in Australia. Pest Manag Sci 74:1094–1100
Nicolia A, Ferradini N, Molla G, Biagetti E, Pollegioni L, Veronesi F, Rosellini D (2014) Expression of an evolved engineered variant of a bacterial glycine oxidase leads to glyphosate resistance in alfalfa. J Biotechnol 184:201–208
Padgette SR, Re DB, Gasser CS, Eichholtz DA, Frazier RB, Hironaka CM, Levine EB, Shah DM, Fraley RT, Kishore GM (1991) Site-directed mutagenesis of a conserved region of the 5-enolpyruvylshikimate-3-phosphate synthase active site. J Biol Chem 266:22364–22369
Padgette SR, Kolacz KH, Delanny X, Re DB, LaVallee BJ, Tinius CN, Rhodes WK, Otero YI, Barry GF, Eichholtz DA, Peschke VM, Nida DL, Taylor NB, Kishore GM (1995) Development, identification, and characterization of a glyphosate-tolerant soybean line. Crop Sci 35:1451–1461
PAN (2016) The pesticide treadmill. Pesticide Action Network. http://www.panna.org/gmos-pesticides-profit/pesticide-treadmill
Pan L, Yu Q, Han H, Mao L, Nyporko A, Fan L, Bai L, Powles SB (2019) Aldo-keto reductase metabolizes glyphosate and confers glyphosate resistance in Echinochloa colona. Plant Physiol 181:1–16
Pedotti M, Rosini E, Molla G, Moschetti T, Savino C, Vallone B, Pollegioni L (2009) Glyphosate resistance by engineering the flavoenzyme glycine oxidase. J Biol Chem 284:36415–36423
Perry ED, Ciliberto F, Hennessy DA, Moschini GC (2016) Genetically engineered crops and pesticide use in US maize and soybeans. Sci Adv 2(8):e1600850
Phillips MWA (2020) Agrochemical industry development, trends in R&D and the impact of regulation. Pest Manag Sci. https://doi.org/10.1002/ps.5728
Pollegioni L, Schonbrunn E, Siehl D (2011) Molecular basis of glyphosate resistance-different approaches through protein engineering. FEBS J 278:2753–2766
Powles SB (2008) Evolved glyphosate-resistant weeds around the world: lessons to be learnt. Pest Manag Sci 64:360–365
Que Q, Chilton M-DM, de Fontes CM, He C, Nuccio M, Zhu T, Wu Y, Chen JS, Shi L (2010) Trait stacking in transgenic crops: challenges and opportunities. GM Crops 1:220–229
Sammons RD, Gaines TA (2014) Glyphosate resistance: state of knowledge. Pest Manag Sci 70:1367–1377
Sammons RD, Giacomini E, Ostrander E, Silva A, Xiang B, Wang D (2016) Mechanisms of glyphosate resistance. Abstr Am Chem Soc 252:96
Schönbrunn E, Eschenburg S, Shuttleworth WA, Schloss JV, Amrhein N, Evans JN, Kabsch W (2001) Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail. Proc Natl Acad Sci U S A 98:1376–1380
Sfiligoj E (2014) The resurgence of crop protection. CropLife. http://www.croplife.com/editorial/eric_sfiligoj/the-resurgence-of-crop-protection/
Shah DM, Rogers SG, Horsch RB, Fraley RT (1990) Glyphosate-resistant plants. US Patent 4,940,835
Siehl DL (1997) Inhibitors of EPSP synthase, glutamine synthetase and histidine synthesis. In: Roe RM et al (eds) Herbicide activity: toxicology, biochemistry and molecular biology. IOS Press, Amsterdam, pp 37–67
Siehl DL, Castle LA, Gorton R, Keenan RJ (2007) The molecular basis of glyphosate resistance by an optimized microbial acetyltransferase. J Biol Chem 282:11446–11455
Sost D, Amrhein N (1990) Substitution of Gly-96 to Ala in the 5-enolpyruvylshikimate-3-phosphate synthase of Klebsiella pneumoniae results in a greatly reduced affinity for the herbicide glyphosate. Arch Biochem Biophys 282:433–436
Spencer M, Mumm R, Gwynn J (2000) Glyphosate resistant maize lines. U. S. Patent 6040497
Stalker DM, Hiatt WR, Comai L (1985) A single amino acid substitution in the enzyme 5-enolpyruvylshikimate-3-phosphate synthase confers resistance to the herbicide glyphosate. J Biol Chem 260:4724–4728
Steinrucken HC, Amrhein N (1984) EPSPS of Klebsiella pneumoniae 2, inhibition by glyphosate. Eur J Biochem 143:351–357
Ungelesbee E (2019) A review of herbicide-tolerant soybean trait options for 2020. Farm Progress. https://www.dtnpf.com/agriculture/web/ag/crops/article/2019/10/17/review-herbicide-tolerant-soybean
Vencill WK, Nichols RL, Webster TM, Soteres JK, Mallory-Smith C, Burgos NR, Johnson WG, McClellan MR (2012) Herbicide resistance: toward an understanding of resistance development and the impact of herbicide-resistant crops. Weed Sci 60:2–30
Villareal-Chiu JF, Quinn JP, McGrath JW (2012) The genes and enzymes of phosphonate metabolism by bacteria, and their distribution in the marine environment. Front Microbiol 3:1–13
Westwood JH, Charudattan R, Duke SO, Fennimore SA, Marrone P, Slaughter DC, Swanton C, Zollinger R (2018) Weed Management in 2050: perspectives on the future of weed science. Weed Sci 66:275–285
Wright TR, Guomin S, Walsh TA, Lira JM, Cui C, Song P, Zhuang M, Arnold NL, Lin G, Russell SM, Cicchillo RM, Peterson MA, Simpson DM, Zhou N, Ponsamuel J, Zhang Z (2010) Robust crop resistance to broadleaf and grass herbicides provided by aryloxyalkanoate dioxygenase transgenes. PNAS 107:20240–20245
WSSA (2019) WSSA board issues statement concerning registration of glyphosate. Weed Science Society of America, Westminster. http://wssa.net/2019/08/wssa-board-issues-statement-concerning-registration-of-glyphosate/
Young BG (2015) Overlapping residual herbicides. FMC. http://www.fmccrop.com/Portals/_default/fmc_pdf/F100-37459_OverlapWhitePaper_MED.pdf?timestamp=1430496575694
Yu Q, Jalaludin A, Han H, Chen M, Sammons RD, Powles SB (2015) Evolution of a double amino acid substitution in the 5-enolpyruvylshikimate-3-phosphate synthase in Eleusine indica conferring high level glyphosate resistance. Plant Physiol 167:1440–1447
Conflict of Interest Statement
The authors state that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Green, J.M., Siehl, D.L. (2021). History and Outlook for Glyphosate-Resistant Crops. In: Knaak, J.B. (eds) Reviews of Environmental Contamination and Toxicology Volume 255. Reviews of Environmental Contamination and Toxicology, vol 255. Springer, Cham. https://doi.org/10.1007/398_2020_54
Download citation
DOI: https://doi.org/10.1007/398_2020_54
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-68482-2
Online ISBN: 978-3-030-68483-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)