Abstract
The present systematic review synthesizes the diverse documentation of research on the occurrence of arsenic in soil–water systems and the human and animal bio-availability scenarios related to food chain contamination by arsenic. Humans and animals may drink arsenic-contaminated groundwater in addition to consuming foods that have been grown in arsenic-contaminated groundwater and soils. Rice grain is a potential arsenic carrier and the staple food in many parts of the world, particularly in Southeast Asian countries. Data have been summarized from 183 articles describing different aspects of arsenic flow in the food chain, that is, the soil–water–rice–human system and the water–crops–animals system and the bio-availability of arsenic to humans and animals. The phyto-availability of arsenic depends on the physicochemical and biological conditions of soil and water. In humans, the bio-accessibility of inorganic arsenic is 63–99%. Arsenic is more bio-available from rice than from other foods: different food materials differ in bio-accessible potential. Additionally, the review identifies trends in research on arsenic contamination and food chain flow considering arsenic species, toxicity assessment, and bio-accessibility studies. This systematic review provides a comprehensive assessment of the documented evidence to be used to guide future research on arsenic availability for the rice plant and subsequent availability to humans from cooked rice that can determine arsenic toxicity. The review also highlights how the focus of research on arsenic as a pollutant has changed in the past decades.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
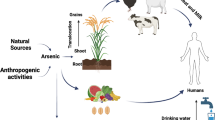
Arsenic contamination in the environment causes serious concern because arsenic is both toxic and carcinogenic (NRC 1999) and is distributed worldwide, including India, Bangladesh, China, the USA, Romania, Chile, Ghana, and Mexico (Smedley and Kinniburgh 2002). Thus, millions of people are affected by arsenic from food and by drinking arsenic-contaminated water (Ravenscroft et al. 2009). Most of these countries face challenges in addressing the health and economic issues related to the occurrence of arsenic. Arsenic in the environment is mostly of geogenic origin (Acharyya et al. 2000; Mukherjee et al. 2008; Yang et al. 2014), but a large amount is introduced from such anthropogenic activities as industries and mining (Benner 2010). In West Bengal, India, the presence of elevated arsenic in groundwater is a major public health concern in 60% of the districts (Nickson et al. 2000; Chakraborti et al. 2001; Das et al. 2008). The behavior of elemental arsenic in the environment is complex and challenging for risk assessment because the exposure routes are different and include drinking water, contaminated foodstuffs, and atmospheric arsenic (NRC 1999). Moreover, arsenic exists in inorganic (arsenate, arsenite) and organic methylated species, which makes its toxicity complex (NRC 1999; WHO 2001). The World Health Organization (WHO) has set the permissible limit of 10 μg l−1 for drinking water, but the major routes of arsenic exposure through foods were not considered (Zavala and Duxbury 2008). From agricultural fields where irrigation groundwater is arsenic contaminated and the soil is loaded with arsenic, contaminated crop products pass through the food chain (Meharg et al. 2009; Zhao et al. 2010). Rice is the staple food for millions of people worldwide and thus became a rich source of arsenic for humans apart from drinking contaminated water (Roychowdhury et al. 2003, 2008; Williams et al. 2007; Rahman et al. 2009; Su et al. 2010; Biswas et al. 2014a, b; Lin et al. 2015), as rice is typically grown under flooded conditions such that it becomes a typical arsenic accumulator. The other concern about arsenic toxicity is arsenic bio-accumulation because the amount of arsenic bio-accumulated in a human depends on the different food matrix or food type. Rice varieties also differ in bio-accumulative fractions of arsenic. In animals, arsenic is accumulated by ingestion of contaminated foods (Datta et al. 2010; Braeuer et al. 2017; Yang et al. 2018). Arsenic toxicity for both humans and animals depends on bio-availability, which is directly related to the types of foods consumed. Considering these facts, the arena of arsenic toxicity assessment research for humans and animals has changed over the years. At first, researchers reported arsenic contamination in the groundwater and drinking water; then, the focus moved to dietary materials and extended to arsenic bio-accessibility and bio-availability from the different dietary matrices for different consumer groups.
The present systematic review has synthesized the recorded evidence of arsenic bio-availability for humans from rice and other food materials, of arsenic phyto-availability for paddy plants, as rice is the staple diet in most of the arsenic endemic regions of the world, with arsenic bio-availability for animals from their fodder. Because publications on arsenic toxicity and risk assessment issues appear frequently, this review was limited mostly to field-based research results.
2 Methods
The review method included such steps as Identification, Screening, Records inclusion as per eligibility, and the Final step-meta analysis (Fig. 1).
2.1 Search Selection
Systematic searches were made in the often-used academic bibliographic databases such as PUBMED (http://www.ncbi.nlm.nih.gov/pubmed), SCIENCEDIRECT (www.sciencedirect.com/), Web of Science (https://webofknowledge.com/), and SCOPUS (www.scopus.com/), plus literature searches using Google Scholar, hand searches of the literature, and references of the included publications.
For the bibliographic database, our search combined comprehensive English terms representing arsenic occurrence and phyto-availability to human bio-availability with the Boolean operator AND. Before conducting the full search, a pilot search was made with required modifications to meet search criteria. The keywords were searched in each bibliographic database. The searched articles had to meet the following criteria to be included in the review process: (a) field-based study published in journals was included; (b) study conducted to identify the arsenic flow chain from soil and water to rice to human was considered; (c) studies on the total and arsenic species of the rice grain were included; (d) arsenic accumulation in animals as reported based on field-based studies; and (e) in vitro arsenic bio-availability study was considered. Articles that met the screening test underwent full text review by the author. During these extensive database searches, if any duplicate document was found in different searches, only one document was considered in detail.
2.2 Documentation, Identification, and Screening
The searched data were categorized on the basis of ‘selected criteria’ to meet the review aim (Table 1). Article titles and keywords were checked thoroughly for this selection. Quality assessment of the selected articles was performed by extending the selection criteria, depth of the data, and exact presentation in the results to discussion section to meet our aim. It is very important to mention here that only field-based research papers were considered as were identified from the methodology section of any selected articles. This selectivity resulted from the enormous numbers of research and review articles available related to arsenic toxicity, bioavailability, phyto-availability, and rice grain arsenic load, etc., so one limitation was required concerning data acceptability and consideration for this particular review.
3 Results
The results of the present review work have been summarized in different sections as per the available data. As the title indicates, the main focus was arsenic bio-availability to humans from different foods as reported from field-based studies. In the background, the results were supported by reports of arsenic phyto-availability, that is, arsenic uptake and accumulation in different crop plants, where rice received more focus, directly or indirectly, because rice is used worldwide as a staple food. Additionally, arsenic bio-availability in animals has been considered, and the differences and/or similarities of their arsenic bioavailability with humans are discussed.
3.1 Phyto-availability of Arsenic
Here I would like to draw the readers’ attention to the fact of that rice is considered primarily in the remainder of this review because rice is known to be a major item of diet (Deb et al. 2013) in most of the arsenic-affected areas worldwide. Arsenic intake and bio-availability from other food materials have also been considered as per research data availability.
3.1.1 Arsenic Uptake in Rice Plants
Chemical processes around the rhizospheric zone may significantly influence and regulate arsenic uptake by paddy plant roots. Radial oxygen loss (ROL) is a well-known phenomenon for paddy rice, resulting in the transfer of oxygen from the aerenchyma into the rhizosphere (Colmer 2003a, b). This ROL can oxidize the ferrous ion to a ferric ion, causing precipitation of iron oxides/hydroxides (FeOOH) around the root surfaces and forming an iron plaque (Smolders and Roelofs 1996). Besides the oxidation capability of the roots, other controlling factors of iron plaque formation are pH, microbial activity, and dissolved ferrous ion concentration in soil solution (Xu et al. 2008; Huang et al. 2012). However, amorphous or crystalline FeOOH is the major component of iron plaque (Hansel et al. 2001; Liu et al. 2006). Iron plaque formation has been frequently reported on the root surfaces of wetland plants including rice (Meharg 2004; Hansel et al. 2001). Several studies have reported less arsenic uptake by rice plants in the presence of iron plaque formation (Liu et al. 2004, 2005; Zhao et al. 2010; Wu et al. 2012). On the other hand, Syu et al. (2013) reported that iron plaque contributed about 73.8–90.4% of the total arsenic uptake from soil as compared to the root (5.8–11.8%) and shoots (2.5–14.3%) in a study to determine the relationship of arsenic sequestration in iron plaque and rice plant accumulation. In contrast, Chen et al. (2005) reported enhanced arsenite uptake in rice plants in the presence of iron plaque formation. Based on element-specific interaction, the addition of sulfur promotes the formation of iron plaque (Hu et al. 2007), whereas phosphate application markedly reduced such formation (Hu et al. 2005). In addition, XANES (X-Ray Absorption Near Edge Structure) analysis showed that arsenate was the dominant species in iron plaque, as was also reported by Liu et al. (2006). A strong co-localization between the root iron plaque and arsenate was also reported by Seyfferth et al. (2010): they applied a combination of techniques, such as X-ray fluorescence imaging, μ-XANES, transmission X-ray microscopy, and tomography, to depict the formation of iron plaque with variable iron coatings influencing the entry of arsenic to the rice roots. Xu et al. (2008) and Zhao et al. (2009) found the dominance of arsenate absorbed on the iron plaque even under flooded conditions. In another study, Syu et al. (2014) compared the effect of iron plaque formation on arsenic uptake in 28 different rice genotypes; and found that 75.7% to 92.8% of arsenic could be sequestered within the iron plaque. In addition, arsenic K-edge XANES spectra indicated the predominance of arsenate in the iron plaque of tested rice genotypes. Some studies have reported the effect of rice genotypes on iron plaque formation (Liu et al. 2004; Lee et al. 2013). Wu et al. (2012) reported that the oxidation capacity of roots controls the formation of iron plaque among different rice genotypes. Pan et al. (2014) investigated the effect of ROL on arsenic sequestration in rice roots; they demonstrated that rice genotypes with higher ROL could sequester a substantial amount of arsenic with more iron plaque formation in the rhizosphere. A lower percentage of arsenite was found in rhizosphere soil solution in rice genotypes with higher ROL from the oxidation of arsenite in the rhizosphere soil. Considering the behavior of iron plaque, Zheng et al. (2012) described that iron plaques act as a buffer in the rhizosphere, especially for uptake of arsenate into rice.
In the soil, the major inorganic arsenic constituents are oxy-anions of arsenite (As3+) and arsenate (As5+), and the presence or occurrence of these arsenic species is governed by pH, redox potential, and the presence of sorbing components (Mandal and Suzuki 2002; Bissen and Frimmel 2003; Haque and Johannesson 2006). For this reason, the adsorption and redox transformation reactions control soil arsenic bioavailability (Hirata et al. 2006; Farooqi et al. 2007).
3.1.2 Arsenic Load in Rice Grains
Arsenic accumulation in rice grains (Oryza sativa L.) has been recognized as a disaster (Meharg 2004). In the rural areas of West Bengal, India, and Bangladesh, such groundwater contamination is alarming because the arsenic-contaminated groundwater is used for drinking and irrigation. Thus, arsenic accumulates in rice plant parts such as the root, shoot, and rice husk, including the rice grains (Roychowdhury et al. 2002a, 2008; Norra et al. 2005; Rahman et al. 2007; Biswas et al. 2012; Biswas and Santra 2012), and arsenic accumulation in paddy roots was 28- and 75-fold higher than in shoots and rice grains, respectively (Rahman et al. 2007). When arsenic-contaminated groundwater is used for irrigation, it affects the soil environment and crop quality as well (Meharg and Rahman 2003). Rice straw, that is, the shoot part, can accumulate up to 92.0 mg kg−1 arsenic (Abedin et al. 2002a).
3.1.2.1 Total Arsenic in Rice Grain
Numerous studies have reported wide variability of rice grain arsenic concentration. Paddy grown on arsenic-contaminated soil has been found to have an arsenic load in the rice grain (Abedin et al. 2002a, b; Meharg and Rahman 2003; Jahiruddin et al. 2004; Islam et al. 2004). Based on a field study in Bangladesh, Williams et al. (2006) reported that arsenic concentration in grain varied from 0.04 to 0.92 mg kg−1 and from 0.04 to 0.91 mg kg−1 for rice growing under Aman and Boro seasons: the names imply the rainy (aman) and the dry (boro) season cultivated rice. Rahman et al. (2006) observed an average concentration of 0.57–0.69 mg kg−1 arsenic in raw rice grain from the arsenic-affected area of Bangladesh. Moreover, Meharg and Rahman (2003) reported an arsenic concentration of 1.7 mg kg−1 in three rice grain samples from Bangladesh, which exceeds the WHO permissible limit (1.0 mg kg−1). This result is consistent with the findings of Islam et al. (2004) where in accumulation of arsenic up to 2.0 mg kg−1 was reported in the Gangetic floodplain of Bangladesh. Large variability in soil arsenic level, in the range 3.1–42.5 mg kg−1, is reflected by elevated grain arsenic in the paddy fields of Bangladesh (Meharg and Rahman 2003). In later studies, higher levels of arsenic in Bangladeshi rice were also reported, as 0.6–0.7 mg kg−1 (Rahman et al. 2007), 0.41–0.98 mg kg−1 (Sun et al. 2008), and 0.02–0.56 mg kg−1 (Rahman et al. 2009), respectively. From the Indian scenario, Roychowdhury et al. (2003) found wide variability in arsenic concentration (from 0.04 to 0.61 mg kg−1) in rice collected from the Murshidabad district, West Bengal. Considerably greater amounts of arsenic were also observed from their further study in the Nadia (0.04–0.39 mg kg−1) and Murshidabad (0.04–0.66 mg kg−1) districts of West Bengal (Roychowdhury et al. 2008). Meharg et al. (2009) reported a grain arsenic concentration range of 0.07–0.31 mg kg−1 for Indian rice based on a market basket study. Bhattacharya et al. (2010) observed grain arsenic accumulation of 0.16–0.58 mg kg−1 in the Aman and Boro seasons from different arsenic-afflicted areas of Nadia district, West Bengal.
Williams et al. (2007) reported a considerable arsenic concentration (0.15–0.66 mg kg−1) in rice grown in south-central US and in rice grown in California (0.10–0.30 mg kg−1) in the United States. This result was consistent with the previous findings reported by Heitkemper et al. (2001a) and Williams et al. (2005). Zavala and Duxbury (2008) found a wide range (0.005–0.710 mg kg−1) of arsenic in commercially available rice grains in different countries. Their study derived a global normal range (0.08–0.20 mg kg−1) of arsenic concentration in rice grain based on the widely variable dataset. They also evaluated the impact of the arsenic-contaminated environment to arsenic concentration in rice grains; grain arsenic concentration increased by 25–45% for Bangladesh studies, whereas a relatively higher increasing trend (47–94%) was found in rice grains from US studies. In the samples for the Bangladesh study, the high arsenic concentration in the rice grains was attributed to the high arsenic concentration in irrigated water, whereas the high arsenic concentration in the US rice grains was linked with the application of arsenic-containing chemicals in the fields and therefore soil arsenic contamination. The link was previously suggested by Williams et al. (2005, 2007). In a very recent field-based study in arsenic-endemic areas of West Bengal, India, Biswas et al. (2018) have concluded that one unit increase of arsenic concentration in soil and irrigated water results in an average 3.660-fold and 1.345-fold increase, respectively, of the arsenic concentration in paddy plants. On the basis of a market basket survey, Meharg et al. (2009) explored inorganic arsenic exposure of white (polished) grain samples from baseline consumption by analyzing 901 samples of white market rice from ten countries including Asia, Europe, and the US to study the distribution of total and inorganic arsenic in rice depending on geographic variation. In baseline market white rice, mean and median values of total arsenic concentration differed by five- to sixfold from different countries of origin. Zavala and Duxbury (2008) and Torres-Escribano et al. (2008) consistently found mean total arsenic concentrations of 0.20 mg kg−1 and 0.21 mg kg−1 in the survey of US rice (n = 112) and Spanish rice (n = 24), respectively. In another interesting report, Rahman et al. (2007) investigated the accumulation and distribution of arsenic in different fractions of rice grain using two widely cultivated rice varieties, namely, BRRI dhan-28 and BRRI hybrid dhan-1. The distribution in different grain fractions was determined in both parboiled and non-parboiled rice: in both varieties, the trend of arsenic concentration in different fractions was rice hull > bran polish > brown rice > raw rice > polished rice. Arsenic concentration in brown rice and polished rice was in the range of 0.3–0.8 mg kg−1 and 0.3–0.5 mg kg−1, respectively. They also suggested that arsenic concentration in polished rice was reduced by parboiling of raw rice before milling. Some previous studies reported that milling of raw rice significantly reduces grain arsenic concentration (Duxbury et al. 2003; Rahman et al. 2006): this may decrease the chance of arsenic intake in the human body. Furthermore, Rahman et al. (2006) stated that cooking can reduce arsenic concentration in the rice, whereas cooking raw rice in arsenic-contaminated water could increase arsenic concentration in rice grains by 10–35% (Misbahuddin 2003). For this dilemma, cooked rice is also of concern in terms of arsenic consumption. Cooking influences arsenic retention in rice (Sengupta et al. 2006; Rahman et al. 2006; Signes et al. 2008; Torres-Escribano et al. 2008; Mondal and Polya 2008) and may remove as much as 57% of the total arsenic from rice grains (Sengupta et al. 2006). In contrast, several studies have reported increased arsenic content in rice cooked in arsenic-contaminated water (Misbahuddin 2003; Rahman et al. 2006; Roychowdhury et al. 2008; Signes et al. 2008; Mondal and Polya 2008). Signes et al. (2008) have reported that the cooking process did not change the arsenic speciation in rice.
Rice grain arsenic concentrations vary with the rice cultivars (Alam et al. 2003; Williams et al. 2006; Rahman et al. 2007; Biswas et al. 2013b). Alam et al. (2003) studied 21 fields of Cumilla district, Bangladesh, and concluded that arsenic concentrations change among rice varieties. Williams et al. (2006) observed arsenic concentration variation in two main rice types, Aman and Boro, as was also observed by Meharg and Rahman (2003), even though it has been reported that a hybrid rice variety has higher root-to-shoot-to-grain translocation of arsenic than the non-hybrid varieties (Rahman et al. 2007). Arsenic in the rice grains is not only harmful to humans, but accumulation of high levels of arsenic in rice straw is a potential threat to cattle that consume the contaminated straw, and thus indirectly to human health, via presumably contaminated bovine meat and milk (Abedin et al. 2002a; Rahman et al. 2008).
3.1.2.2 Arsenic Species in Rice Grain
Arsenite (As3+) is the dominant arsenic species in rice grain, followed by arsenate (As5+) and dimethylarsinic acid (DMA) (Williams et al. 2006; Meharg et al. 2008; Roychowdhury et al. 2008; Biswas et al. 2013c). Khan et al. (2010a, b) investigated the bio-availability of arsenic to rice by conducting a glasshouse pot experiment with different soil samples varying in the source of arsenic contamination collected from Bangladesh, China, and the UK. The grain total arsenic concentration ranged from 0.24 to 1.09 mg kg−1, whereas inorganic arsenic and DMA accounted for 33–77% and 23–67%, respectively. Rice cultivars grown in Bangladeshi soils were found to have higher grain bioavailability, which could be related to the use of arsenic-laden irrigation water. This range of grain arsenic concentration is consistent with previous reports (Xu et al. 2008; Khan et al. 2009; Li et al. 2009) in which inorganic and organic arsenic species increased simultaneously with increased arsenic load from irrigation water, but DMA was the dominant arsenic species above 0.4 mg kg−1 of total arsenic, because a relatively higher percentage of DMA was found in rice grain growing under the greenhouse pot experiment as compared to grain samples collected from the paddy field. This increasing trend of DMA is consistent with the findings of Meharg et al. (2008), Xu et al. (2008), and Norton et al. (2009). In contrast, Li et al. (2009) and Carey et al. (2010) reported much higher translocation efficiency of DMA from root to shoot and from shoot to grain than inorganic arsenic. The study also indicated a strong environmental influence on arsenic speciation in rice grain for two rice cultivars from Bangladesh.
3.1.3 Arsenic Phyto-availability in Crops and Vegetables Other Than Rice
Arsenic concentration in different salad and vegetable crops from a historical mining area in the UK was estimated wherein the authors found elevated arsenic in the edible parts of the vegetables. In beetroot, lettuce, onion, and peas, elevated arsenic was correlated with soil arsenic concentration (Xu and Thornton 1985). Arsenic uptake depended on arsenic concentration in the nutrient solution, and arsenic accumulation in roots increased in the tomato (Lycopersicon esculentum Mill., cv. Marmande) plants; increased arsenic decreased plant growth and fruit yields (Carbonell-Barrachina et al. 1995). Bean plants (Phaseolus vulgaris L.) were shown (Carbonell-Barrachina et al. 1997) as a good arsenic accumulator from applied arsenic source, as half the absorbed arsenic was transported to the upper parts of the plant, although the fruits, the main vegetable, was arsenic safe; the root was a very high accumulator, sometimes more than the arsenic concentration applied. In a large data set (Biswas et al. 2012) of arsenic accumulation in different vegetables collected from the fields and the surrounding local markets, the authors found that spinach had the maximum arsenic accumulation (0.910 mg kg−1 wet weight) among the leafy vegetables, whereas among the non-leafy vegetables the highest arsenic accumulation was found in the tomato (Lycopersicon, 0.551 mg kg−1 wet weight). Among the roots and tubers, Arum had the highest arsenic accumulation (0.558 mg kg−1 wet weight). Among the pulses, Bengal gram (Cicer sp.) had the highest arsenic accumulation (0.891 mg kg−1 wet weight). In carrot (Mayorga et al. 2013), arsenic concentration in the leaves and roots increased with increased arsenic concentration of irrigation water, with a high magnitude of arsenic translocation from roots to leaves, although the leaves had higher arsenic affinity than the roots, and overall it was a slow and continuous translocation to be of environmental risk. Rahaman et al. (2013) reported the arsenic concentrations in different vegetable crops of Malda district, West Bengal, and found the highest arsenic accumulation in potato, 0.456 mg kg−1, followed by amaranth, radish, cauliflower, carrot, tomato, bitter gourd, chili, spinach, and cabbage. Kar et al. (2013) described arsenic accumulation in different vegetable crops in Taiwan and found eggplant to be the highest arsenic accumulator (0.140 mg kg−1) followed by amaranth, tomatoes, spinach, and cabbage.
In a comparison study (McBride et al. 2015) among vegetables, lettuce was the highest arsenic accumulator and tomato was the lowest, and the tomato proved to be safe in terms of arsenic accumulation even when fields were highly loaded with arsenic. For the other vegetables, however, arsenic accumulation exceeded the mark of safety.
It is noticeable that arsenic accumulation in vegetable crops depends on the availability of arsenic in the soil–water system; otherwise, the same crops or vegetables should contain the same amount of arsenic when cultivated in different arsenic-laden crop fields. From the foregoing discussion, another noticeable point is that different parts of plants (roots, stems, leaves, fruits) accumulate different amounts of arsenic in their tissues. In general, the accumulation follows the trends of arsenic load in root > stem and leaves > fruits (Roychowdhury et al. 2005; Dahal et al. 2008; Biswas et al. 2012; Kar et al. 2013). A study by Intamat et al. (2017) assessed arsenic bioaccumulation in aquatic plants and found the highest arsenic accumulation in Limnocharis flava (0.78 ± 0.31 mg kg−1 dry weight) with a bio-accumulation factor of 131.30 ± 15.35; this accumulation was from aquatic sediments.
3.2 Arsenic Bio-Availability and Bio-Accessibility
Bio-availability is the gastrointestinal (GI) digestion, absorption, metabolism, and tissue distribution of any wanted nutrient or bioactive compounds (Galanakis 2017). So, it is the rate at which the therapeutic substance is absorbed and becomes available at the active site or the fraction of the stored nutrient being available in physiological functions, that is, either reaches the systemic circulation and is ultimately utilized. Bio-accessibility is the quantity of a compound that is released from its matrix in the gastrointestinal tract and is available for absorption, that is, enters the bloodstream. So, this is digestive transformation of foods into ready material available for assimilation. Not all the arsenic present in food, including arsenic species, is bio-available from the corresponding food matrix (Bastias et al. 2013). Element bio-accessibility depends on the matrix and chemical form and, in the in vitro studies, on the model used. In the past few years different studies have reported different methods and models to assess trace element bio-accessibility (Laparra et al. 2003; Moreda-Pineiro et al. 2011; Ruby et al. 1996; Intawongse and Dean 2006). Most of the researchers used batch gastrointestinal models, wherein samples are sequentially exposed to artificial saliva and gastric and intestinal fluids. Some dynamic models have also been reported, viz., TIM (TNO GastroIntestinal Model), a computer-controlled system with several chambers simulating conditions in the stomach, duodenum, jejunum, ileum, and large intestine (Minekus et al. 1999; Torres-Escribano et al. 2011). Chu and Beauchemin (2004) reported a continuous leaching bio-accessible method for successive leaching of the food sample by artificial saliva, gastric fluid, then intestinal juices in order.
3.2.1 Bio-Accessibility of Arsenic: From Rice to Human
Bio-accessibility studies of arsenic can help to evaluate the bio-availability and health effects of dietary arsenic exposure. Different researchers have defined the term “bio-accessibility” in different ways based on the scope and objective of the study performed. Koch et al. (2007) and Wragg et al. (2011) described bio-accessibility of arsenic as the fraction of arsenic that is released into the aqueous phase within the gastrointestinal tract and is readily available for absorption. It is also defined as the fraction that is mobilized into the gut fluids after ingestion and is available for assimilation (Ruby et al. 1999; Peijnenburg and Jager 2003). Bio-accessibility tests are often used as an approximation of bio-availability. A contaminant is said to be bio-available if it, as a whole or in parts, becomes available at the site of action after ingestion, inhalation, or contact with the skin. The bio-accessibility of arsenic depends on several factors such as the properties of the matrix, the oxidative state of arsenic, and the conditions of the surrounding medium. For instance, in soil, its bio-accessibility is affected by the ubiquitous and sequestering properties of soil (Stewart et al. 2005), whereas in the GI tract, physiological conditions such as pH (Conklin et al. 2008; Sharma and Sohn 2009) and bile concentration determine its solubility (Oomen et al. 2003). Bio-accessibility studies help in identifying and developing different scenarios to which humans can be exposed. These determinants of bio-availability and bio-accessibility must be understood if one is to monitor or, ultimately, predict the effects of contaminants (Peijnenburg and Jager 2003). In vitro, research has been done to assess bio-accessibility of heavy metals such as lead (Koch et al. 2011), cadmium (Waisberg et al. 2004), and arsenic (Laparra et al. 2005; Trenary et al. 2012; Sun et al. 2012) in different media such as soil, plants, and water. The first release from the rice matrix takes place in the mouth; thereafter, a fraction of arsenic is mobilized from the matrix through the digestive juices in the GI tract. The mobilized arsenic could then be transported into the portal vein via the epithelial membrane. Finally, the fraction of arsenic that reaches the systematic circulation from the liver without being metabolized is the bio-available fraction.
Rice has been found to provide a major quantified exposure of inorganic arsenic to populations living on a rice diet (Meliker et al. 2006; Tsuji et al. 2007). Daily consumption of rice with a total arsenic level of 0.08 mg kg−1 would be the equivalent to drinking contaminated water with an arsenic concentration of 0.01 mg l−1 (Williams et al. 2006). With the growing concern about the presence of arsenic in drinking water, although people had changed their drinking water sources to less contaminated ones, that had not served to prevent arsenic toxicity. The average contribution to total arsenic intake from drinking water was only 13%, whereas from cooked rice was 56%, which made it clear that cooked rice contributed most to the daily arsenic intake (Ohno et al. 2007). Later, that fact was repeated in several studies from West Bengal, India (Guha Mazumder et al. 2013, 2014; Biswas et al. 2014a, b). Because paddy soil is anaerobic, rice has the ability to accumulate higher levels of arsenic into grain than other cereal crops (Meharg and Rahman 2003; Williams et al. 2006, 2007; Su et al. 2010). The risk posed by inorganic arsenic exposure from rice is governed by the inorganic arsenic concentration in rice grain and the amount ingested through the grain (Kile et al. 2007; Ohno et al. 2007; Meharg et al. 2009). Generally, it has been found that inorganic arsenic is more toxic than the pentavalent methylated arsenic species (Schoof et al. 1999). The rice grain can contain an undesirable amount of methylated arsenic species, especially DMA (Meharg et al. 2009). However, the relative concentration of inorganic and organic arsenic in rice grain depends on rice genotypes and the rice-growing environment (Liu et al. 2006; Xu et al. 2008; Li et al. 2009; Norton et al. 2009).
A study examining the bio-accessibility and bio-availability of arsenic in rice cooked in arsenic-contaminated water, using simulated in vitro gastrointestinal digestion and Caco-2 cells, found that arsenic bio-accessibility ranged from 63% to 99% (Laparra et al. 2005). However, arsenic uptake by Caco-2 cells varied from 3.9% to 17.8%, suggesting that other soluble components of the rice may limit the extent of arsenic absorption. Sometimes in vitro assessments do not fully follow the results of the real scale, as Laparra et al. (2005) found only 12% arsenic absorption in case of an in vitro model but this was much higher in the real-time cell line study. Trenary et al. (2012) showed 61% bio-available arsenic from market-available contaminated rice in the US market, and the trend was somewhat similar to the report of Sun et al. (2012), wherein bio-available arsenic was 37% to 57% in the market rice of China. On the other hand, Esther et al. (2014) observed very high, that is, 83% bio-available arsenic in rice collected in Thailand. Not only from rice, but arsenic is also bio-available from the soil; Jeong et al. (2013) reported 0.64%, 11.9%, and 2.02% arsenic bio-availability from Korean soil. At cattle tick dip sites, in northeastern New South Wales, Australia, Juhasz et al. (2007) showed bio-availability of arsenic of 45.37% at the highest. It is clear that soil arsenic is less bio-available compared to arsenic in rice. These results show that in endemic areas with subsistence rice diets, the contribution of inorganic arsenic from cooked rice should be considered in assessments of arsenic health risk (Laparra et al. 2005).
3.2.2 Arsenic Bio-Availability in Humans from Foods Other Than Rice
Few studies have focused on the issues of arsenic bio-availability from foods and vegetables other than rice. In a very recent study, Pizarro et al. (2016) have compared the total arsenic content and total arsenic bio-accessibility from carrot, beet, and quinoa. Almost all the arsenic from carrots (98%) and beets (90%) is bio-accessible following the in vitro gastrointestinal digestion model, but in quinoa only 40% of arsenic is bio-accessible. Leufroy et al. (2012) assessed arsenic bio-accessibility of some seafood materials and found more than 50% bio-accessible arsenic considering all the samples, such as 53–83% in fish and 58–117% in shellfish. The highest bio-accessibility was observed for scallop and crab (117% and 98%, respectively); from canned tuna and salmon, bio-accessibility was between 53% and 65%.
3.3 Arsenic Uptake and Bio-Availability in Animals
Only field study data have been considered here, and the limitations were that most of the reports and experiments were in-house laboratory experiments. So, as per availability in the web database and depending on our search criteria, we have summarized the arsenic bio-availability in animals.
Arsenic is a severe alimentary tract irritant in domestic animals, and the most frequent intoxication has been observed in dogs, cats, horses, and pigs (Selby et al. 1977). Arsenic can be taken up by plants and transferred into the food chain, causing severe effects in animals (Somasundaram et al. 2005). In endemic areas, animals, especially livestock, are considered to be affected by the food chain path. Besides the presence of arsenic in soil, domestic and agricultural use of cow dung fertilizer leads to arsenic contamination of the crops (Pal et al. 2007). Most species of livestock and pet animals apparently excrete arsenic rapidly (People 1964). Arsenic distribution in an animal body indicates feces and urine as major excretory paths, in addition to milk in the case of domestic cattle (Datta et al. 2012). Milk products also contain arsenic if cattle are fed with arsenic-contaminated water and straw. Arsenite was the main species to be eliminated through milk, and organo-arsenic species were the main species in the feces (Datta et al. 2010).
Bertin et al. (2013) reported death, diarrhea, ataxia, dehydration, and respiratory distress as the most common clinical signs in cattle with acute arsenic poisoning. Among cattle, the most common clinicopathological abnormalities include azotemia (100%), hematuria (100%), increased liver enzyme activity (86%), and increased hematocrit (60%), although the antidote treatment gave better outcomes and survival with no abnormalities.
Few reports have been published on the accumulation of arsenic in sea turtles. The highest arsenic accumulation has been reported in liver samples of the hawksbill turtle (Eretmochelys imbricata, mean, 20.9 μg g−1 dry weight) followed by the loggerhead turtle (Caretta caretta, mean, 9.0 μg g−1 dry weight), and the green turtle (Chelonia mydas, mean, 2.9 μg g−1 dry weight) (Saeki et al. 2000; Kubota et al. 2003; Fujihara et al. 2003; Agusa et al. 2008). Carnivorous turtle species (hawksbill and loggerhead turtles) tended to show higher arsenic levels than herbivorous sea turtles (the green turtle). Similar differences have been observed among herbivorous and carnivorous mollusks (Cullen and Reimer 1989).
Edmonds et al. (1994) for the first time reported arsenic species in sea turtles: arsenobetaine, As3+, and arsenocholine accounted for 50%, 35%, and 15%, respectively, of water-extractable arsenic in liver of the leatherback turtle (Dermochelys coriacea), but this does not occur in marine mammals and seabirds. In 1998, Goessler et al. first reported arsenic in marine mammals and arsenobetaine as the predominant arsenic species in samples of seal (Pusa hispida), bearded seal (Erignathus barbatus), pilot whale (Globicephala melas), and beluga (Delphinapterus leucas).
Limited studies have reported the presence of arsenic species in birds. Arsenic levels are higher in seabirds compared to terrestrial birds (Kunito et al. 2008). Kubota et al. (2002), for the first time, reported arsenic species in different seabirds such as the black-footed albatross (Phoebastria nigripes, maximum 26.7 μg g−1) and black-tailed gull (Larus crassirostris, maximum 2.25 μg g−1). Bio-accumulation of arsenic or the trophic transfer coefficient (TTC, defined as the ratio of the concentration in a consumer’s body to the concentration in diet) (Suedel et al. 1994), was found to be 1.0 for the black-footed albatross considering arsenic concentration in 17 tissues of the body (Fujihara et al. 2004). Although high arsenic bio-accumulation was observed, there were no bio-magnifications.
Chickens rapidly excrete arsenicals, but the feathers retained the highest residue of arsenic in poultry birds in a study from the arsenic-affected Chakdaha block of West Bengal, India (Datta et al. 2012). In a recent work by Intamat et al. (2017), the fish Oreochromis niloticus was found to be the highest arsenic accumulator (0.16 ± 0.16 mg kg−1 wet weight) with a bio-accumulation factor of 228.21 ± 26.99 among the four fish species sampled. This study also reported one snail species, Filopaludina sumatrensis, with maximum arsenic bio-accumulation (0.18 ± 0.06 mg kg−1 wet weight) from sediment rather than water, where the bio-accumulation factor was 33.04 ± 10.58.
4 Discussion
The present systematic review provides an extensive look into arsenic bio-availability in both humans and animals. Considering that only the field-based study was of the utmost need for better understanding of the arsenic load of soil and water, articles on the phyto-availability (for rice plant) of arsenic, articles on rice grain arsenic status, and finally, articles on human arsenic bio-availability were discussed. The reason for a broader discussion about arsenic bio-availability in humans from rice was that rice was already known as the major dietary component among arsenic-affected populations worldwide. Other food materials were also considered, but rice proved to be the most favorable food matrix for arsenic bio-availability. Besides the food matrix, variety within the food materials (e.g., different rice varieties) has different bio-accessible amounts of arsenic; the presence of thiol groups in certain rice varieties strongly bound arsenite and make it less bio-accessible. Considering all the foregoing criteria and facts, the best matches relating to our search criteria were selected. This review is also important in noting how research areas on arsenic as a single pollutant have changed over decades, changes that are still going on.
From Table 2 and Fig. 2, one interesting finding is the year-wise change of research trends, in that the first studies reported arsenic occurrence in rice grain from which the risk actually comes for humans. Then, researchers started to consider the matter of arsenic loading in the soil. Soon, researchers became concerned about arsenic bio-availability from soil and from rice, and subsequently from other food materials.
5 Conclusion
This review has attempted to find the major features responsible for the bio-availability of arsenic in humans and animals. Irrigation groundwater is the major arsenic source, which in turn transfers through water and soil systems into plants and thereafter up through the food chain to humans and animals. To date no permanent solution has been described to restrict the entry of arsenic into the food chain. Several alternative strategies have been proposed to reduce arsenic contamination in water and foodstuffs, such as use of less groundwater, choosing an alternative rice season (Biswas et al. 2013a), choosing low arsenic accumulator rice cultivars (Biswas et al. 2013b), etc.
This risk to food safety, particularly in crop production, emphasizes the need to develop appropriate management practices to minimize the input of contaminants to the environment. This realization indicates a significant need to restrict the entry of arsenic to the food chain by addressing proper soil, crop, and irrigation water management strategies. Some potential measures to reduce higher arsenic exposure in soil and rice based on future research activities are stated here:
-
If the present rice cultivation process can be altered with minimum groundwater use, laboratory-based arsenic removal technologies should be field-scale trials in the endemic areas so that the potential technology or plan can be evaluated with a feasibility and sustainability study.
-
Crop alteration or choosing low arsenic accumulator varieties may be a good solution in many crops. Adequate knowledge should be developed on the genetic variability of arsenic uptake.
-
Factors in the soil and water system that make arsenic labile and phyto-available, and the food chain contamination, need to be minimized.
6 Summary
The present review highlighted arsenic phyto-accumulation, especially in rice and other crop plants, and arsenic bio-accumulation in humans and animals through the food chain. Drinking of arsenic-contaminated water is the prime source of arsenic for humans and animals. Contaminated foodstuffs are also a potential source, as shown by the past 20 years of research. Special attention has been given to arsenic accumulation and food chain contamination through the major crop rice, as it is the staple food in most of the arsenic-affected areas of the world. The discussion has been continued to arsenic accumulation in other food crops. For the first time, an elaborate discussion has been produced on arsenic bio-accessibility from rice and other food crops, as there are differences in total arsenic content (including arsenic species) of the food materials and their bio-availability; the entire content is not bio-available. Bio-availability directly depends on dietary material types. The adverse health effects from arsenic poisoning are directly related to its bio-available fraction, as the maximum portion of ingested arsenic in humans and animals is released through their excreta and urine. Risk assessment should be considered depending on continuous monitoring, and serious preventive measures should be undertaken considering the respective food chain of humans and other animals. Because arsenic accumulation in rice and vegetables (the first and the second major dietary items, respectively) are subject to different accumulation levels (potential) in different edible parts, so special care is needed during analysis and reporting of the arsenic load and corresponding risk assessment by the same, considering the most edible parts may be the whole plant or parts of a plant.
Abbreviations
- DMA:
-
Dimethylarsinic acid
- NRC:
-
National Research Council
- ROL:
-
Radial oxygen loss (ROL)
- TIM:
-
TNO GastroIntestinal model
- TTC:
-
Trophic transfer coefficient
- WHO:
-
World Health Organization
- XANES:
-
X-ray absorption near edge structure
References
Abedin MJ, Cotter-Howells J, Meharg AA (2002a) Arsenic uptake and accumulation in rice (Oryza sativa L.) irrigated with contaminated water. Plant Soil 240:311–319
Abedin MJ, Feldmann J, Meharg AA (2002b) Uptake kinetics of arsenic species in rice plants. Plant Physiol 128(3):1120–1128
Acharyya S, Lahiri S, Raymahashay B, Bhowmik A (2000) Arsenic toxicity of groundwater in parts of the Bengal basin in India and Bangladesh: the role of Quaternary stratigraphy and Holocene sea-level fluctuation. Environ Geol 39:1127
Adomako EE, Williams PN, Deacon C, Mehrag AA (2011) Inorganic arsenic and trace elements in Ghanaian grain staples. Environ Pollut 159:2435–2442
Agusa T, Takagi K, Kubota R, Anan Y, Iwata H, Tanabe S (2008) Specific accumulation of arsenic compounds in green turtles (Chelonia mydas) and hawksbill turtles (Eretmochelys imbricata) from Ishigaki Island, Japan. Environ Pollut 153:127–136. (in press)
Al Rmalli SW, Haris PI, Harrington CF, Ayub M (2005) A survey of arsenic in foodstuffs on sale in the United Kingdom and imported from Bangladesh. Sci Total Environ 337:23–30
Alam MGM, Snow ET, Tanaka A (2003) Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci Total Environ 308:83–96
Bastias J, Jambon P, Muñoz O, Manquian N, Bahamonde P, Neira M (2013) Honey as a bioindicator of arsenic contamination due to volcanic and mining activities in Chile. Chilean J Agric Res 73:147–153
Benner S (2010) Hydrology: anthropogenic arsenic. Nat Geosci 3:5–6
Bertin FR, Baseler LJ, Wilson CR, Kritchevsky JE, Taylor SD (2013) Arsenic toxicosis in cattle: meta-analysis of 156 cases. J Vet Intern Med 27:977–981
Bhattacharya P, Samal AC, Majumdar J, Santra SC (2010) Accumulation of arsenic and its distribution in rice plants (Oryza sativa L.) in Gangetic West Bengal, India. Paddy Water Environ 8:63–70
Bissen M, Frimmel FH (2003) Arsenic: a review. Part I: Occurrence, toxicity, speciation, mobility. Acta Hydrochim Hydrobiol 31:9–48
Biswas A, Santra SC (2012) Arsenic distribution in winter rice and vegetable crops – in vivo micro level study in a contaminated region. Int Res J Agric Sci Soil Sci 1(6):205–210
Biswas A, Biswas S, Santra SC (2012) Risk from winter vegetables and pulses produced in arsenic endemic areas of Nadia District: field study comparison with market basket survey. Bull Environ Contam Toxicol 88(6):909–914
Biswas A, Basu B, Bhattacharya K, Guha Mazumder DN, Santra SC (2013a) Species level study on arsenic availability from dietary components. Toxicol Environ Chem 95(3):529–540
Biswas A, Biswas S, Lavu RVS, Gupta PC, Santra SC (2013b) Arsenic prone rice cultivars: a study in endemic region. Paddy Water Environ 12:379–386
Biswas A, Biswas S, Santra SC (2013c) Arsenic in irrigated water, soil, and rice: perspective of the cropping seasons. Paddy Water Environ 12:407–412
Biswas A, Deb D, Ghose A, Du Laing G, De Neve J, Santra SC, Guha Mazumder DN (2014a) Dietary arsenic consumption and urine arsenic in an endemic population: response to improvement of drinking water quality in a 2-year consecutive study. Environ Sci Pollut Res Int 21:609–619
Biswas A, Deb D, Ghose A, Santra SC, Guha Mazumder DN (2014b) Seasonal perspective of dietary arsenic consumption and urine arsenic in an endemic population. Environ Monit Assess 186:4543–4551
Biswas A, Biswas S, Das A, Roychowdhury T (2018) Spatial variability and competing dynamics of arsenic, selenium, iron and bioavailable phosphate from ground water and soil to paddy plant parts. Groundwater Sustain Dev. https://doi.org/10.1016/j.gsd.2018.08.001
Braeuer S, Dungl E, Hoffmann W, Li D, Wang C, Zhang H, Goessler W (2017) Unusual arsenic metabolism in giant pandas. Chemosphere 189:418–425
Brandon EF, Janssen PJ, de Wit-Bos L (2014) Arsenic: bioaccessibility from seaweed and rice, dietary exposure calculations and risk assessment. Food Addit Contam Part A 31(12):1993–2003
Carbonell-Barrachina A, Burlo Carbonell F, Mataix Beneyto J (1995) Arsenic uptake, distribution, and accumulation in tomato plants: effect of arsenite on plant growth and yield. J Plant Nutr 18(6):1237–1250
Carbonell-Barrachina AA, Burló-Carbonell F, Mataix-Beneyto J (1997) Arsenic uptake, distribution, and accumulation in bean plants: effect of arsenite and salinity on plant growth and yield. J Plant Nutr 20(10):1419–1430
Carey AM, Scheckel KG, Lombi E, Newville M, Choi Y, Norton GJ, Charnock JM, Feldmann J, Price AH, Meharg AA (2010) Grain unloading of arsenic species in rice. Plant Physiol 152:309–319
Chakraborti D, Basu GK, Biswas BK, Chowdhury UK, Rahman MM, Paul K, Roychowdhury T, Chanda CR, Lodh D, Ray SL (2001) Characterization of arsenic-bearing sediments in the Gangetic delta of West Bengal, India. In: Chappell WR, Abernathy CO, Calderon RA (eds) Arsenic exposure and health effects, vol IV. Elsevier Science, Amsterdam, pp 27–52
Chowdhury UK, Rahman MM, Mondal BK, Paul K, Lodh D, Biswas BK, Basu GK, Chanda CR, Saha KC, Mukherjee SK, Roy S, Das R, Kaies I, Barua AK, Palit SK, Quamruzzaman Q, Chakraborti D (2001) Groundwater arsenic contamination and human suffering in West Bengal, India and Bangladesh. Environ Sci 8:393–415
Chu M, Beauchemin D (2004) Simple method to assess the maximum bio-accessibility of elements from food using flow injection and inductively coupled plasma mass spectrometry. J Anal At Spectrom 19(9):1213–1216
Colmer TD (2003a) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deepwater rice (Oryza sativa L.). Ann Bot 91(2):301–309
Colmer TD (2003b) Longdistance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26(1):17–36
Conklin SD, Fricke MW, Creed PA, Creed JT (2008) Investigation of the pH effects on the formation of methylated thio-arsenicals, and the effects of pH and temperature on their stability. J Anal At Spectrom 23(5):711–716
Cullen WR, Reimer KJ (1989) Arsenic speciation in the environment. Chem Rev 89:713–764
Dahal BM, Fuerhacker M, Mentler A, Karki KB, Shrestha RR, Blum WEH (2008) As contamination of soils and agricultural plants through irrigation water in Nepal. Environ Pollut 155:157–163
Das HK, Mitra AK, Sengupta PK, Hossain A, Islam F, Rabbani GH (2004) Arsenic concentrations in rice, vegetables, and fish in Bangladesh: a preliminary study. Environ Int 30:383–387
Das DK, Sur P, Das K (2008) Mobilization of arsenic in soils and in rice (Oryza sativa L.) plants affected by organic matter and zinc application in irrigation water contaminated with arsenic. Plant Soil Environ 54:30–37
Datta BK, Mishra A, Singh A, Sar TK, Sarkar S, Bhatacharya A, Chakraborty AK, Mandal TK (2010) Chronic arsenicosis in cattle with special reference to its metabolism in arsenic endemic village of Nadia district, West Bengal, India. Sci Total Environ 409(2):284–288
Datta BK, Bhar MK, Patra PH, Majumdar D, Dey RR, Sarkar S, Mandal TK, Chakraborty AK (2012) Effect of environmental exposure of arsenic on cattle and poultry in Nadia District, West Bengal, India. Toxicol Int 19(1):59–62
Deb D, Biswas A, Ghose A, Das A, Majumdar KK, Guha Mazumder DN (2013) Nutritional deficiency and arsenical manifestations: a perspective study in an arsenic endemic region of West Bengal, India. Public Health Nutr 16(9):1644–1655
Duxbury JM, Mayer AB, Lauren JG, Hassan N (2003) Food chain aspects of arsenic contamination in Bangladesh: effects on quality and productivity of rice. J Environ Sci Health 38:61–69
Edmonds JS, Shibata Y, Prince RIT, Francesconi KA, Morita M (1994) Arsenic compounds in tissues of the leatherback turtle, Dermochelys coriacea. J Mar Biol Assoc UK 74:463–466
Farooqi A, Masuda H, Firdous N (2007) Toxic fluoride and arsenic contaminated groundwater in the Lahore and Kasur districts, Punjab, Pakistan and possible contaminant sources. Environ Pollut 145:839–849
Fujihara J, Kunito T, Kubota R, Tanabe S (2003) Arsenic accumulation in livers of pinnipeds, seabirds, and sea turtles: subcellular distribution and interaction between arsenobetaine and glycine betaine. Comp Biochem Physiol C 136:287–296
Fujihara J, Kunito T, Kubota R, Tanaka H, Tanabe S (2004) Arsenic accumulation and distribution in tissues of black-footed albatrosses. Mar Pollut Bull 48:1153–1160
Galanakis C (2017) What is the difference between bioavailability bioaccessibility and bioactivity of food components? Posted on: April 27, 2017, Elsevier SciTech Connect. Accessed 28 Aug 2018
Guha Mazumder DN, Deb D, Biswas A, Saha C, Nandy A, Ganguly B, Ghose A, Bhattacharya K, Majumdar KK (2013) Evaluation of dietary arsenic exposure and its biomarkers: a case study of West Bengal, India. J Environ Sci Health Part A 48:1–9
Guha Mazumder DN, Deb D, Biswas A, Saha C, Nandy A, Das A, Ghose A, Bhattacharya K, Mazumdar KK (2014) Dietary arsenic exposure with low level of arsenic in drinking water and biomarker: a study in West Bengal. J Environ Sci Health A 49:1–10
Halder D, Bhowmick S, Biswas A, Chatterjee D, Nriagu J, Guha Mazumder DN, Šlejkovec Z, Jacks G, Bhattacharya P (2013) Risk of arsenic exposure from drinking water and dietary components: implications for risk management in rural Bengal. Environ Sci Technol 47(2):1120–1127
Hansel CM, Fendorf S, Sutton S, Newville M (2001) Characterization of Fe plaque and associated metals on the roots of minewaste impacted aquatic plants. Environ Sci Technol 35(19):3863–3868
Haque S, Johannesson KH (2006) Arsenic concentrations and speciation along a groundwater flow path: the Carrizo sand aquifer, Texas, U.S.A. Chem Geol 228:57–71
Heitkemper DT, Vela NP, Stewart KR (2001a) Determination of total and speciated arsenic in rice by ion chromatography and inductively coupled plasma mass spectrometry. J Anal At Spectrom 16:299–306
Heitkemper DT, Vela NP, Stewart KR, Westphal CS (2001b) Determination of total and speciated arsenic in rice by ion chromatography and inductively coupled plasma mass spectrometry. J Anal At Spectrom 16:299–306
Hirata S, Toshimitsu H, Aihara M (2006) Determination of arsenic species in marine samples by HPLC-ICP-MS. Anal Sci 22:39
Hu Y, Li JH, Huang YZ, Hu HQ, Christie P (2005) Sequestration of arsenic by iron plaque on the roots of three rice (Oryza sativa L.) cultivars in a low phosphorus soil with or without fertilizer. Environ Geochem Health 27:169–176
Hu ZY, Zhu YG, Li M, Zhang LG, Cao ZH, Smith FA (2007) Sulphur (S) induced enhancement of iron plaque formation in the rhizosphere reduces arsenic accumulation in rice (Oryza sativa L.) seedlings. Environ Pollut 147:387–393
Huang H, Jia Y, Sun GX, Zhu YG (2012) Arsenic speciation and volatilization from flooded paddy soils amended with different organic matters. Environ Sci Technol 46(4):2163–2168
Huq SMI, Joardar JC, Parvin S, Correll R, Naidu R (2006) Arsenic contamination in food-chain: transfer of arsenic into food materials through groundwater irrigation. J Health Popul Nutr 24:305–316
Intamat S, Buasriyot P, Sriuttha M, Tengjaroenkul B, Neeratanaphan L (2017) Bioaccumulation of arsenic in aquatic plants and animals near a municipal landfill. Int J Environ Stud 74(2):303–314
Intawongse M, Dean JR (2006) In-vitro testing for assessing oral bioaccessibility of trace metals in soil and food samples. TrAC Trends Anal Chem 25(9):876–886
Islam FS, Gault AG, Boothman C, Polya DA, Charnock JM, Chatterjee D (2004) Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430:68–71
Jahiruddin M, Islam MA, Islam MR, Islam S (2004) Effects of arsenic contamination on rice crop (Oryza sativa L). Environ Forensic 1:104–110
Jeong S, Moon HS, Nam K (2013) Differential in vitro bioaccessibility of residual As in a field-aged former smelter site and its implication for potential risk. Sci Total Environ 463-464:348–354
Juhasz AL, Smith E, Weber J, Rees M, Rofe A, Kuchel T, Sansom L, Naidu R (2007) In vitro assessment of arsenic bioaccessibility in contaminated (anthropogenic and geogenic) soils. Chemosphere 69:69–78
Kar S, Das S, Jean JS, Chakraborty S, Chuan-Liu C (2013) As in the water-soil-plant system and the potential health risks in the coastal part of Chianan plain, Southwestern Taiwan. J Asian Earth Sci 77:295–302
Khan MA, Islam MR, Panaullah GM, Duxbury JM, Jahiruddin M, Loeppert RH (2009) Fate of irrigation water arsenic in rice soils of Bangladesh. Plant Soil 322:263–2772
Khan MA, Islam MR, Panaullah GM, Duxbury JM, Jahiruddin M, Loeppert RH (2010a) Accumulation of arsenic in soil and rice under wetland condition in Bangladesh. Plant Soil 333:263–274
Khan MA, Stroud J, Zhu YG, McGrath SP, Zhao FJ (2010b) Arsenic bioavailability to rice is elevated in Bangladeshi paddy soils. Environ Sci Technol 44:8515–8521
Kile ML, Houseman EA, Breton CV, Smith T, Quamruzzaman O, Rahman M, Mahiuddin G, Christiani DC (2007) Dietary arsenic exposure in Bangladesh. Environ Health Perspect 115:889–893
Kim J, Kim W, Kunhikrishnan A, Kang DW, Kim DH, Lee YJ, Kim YJ, Kim CT (2013) Determination of arsenic species in rice grains using HPLC-ICP-MS. Food Sci Biotechnol 22:1509
Koch I, McPherson K, Smith P, Easton L, Doa KG, Reimer KJ (2007) Arsenic bioaccessibility and speciation in clams and seaweed from a contaminated marine environment. Mar Pollut Bull 54(5):586–594
Koch I, Moriarty M, House K, Sui J, Cullen WR, Saper RB, Reimer KJ (2011) Bioaccessibility of lead and arsenic in traditional Indian medicines. Sci Total Environ 409(21):4545–4552
Kubota R, Kunito T, Tanabe S, Ogi H, Shibata Y (2002) Maternal transfer of arsenic to eggs of blacktailed gull (Larus crassirostis) from Rishiri Island, Japan. Appl Organomet Chem 16:463–468
Kubota R, Kunito T, Tanabe S (2003) Occurrence of several arsenic compounds in the liver of birds, cetaceans, pinnipeds, and sea turtles. Environ Toxicol Chem 22:1200–1207
Kunito T, Kubota R, Fujihara J, Agusa T, Tanabe S (2008) Arsenic in marine mammals, seabirds, and sea turtles. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology. Springer, New York, pp 31–71
Laparra JM, Velez D, Montoro R, Barbera R, Farre R (2003) Estimation of arsenic bioaccessibility in edible seaweed by an in vitro digestion method. J Agric Food Chem 51(20):6080–6085
Laparra JM, Veälez D, Barbera R, Farre R, Montoro R (2005) Bioavailability of inorganic arsenic in cooked rice: practical aspects for human health risk assessments. J Agric Food Chem 53:8829–8833
Leufroy A, Noël L, Beauchemin D, Guérin T (2012) Bioaccessibility of total arsenic and arsenic species in seafood as determined by a continuous online leaching method. Anal Bioanal Chem 402:2849–2859
Lee CH, Hsieh YC, Lin TH, Lee DY (2013) Iron plaque formation and its effect on arsenic uptake by different genotypes of paddy rice. Plant Soil 363:231–241
Li RY, Stroud JL, Ma JF, McGrath SP, Zhao FJ (2009) Mitigation of arsenic accumulation in rice with water management and silicon fertilization. Environ Sci Technol 43:3778–3783
Lin HT, Wong SS, Li GC (2004) Heavy metal content of rice and shell in Taiwan. J Food Drug Anal 12:67–74
Lin SC, Chang TK, Huang WD, Lur HS, Shyu GS (2015) Accumulation of arsenic in rice plant: a study of an arseniccontaminated site in Taiwan. Paddy Water Environ 13:11–18
Liu WJ, Zhu YG, Smith FA, Smith SE (2004) Do iron plaque and genotypes affect arsenate uptake and translocation by rice seedlings (Oryza sativa L.) grown in solution culture? J Exp Bot 55(403):1707–1713
Liu WJ, Zhu YG, Smith FA (2005) Effects of iron and manganese plaques on arsenic uptake by rice seedlings (Oryza sativa L.) grown in solution culture supplied with arsenate and arsenite. Plant Soil 277:127–138
Liu WJ, Zhu Y, Hu Y, Williams PH, Gault AG, Meharg AA, Charnock JM, Smith FA (2006) Arsenic sequestration in iron plaque, its accumulation and speciation in mature rice plants (Oryza sativa L.). Environ Sci Technol 40(18):5730–5736
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Mayorga P, Moyano A, Anawar HM, Garcia-Sanchez A (2013) Uptake and accumulation of arsenic in different organs of carrot irrigated with As-rich water. Clean Soil Air Water 41(6):587–592
McBride MB, Shayler HA, Russell-Anelli JM, Spliethoff HM, Marquez-Bravo LG (2015) Arsenic and lead uptake by vegetable crops grown on an old orchard site amended with compost. Water Air Soil Pollut 226(8):265. https://doi.org/10.1007/s11270-015-2529-9
Meharg AA (2004) Arsenic in rice: understanding a new disaster for SouthEast Asia. Trends Plant Sci 9(9):415–417
Meharg AA, Rahman MM (2003) Arsenic contamination of Bangladesh paddy field soils: implications for rice contribution to arsenic consumption. Environ Sci Technol 37:229–234
Meharg AA, Adomako E, Lawgali Y, Deacon C, Williams P (2008) Food Standards Agency contract C101045: levels of arsenic in rice-literature review
Meharg AA, Williams PN, Adomako A, Lawgali YY, Deacon C, Villada A, Campbell RCJ, Sun G, Zhu YG, Feldmann I, Rabb A, Zhao FJ, Islam R, Hossain S, Yanai I (2009) Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ Sci Technol 43:1612–1617
Meliker JR, Franzblau A, Slotnick MJ, Nriagu JO (2006) Major contributors to inorganic arsenic intake in southeastern Michigan. Int J Hyg Environ Health 209:399–411
Minekus M, Smeets-Peeters M, Havenaar R, Bernalier A, Fonty G, Marol-Bonnin S, Alric M, Marteau P, Huis In’t Veld JHJ (1999) A computer-controlled system to simulate conditions of the large intestine with peristaltic mixing, water absorption and absorption of fermentation products. Appl Microbiol Biotechnol 53(1):108–114
Misbahuddin M (2003) Consumption of arsenic through cooked rice. Lancet 361:435–436
Mondal D, Polya DA (2008) Rice is a major exposure route for arsenic in Chakdaha block, Nadia district, West Bengal, India: a probabilistic risk assessment. Appl Geochem 23:2987–2998
Moreda-Pineiro J, Moreda-Pineiro A, Romaris-Hortas V, Moscoso-Perez C, Lopez-Mahia P, Muniategui-Lorenzo S, Bermejo-Barrera P, Prada-Rodriguez D (2011) In-vivo and in-vitro testing to assess the bioaccessibility and the bioavailability of arsenic, selenium and mercury species in food samples. TrAC Trends Anal Chem 30(2):324–345
Mukherjee A, Brömssen M, Scanlon BR, Bhattacharya P, Fryar AE, Hasan MA, Ahmed KM, Chatterjee D, Jacks G, Sracek O (2008) Hydrogeochemical comparison and effects of overlapping redox zones on groundwater arsenic near the Western (Bhagirathi subbasin, India) and Eastern (Meghna subbasin, Bangladesh) margins of the Bengal Basin. J Contam Hydrol 99:31–48
Nickson R, MacArthur JM, Ravenscroft P, Burgess WG, Ahmed KM (2000) Mechanism of arsenic release in groundwater, Bangladesh and West Bengal. Appl Geochem 15:403–413
Norra S, Berner ZA, Agarwala P, Wagner F, Chandrasekharam D, Stuben D (2005) Impact of irrigation with arsenic rich groundwater on soil and crops: a geochemical case study in West Bengal Delta plain, India. Appl Geochem 20:1890–1906
Norton GJ, Duan GL, Dasgupta T, Islam MR, Lei M, Zhu YG, Deacon CM, Moran AC, Islam S, Zhao FJ, Stroud JL, McGrath SP, Feldmann J, Price AH, Meharg AA (2009) Environmental and genetic control of arsenic accumulation and speciation in rice grain: comparing a range of common cultivars grown in contaminated sites across Bangladesh, China, and India. Environ Sci Technol 43:8381–8386
NRC (National Research Council) (1999) Health effects of arsenic. Arsenic in drinking water. National Academic Press, Washington, pp 83–149
Ohno K, Yanase T, Matsuo Y, Kimura T, Rahman MH, Magara Y, Matsui Y (2007) Arsenic intake via water and food by a population living in an arsenic affected area of Bangladesh. Sci Total Environ 381:68–76
Oomen AG, Tolls J, Sips AM, Van den Hoop MA (2003) Lead speciation in artificial human digestive fluid. Environ Contami Toxicol 44:107–115
Pal A, Nayak B, Das B, Hossain MA, Ahmed S, Chakraborti D (2007) Additional danger of arsenic exposure through inhalation from burning of cow dung cakes laced with arsenic as a fuel in arsenic affected villages in Ganga-Meghna-Brahmaputra plain. J Environ Monit 9(10):1067–1070
Pan W, Wu C, Xue S, Hartley W (2014) Arsenic dynamics in the rhizosphere and its sequestration on rice roots as affected by root oxidation. J Environ Sci 26:892–899
Peijnenburg WJ, Jager T (2003) Monitoring approaches to assess bioaccessibility and bioavailability of metals: matrix issues. Ecotoxicol Environ Saf 56(1):63–77
People SA (1964) Arsenic toxicity in cattle. Ann N Y Acad Sci 111:644
Phuong TD, Chuong PV, Khiem DT, Kokot S (1999) Elemental content of Vietnamese rice. Part 1. Sampling, analysis and comparison with previous studies. Analyst 124:553–560
Pizarro I, Gómez MG, León J, Román D, Palacios MA (2016) Bioaccessibility and arsenic speciation in carrots, beets and quinoa from a contaminated area of Chile. Sci Total Environ 565:557–563
Rahaman S, Sinha AC, Pati R, Mukhopadhyay D (2013) As contamination: a potential hazard to the affected areas of West Bengal, India. Environ Geochem Health 35:119–132
Rahman MM, Mandal BK, Chowdhury TR, Sengupta MK, Chowdhury UK, Lodh D, Chanda CR, Basu GK, Mukherjee SC, Saha KC, Chakraborti D (2003) Arsenic groundwater contamination and sufferings of people in north 24-Parganas, one of the nine arsenic affected districts of West Bengal, India. J Environ Sci Health Part A 28:25–59
Rahman MA, Hasegawa H, Rahman MA, Rahman MM, Miah MAM (2006) Influence of cooking method on arsenic retention in cooked rice related to dietary exposure. Sci Total Environ 370:51–60
Rahman MA, Hasegawa H, Rahman MM, Rahman MA, Miah MAM (2007) Accumulation of arsenic in tissues of rice plant (Oryza sativa L.) and its distribution in fractions of rice grain. Chemosphere 69:942–948
Rahman MA, Hasegawa H, Rahman MM, Miah MA, Tasmin A (2008) Arsenic accumulation in rice (Oryza sativa L.): human exposure through food chain. Ecotoxicol Environ Saf 69:317–324
Rahman MM, Owens G, Naidu R (2009) Arsenic levels in rice grain and assessment of daily dietary intake of arsenic from rice in arsenic-contaminated regions of Bangladesh: implications to groundwater irrigation. Environ Geochem Health 31:179–187
Ravenscroft P, Brammer H, Richards KS (2009) Arsenic pollution: a global synthesis. Wiley-Blackwell, Hoboken
Roychowdhury T, Uchino T, Tokunaga H, Ando M (2002a) Survey of arsenic in food composites from an arsenic affected area of West Bengal, India. Food Chem Toxicol 40:1611–1621
Roychowdhury T, Uchino T, Tokunaga H, Ando M (2002b) Arsenic and other heavy metals in soils from an arsenic-affected area of West Bengal, India. Chemosphere 49:605–618
Roychowdhury T, Tokunaga H, Ando M (2003) Survey of arsenic and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from an arsenic-affected area of West Bengal, India. Sci Total Environ 308:15–35
Roychowdhury T, Tokunaga H, Uchino T, Ando M (2005) Effect of arsenic contaminated irrigation water on agricultural land soil and plants in West Bengal, India. Chemosphere 58:799–810
Roychowdhury T, Uchino T, Tokunaga H (2008) Effect of arsenic on soil, plant and foodstuffs by using irrigated groundwater and pond water from Nadia district, West Bengal. Int J Environ Pollut 33(2/3):218–234
Ruby MV, Davis A, Schoof R, Eberle S, Sellstone CM (1996) Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ Sci Technol 30(2):422–430
Ruby MV, Schoof R, Brattin W, Goldade M, Post G, Harnois M, Mosby DE, Casteel SW, Berti W, Carpenter M, Edwards D, Cragin D, Chappell W (1999) Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environ Sci Technol 33(21):3697–3705
Saeki K, Sakakibara H, Sakai H, Kunito T, Tanabe S (2000) Arsenic accumulation in three species of sea turtles. Biometals 13:241–250
Schoof RA, Yost LJ, Crecelius E, Irgolic K, Goessler W, Guo HR, Greene H (1998) Dietary arsenic intake in Taiwanese districts with elevated arsenic in drinking water. Human Ecol Risk Assess 4:117–135
Schoof RA, Yost LJ, Eickhoff J, Crecelius EA, Meacher DM, Menzel DB (1999) A market basket survey of inorganic arsenic in food. Food Chem Toxicol 37:839–836
Selby LA, Case AA, Osweiler GD, Hayes HM Jr (1977) Epidemiology and toxicology of arsenic poisoning in domestic animals. Environ Health Perspect 19:183–189
Sengupta MK, Hossain MA, Mukherjee A, Ahamed S, Das B, Nayak B, Pal A, Chakraborti D (2006) Arsenic burden of cooked rice: traditional and modern methods. Food Chem Toxicol 44:1823–1829
Seyfferth AL, Webb SM, Andrews JC, Fendorf S (2010) Arsenic localization, speciation, and co-occurrence with iron on rice (Oryza sativa L.) roots having variable Fe coatings. Environ Sci Technol 44:8108–8113
Seyfferth AL, McCurdy S, Schaefer MV, Fendorf S (2014) Arsenic concentrations in paddy soil and rice and health implications for major ricegrowing regions of Cambodia. Environ Sci Technol 48:4699–4706
Sharma V, Sohn M (2009) Aquatic arsenic: toxicity, speciation, transformation and remediation. Environ Int 35:743–759
Signes A, Mitra K, Burlo F, Carbonell-Barrachina AA (2008) Contribution of water and cooked rice to an estimation of the dietary intake of inorganic arsenic in a rural village of West Bengal, India. Food Addit Contam 25:41–50
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Smith PG (2006) Arsenic biotransformation in terrestrial organisms – a study of the transport and transformation of arsenic in plants, fungi, fur and feathers, using conventional speciation analysis and X-ray absorption spectroscopy. Doctoral dissertation, Queen’s University, Canada
Smolders AJP, Roelofs JGM (1996) The roles of internal iron hydroxide precipitation, sulphide toxicity and oxidizing ability in the survival of Stratiotes aloides roots at different iron concentrations in sediment pore water. New Phytol 133(2):253–260
Somasundaram J, Krishnasamy R, Savithri P (2005) Biotransformation of heavy metals in Jersey cows. Indian J Anim Sci 75:1257–1260
Stewart MA, Jardine PM, Fendorf SE, Barnett MO (2005) Evaluation of soil properties to reduce bioaccessibility of heavy metals. In: International conference on the remediation of contaminated sediments; New Orleans, Louisiana, January 24–27, 2005
Stroud JL, Norton GJ, Islam MR, Dasgupta T, White RP, Price AH, Meharg AA, Mcgrath SP, Zhao FJ (2011) The dynamics of arsenic in four paddy fields in the Bengal delta. Environ Pollut 159(4):947–953
Su YH, McGrath SP, Zhao FJ (2010) Rice is more efficient in arsenite uptake and translocation than wheat and barley. Plant Soil 328:27–34
Suedel BC, Boraczek JA, Peddicord RK, Clifford PA, Dillon TM (1994) Trophic transfer and biomagnification potential of contaminants in aquatic ecosystems. Rev Environ Contam Toxicol 136:21–89
Sun GX, Williams PN, Carey AM, Zhu YG, Deacon C, Raab A, Feldmann J, Islam RM, Meharg AA (2008) Inorganic arsenic in rice bran and its products are an order of magnitude higher than in bulk grain. Environ Sci Technol 42:7542–7546
Sun GX, van de Wiele T, Alava P, Tack F, Du Laing G (2012) Arsenic in cooked rice: effect of chemical, enzymatic and microbial processes on bioaccessibility and speciation in the human gastrointestinal tract. Environ Pollut 162:241–246
Syu CH, Jiang PY, Huang HH, Chen WT, Lin TH, Lee DY (2013) Arsenic sequestration in iron plaque and its effect on arsenic uptake by rice plants grown in paddy soils with high contents of arsenic, iron oxides and organic matter. Soil Sci Plant Nutr 59:463–471
Syu CH, Lee CH, Jiang PY, Chen MK, Lee DY (2014) Comparison of arsenic sequestration in iron plaque and uptake by different genotypes of rice plants grown in arsenic contaminated paddy soils. Plant Soil 374:411–422
Torres-Escribano S, Leal M, Velez D, Montoro R (2008) Total and inorganic arsenic concentrations in rice sold in Spain: effect of cooking and risk assessments. Environ Sci Technol 42:3867–3872
Torres-Escribano S, Denis S, Blanquet-Diot S, Calatayud M, Barrios L, Velez D, Alric M, Montoro R (2011) Comparison of a static and a dynamic in vitro model to estimate the bioaccessibility of As, Cd, Pb and Hg from food reference materials: Fucus sp. (IAEA-140/TM) and lobster hepatopancreas (TORT-2). Sci Tot Env 409(3):604–611
Trenary HR, Creed PA, Young AR, Mantha M, Schwege CA, Xue J, Kohan MJ, Davis KH, Thomas DJ, Caruso JA, Creed JT (2012) An in vitro assessment of bioaccessibility of arsenicals in rice and the use of this estimate within a probabilistic exposure model. J Exp Sci Environ Epidemiol 2012:1–7
Tsuji JS, Yost LJ, Barraj LM, Scrafford CG, Mink PJ (2007) Use of background inorganic arsenic exposures to provide perspective on risk assessment results. Regul Toxicol Pharmacol 48:59–68
Waisberg M, Black WD, Waisberg CM, Hale B (2004) The effect of pH, time and dietary source of cadmium on the bioaccessibility and adsorption of cadmium to/from lettuce. Food Chem Toxicol 42(5):835–842
WHO (2001) Arsenic and arsenic compounds. Environmental health criteria 224. World Health Organization, Geneva
Williams PN, Price AH, Raab A, Hossain SA, Feldmann J, Meharg AA (2005) Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ Sci Technol 39:5531–5540
Williams PN, Islam MR, Adomako EE, Raab A, Hossain SA, Zhu YG, Feldmann J, Meharg AA (2006) Increase in rice grain arsenic for regions of Bangladesh irrigating paddies with elevated arsenic in groundwaters. Environ Sci Technol 40:4903–4908
Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmaan J, Meharg AA (2007) Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol 41:6854–6859
Wragg J, Cave M, Basta N, Brandon E, Casteel S, Denys S, Gron C, Oomen A, Reimer K, Tack K, Van de Wiele T (2011) An inter-laboratory trial of the unified BARGE bioaccessibility method for arsenic, cadmium and lead in soil. Sci Total Environ 409(19):4016–4030
Wu C, Ye ZH, Wu SC, Deng D, Zhu YG, Wong MH (2012) Do radial oxygen loss and external aeration affect iron plaque formation and arsenic accumulation and speciation in rice? J Exp Bot 63:2961–2970
Xie ZM, Huang CY (1998) Control of arsenic toxicity in rice plants grown on an arsenic-polluted paddy soil. Commun Soil Sci Plant Anal 29:2471–2477
Xu J, Thornton I (1985) Arsenic in garden soils and vegetable crops in Cornwall, England: implications for human health. Environ Geochem Health 7(4):131–133
Xu XY, McGrath SP, Meharg AA, Zhao FJ (2008) Growing rice aerobically markedly decreases arsenic accumulation. Environ Sci Technol 42:5574–5579
Yang N, Winkel LHE, Johannesson KH (2014) Predicting geogenic arsenic contamination in shallow groundwater of South Louisiana, United States. Environ Sci Technol 48(10):5660–5666
Yang F, Xie S, Liu J, Wei C, Zhang H, Chen T, Zhang J (2018) Arsenic concentrations and speciation in wild birds from an abandoned realgar mine in China. Chemosphere 193:777–784
Zavala YJ, Duxbury JM (2008) Arsenic in rice: I. Estimating normal levels of arsenic in rice. Environ Sci Technol 42:3856–3860
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Zhao FJ, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanism of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559
Zheng MZ, Cai C, Hu Y, Sun GX, Williams PN, Cui HJ, Duan GL, Zhao FJ, Zhu YG (2012) Spatial distribution of arsenic and temporal variation of its concentration in rice. New Phytol 189:200–209
Zhu YG, Williams PN, Meharg AA (2008) Exposure to inorganic arsenic from rice: a global health issue? Environ Pollut 154:167–171
Acknowledgments
The author acknowledges the Science and Engineering Research Board, Department of Science and Technology (DST-SERB), Government of India for providing research funding as National Postdoctoral Fellowship (File No. PDF/2016/000699). The author also acknowledges the authors whose works have been considered in the present work. We also acknowledge the anonymous persons responsible for making the web databases available relating to our database search.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Biswas, A. (2019). A Systematic Review on Arsenic Bio-Availability in Human and Animals: Special Focus on the Rice–Human System. In: Reviews of Environmental Contamination and Toxicology. Springer, New York, NY. https://doi.org/10.1007/398_2019_28
Download citation
DOI: https://doi.org/10.1007/398_2019_28
Published:
Publisher Name: Springer, New York, NY