Abstract
Due to its intrinsic beauty and unique properties, bis(η5-cyclopentadienyl)iron or ferrocene continues to attract the attention of chemists even after seven decades from its discovery. One of the particularly attractive and active fields is the preparation of planar-chiral ferrocene derivatives, which found manifold use as auxiliary ligands in enantioselective transition metal catalysis and organocatalysis. This chapter briefly illustrates the historical context and recent trends in this area, paying particular attention to the development of synthetic routes leading to planar-chiral ferrocenes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Cyclopentadienide anions have been widely applied as versatile auxiliary ligands for transition metals [1]. The first compound featuring π-coordinated C5H5− anion, bis(η5-cyclopentadienyl)iron or ferrocene, [Fe(η5-C5H2)2] (1), was discovered and structurally characterized in the early 1950s [2]. Since then the chemistry of cyclopentadienyl complexes rapidly developed, resulting in a vast family of structurally diverse and widely practically utilized compounds [3,4,5,6,7]. When it comes to chiral metallocene derivatives, however, compounds derived from the exceptionally stable ferrocene, which is the archetypal representative of cyclopentadienyl metal complexes, still dominate due to their applications in catalysis. This chapter, which was partly adapted from Ref. [2] with permission from the Royal Society of Chemistry, attempts to briefly illustrate the chemistry of chiral ferrocenes, mainly focusing on the synthetic routes leading to these compounds. Given the enormous number of chiral ferrocene derivatives synthesized to date and their manifold applications, this chapter cannot adequately cover all aspects. Therefore, the reader is referred to review articles and books cited here that summarize the chemistry of chiral ferrocene compounds in more detail [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21].
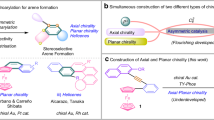
Generally, there are three types of chirality encountered in ferrocene derivatives: central, planar, and helical (Scheme 1). While central chirality is usually associated with the substituent(s) appended to the ferrocene core, the other two chirality types reflect the specific steric properties of the ferrocene unit. Generally, planar chirality in cyclopentadienyl complexes is enabled by the coordination of a metal center, formally [(C5H5)Fe+–C5H5−] for ferrocene, which differentiates the two enantiotopic faces of the planar and aromatic cyclopentadienide anion. In contrast, helical chirality results from blocking the rotation of the parallel cyclopentadienyl rings along the molecular axis in heteroannularly substituted ferrocene derivatives, either in the solid state or by bridging the cyclopentadienyl rings (such as in ferrocenophanes).
There are several main routes toward chiral ferrocenes that differ depending on the type of chirality introduced (Scheme 2). Ferrocene derivatives with central chirality are synthesized in conventional manner by attaching or generating a chiral center in a molecule containing the ferrocene moiety. In contrast, planar chiral derivatives are typically obtained by diastereoselective methods when a suitable functional group at the ferrocene unit is used to direct further functionalization to the adjacent position at the ferrocene core (usually position 2 and rarely position 3). Without any chiral “influence,” this functionalization expectedly gives rise to a racemic mixture of planar chiral products. In contrast, reactions performed with a chiral directing group or a chiral reagent can produce mixtures enriched with one of the two stereoisomers. These reactions often employ the lithiation/electrophilic quenching approach, using either a chiral ferrocene derivative and common organolithium compound or, alternatively, a simple ferrocene derivative and a chiral metalating agent during the metalation step. More recently, this approach was extended toward C−H bond activation reactions at the ferrocene moiety. Methods relying on kinetic resolution and desymmetrization of suitable ferrocene derivatives remain less widely explored and practically utilized [22, 23].
Synthesis of planar-chiral ferrocenes: (a) diastereoselective lithiation/functionalization of ferrocenes with chiral ortho-directing groups (ODG*), (b) diastereoselective lithiation/functionalization of ferrocenes with ortho-directing groups (ODG) and chiral bases, (c) kinetic resolution of racemic ferrocenes, and (d) desymmetrization of achiral ferrocenes (X, Y, Z = various substituents, E+ = electrophile). Routes (a) and (b) were equally well applied for the synthesis of planar-chiral ferrocenes through the C−H bond activation/functionalization sequence
Initial attempts at preparing planar-chiral ferrocenes were based on the conventional synthetic approaches developed for organic compounds [24, 25]. These methods typically afforded racemic mixtures of enantiomers, which were resolved either via diastereoisomeric derivatives or, less commonly, by chromatography over chiral stationary phases [26]. The first planar chiral ferrocene derivative resolved into (Rp)- and (Sp)-enantiomers was 1,2-(α-ketotetramethylene)ferrocene (2), which is accessible (in racemic form) by acid-catalyzed intramolecular acylation from 4-ferrocenylbutanoic acid (3) [27]. This compound was resolved via diastereomeric hydrazones obtained from (−)-menthylhydrazine [28], and the absolute configuration of the (+)-isomer was established using chemical methods (Scheme 3) [29].
Later on, the development of synthetic routes toward planar chiral ferrocenes became intimately associated with the design, synthesis, and applications of chiral ferrocene ligands, mostly phosphines, in homogeneous transition metal catalysis. These compounds are usually obtained by diastereoselective functionalization of chiral starting materials and, hence, combine central chirality with chirality at the cyclopentadienyl plane. Compounds featuring only planar chirality remain less common.
Access to such planar chiral ferrocenes was opened in 1965 through the research of Hauser and coworkers [30], showing that initial lithiation of [(dimethylamino)methyl]ferrocene (4) [31, 32] with n-butyllithium n-BuLi occurs preferentially in position 2 of the ferrocene unit and that an excess of n-BuLi is needed to achieve lithiation at the unsubstituted cyclopentadienyl ring (Scheme 4). Soon afterwards, the concept of directed ortho-lithiation was extended into diastereoselective variants using chiral aminoferrocenes 6 [33, 34] and 7 [35] (Scheme 5). Of these compounds, N,N-dimethyl-1-ferrocenylethylamine, Ugi’s amine (7), proved particularly useful for further synthesis because of its synthetic accessibility in optically pure form, high stereoselectivity of the lithiation reaction (dr = 96:4), and ability to undergo nucleophilic replacement of the NMe2 group (SN1-type substitution) that proceeds with the retention of configuration at the stereogenic carbon [36]. The latter property, a result of the exceptional stabilization of ferrocenylmethylium cations as plausible and even isolable intermediates [37], is preserved for 2-substituted derivatives and was observed for analogous compounds containing other easily leaving groups (e.g., NMe3+ and OAc instead of NMe2 in the side chain), thus widening the scope of accessible compounds.
Already in the 1970s, Kumada and coworkers utilized lithiation of Ugi’s amine to prepare chiral ferrocene P,N-donors, (R,Sp)-PPFA and (R,Sp)-BPPFA (Scheme 6) [38], which were evaluated as efficient chiral supporting ligands in asymmetric alkene hydrogenation [39, 40] and ketone hydrosilylation [38] over chiral rhodium catalysts and in alkene hydrosilylation [41] and Kumada cross-coupling using palladium catalysts [42, 43]. The family of chiral phosphinoferrocene ligands (Scheme 7) was further widened via stereoconservative nucleophilic replacement of the NMe2 group that resulted in a range of multi-donor (usually hybrid [44]) derivatives combining phosphine and other ligating groups (N-, P-, S-, O-donors, etc.) [45], which were also applied in transition metal-mediated asymmetric organic transformations [46].
In particular, replacing the NMe2 group in PPFA-type compounds with another phosphine moiety produced chiral diphosphines from the Josiphos family (compounds 9 in Scheme 8) [47]. The possibility of independently altering the chirality at the cyclopentadienyl plane and in the side chain as well as the phosphine substituents (R1 and R2) in these ligands enabled their tuning according to the particular use, which consequently opened ways to their massive applications in transition-metal catalysis in laboratory and even industry scale [48, 49] and also stimulated the synthesis of numerous, structurally related P,N- and P,P-ligands with varied spacer and donor groups [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21].
The high efficiency and robustness of the synthetic methods based on diastereoselective ortho-lithiation/functionalization of chiral ferrocene amines obviously initiated a search for other applicable chiral directing groups [50]. Among the approaches developed to date, C-chiral ferrocene oxazolines 10-R [51,52,53], S-chiral sulfoxides 11-R [54, 55], and acetal 12 [56, 57] (Scheme 9) proved particularly attractive because they are practical in terms of accessibility and subsequent transformation, produce valuable synthetic intermediates, or even provide a direct access to new ligands (viz., chiral ferrocene oxazolines [51,52,53, 58, 59]).
Examples of chiral ferrocenes used in diastereoselective ortho-lithiation reactions (Note: the synthesis of 10-R and 12 makes use of common chiral pool: while compounds 10-R are prepared from β-amino alcohols accessible from α-amino acids, (S)-1,2,4-butanetriol required for the synthesis of 12 is obtained by reduction of L-malic acid)
As mentioned above, complementary synthetic approaches (Scheme 10) toward planar chiral ferrocenes were developed based on the metalation of prochiral substrates bearing suitable directing groups such as amine 4 [60], tertiary amide 15 [61,62,63,64], and phosphine oxide 18 [65, 66] using adducts generated from organolithium compounds and chiral amines (e.g., (1R,2R)-N,N,N′,N′-tetramethylcyclohexane-1,2-diamine (14) and (−)-sparteine (17)) and chiral amides such as (R,R)-lithium bis(1-phenylethyl)amide (see the last entry in Scheme 10; 95% yield, ee 54%). Recently, mixed Li-Zn and Li-Cd amides resulting from bis(1-phenylethyl)amine and analogs, where one 1-phenylethyl substituent was replaced for a terpene residue, were successfully applied in directed lithiation of alkyl ferrocene carboxylates FcCO2R (Fc = ferrocenyl). The best results (ee’s up to 71%) were obtained when LiNR2 and ZnR2 were combined (E = CH(Me)Ph) [67].
Chiral directing groups or chiral transition metal catalysts were also used to accomplish enantioselective C−H bond activation at the ferrocene moiety [68]. In the 1970s, Sokolov et al. demonstrated that orthopalladation of Ugi’s amine produced two diastereoisomeric palladium complexes, analogous to 20 (Scheme 11, top), in an 85:15 ratio [69] and that palladation of achiral amine 4 [70] can be achieved in an asymmetric manner when using N-acetyl-L-valine/NaOH as a stoichiometric additive [71, 72]. Several decades later, asymmetric C−H activation of 4 with concomitant C−C bond formation was achieved with the composite Pd-catalyst and boronic acid to give aryl-substituted compounds 21 (Scheme 11) [73].
In 1997, Siegel and Schmalz reported that insertion of a carbene in situ-generated from diazo compound 22 in the presence of a chiral copper catalyst produces planar-chiral ketone 23 with a good yield and ee (72% and 78%, respectively; see Scheme 12a) [74]. An intermolecular arylation based on C−H activation/C−C bond formation was observed when reacting chiral oxazoline (S)-10-iPr with benzene in the presence of a palladium catalyst and a base. This reaction produced compound 24 as single diastereoisomer in 24% yield (Scheme 12b). Minor amounts of the doubly arylated product (2,5-isomer) were also detected [75].
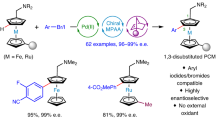
During the subsequent research, the array of the directing groups suitable for efficient catalytic C−H bond functionalization reactions of ferrocenes proceeding under the formation of new C−C bonds in enantioselective manner was markedly expanded, e.g., towards ferrocene N-heterocycles, amides, thioamides, azines, and acyl derivatives and even the palette of the tandem reactions was considerably widened [68, 76,77,78,79,80,81,82,83,84]. As an illustrative example can serve the recently disclosed C−H activation/alkenylation of ferrocene amide 25 containing an extended, pyridine-based directing group. This reaction occurs in position 3 of the ferrocene core and thus offers an alternative access to the difficult-to-prepare 1,3-disubstituted ferrocenes in racemic form [85, 86] (Scheme 13).
An alternative approach to 1,3-disubstituted ferrocenes 27-Ar has been developed based on remote arylation of amine 4 utilizing a Pd(OAc)2/Boc-L-Val-OH catalyst and a norbornene derivative (racemic), which serves as a temporary blocking group (in position adjacent to the amine substituent) and a mediator in the subsequent functionalization at position 3 of the ferrocene core (Scheme 14). The scope of compounds accessible by this method is very wide because many substituents are tolerated at the aryl halide and the products retain the reactivity of the parent amine (including ortho-functionalization).
In a recent paper, You and coworkers reported on asymmetric, Rh-catalyzed arylation of imine generated in situ from ferrocene carboxaldehyde (29) and benzylamine (Scheme 15), which produces (after hydrolysis) 2-arylated ferrocene carboxaldehydes 30-Ar with up to 83% yield and >99% ee under optimized conditions (10 mol-% Rh and 20 mol-% of chiral phosphoramidite ligand 31 at 80°C). This method tolerates various substituents at the aryl bromide (alkyl, OMe, NMe2, SMe, halide, ester, or heterocycle) and on the unsubstituted C5H5 ring of the starting aldehyde (e.g., alkyl, vinyl, aryl) and thus offers an efficient complementary alternative to synthetic routes employing chiral acetal 12.
Planar chirality is also encountered in suitably annellated ferrocenes [87] (see compound 2 mentioned above). Further examples of such compounds include planar chiral analogs of 3-(dimethylamino)pyridine (DMAP), compounds 32, which were extensively studied as organocatalysts [87,88,89,90]. Initially, these compounds were obtained by sequential addition of cyclopentadienide reagents to FeCl2 (Scheme 16), and the product mixture was separated using chemical methods (via diastereoisomers) or chiral HPLC. Later on, convergent asymmetric routes to these compounds were devised, employing chiral acetal 12 and the analogous compound permethylated at the other cyclopentadienyl ring as the starting materials [91, 92]. Further attractive examples of chiral annellated ferrocenes include chiral ferrocene-based carbene ligands [93,94,95] represented by compounds 33–37 (Scheme 17). The synthesis of 33 involved the preparation of a racemic annellated precursor and its resolution into enantiomers. The free carbene was isolated and utilized as both an organocatalyst and an auxiliary ligand in transition metal-mediated reactions [96, 97]. Conversely, carbene 34 was prepared by diastereoselective functionalization of a chiral ferrocene precursor and was isolated in the form of an Ir(I) complex, subsequently tested in Ir-catalyzed quinoline hydrogenation (free carbene was not isolated) [98]. In a similar vein, the planar chiral carbene 35 was obtained in several steps from acetal 12 and isolated in the form of a CuCl complex [99]. The same starting material was used to prepare the homologous compounds 36 and 37, which were evaluated as ligands in Ir-catalyzed asymmetric transfer hydrogenation and in Cu-catalyzed borylation of tert-butyl cinnamate [100,101,102,103].
Although far from exhaustive, this overview of the routes leading to planar chiral ferrocene derivatives illustrates the rapid and extensive developments in the area of planar chiral ferrocenes during the approximately seven decades, which passed since the discovery of the parent compound. These developments, which changed chiral ferrocenes from mere laboratory curiosities to useful molecules are driven by wide and rapidly emerging applications of these compounds in fields as diverse as molecular synthesis, catalysis, material science, and bioorganometallic chemistry [104,105,106]. Even though the currently available synthetic methods offer reliable access to a wide array of ferrocene derivatives, despite revolving around relatively few synthetic principles, further research is still highly desirable as it may result in alternative and possibly more efficient (in terms of both the yield and stereoselectivity) and atom economical processes. These should further widen the scope of accessible compounds and also mitigate problems with subsequent manipulation of the auxiliary substituents. In this view, approaches based on the functionalization of ferrocene C−H bonds appear particularly attractive. In turn, this research can open further application fields and enable wider applications of chiral ferrocenes in transition metal catalysis as well as in organocatalysis.
Abbreviations
- Ac:
-
Acetyl
- Ar:
-
Aryl
- Boc:
-
tert-butyloxycarbonyl
- BPPFA:
-
2-[1-(Dimethylamino)ethyl]-1,1′-bis(diphenylphosphino)ferrocene
- DMAP:
-
4-(Dimethylamino)pyridine
- DMF:
-
N,N-dimethylformamide
- DMSO:
-
Dimethyl sulfoxide
- dr:
-
Diastereomeric ratio
- ee:
-
Enantiomeric excess
- Fc:
-
Ferrocenyl
- H-L-Val-OH:
-
L-valine
- HPLC:
-
High-performance liquid chromatography
- n-BuLi:
-
n-butyllithium
- ODG:
-
Ortho-directing group
- Ph:
-
Phenyl
- PPFA:
-
2-[1-(Dimethylamino)ethyl]-1-(diphenylphosphino)ferrocene
- R:
-
An unspecified hydrocarbyl substituent
- t-Bu:
-
tert-butyl
- TMEDA:
-
N,N,N′,N′-tetramethyl-1,2-diaminoethane
References
Elschenbroich C (2006) Organometallics.3rd edn. Wiley-VCH, Weinheim
Štěpnička P (2022) Dalton Trans 51:8085
Togni A, Haltermann RL (eds) (1998) Metallocenes: synthesis, reactivity, applications. Wiley-VCH, Weinheim
Long NJ (1998) Metallocenes: introduction to sandwich complexes. Wiley-Blackwell, London
Togni A, Hayashi T (eds) (1995) Ferrocenes: homogeneous catalysis, organic synthesis materials science. Wiley-VCH, Weinheim
Štěpnička P (ed) (2008) Ferrocenes: ligands, materials and biomolecules. Wiley, Chichester
Dai L-X, Hou X-L (eds) (2010) Chiral ferrocenes in asymmetric catalysis. Wiley-VCH, Weinheim
Richards CJ, Locke AJ (1998) Tetrahedron Asymm 9:2377
Bandoli G, Dolmella A (2000) Coord Chem Rev 209:161
Colacot TJ (2003) Chem Rev 103:3101
Barbaro P, Bianchini C, Giambastiani G, Parisel SL (2004) Coord Chem Rev 248:2131
Atkinson RCJ, Gibson VC, Long NJ (2004) Chem Soc Rev 33:313
Arrayás RG, Adrio J, Carretero JC (2006) Angew Chem Int Ed 45:7674
Young DJ, Chien SW, Hor TSA (2012) Dalton Trans 41:12655
Noël T, Van der Eycken J (2013) Green Process Synth 2:297
Toma Š, Csizmadiová J, Mečiarová M, Šebesta R (2014) Dalton Trans 43:16557
Arae S, Ogasawara M (2015) Tetrahedron Lett 56:1751
Dwadnia N, Roger J, Pirio N, Cattey H, Hierso J-C (2018) Coord Chem Rev 355:74
Zhu J-C, Cui D-X, Li Y-D, Jiang R, Chen W-P, Wang P-A (2018) ChemCatChem 10:907
Cunningham L, Benson A, Guiry PJ (2020) Org Biomol Chem 18:9329
Dey S, Pietschnig R (2021) Coord Chem Rev 437:213850
Alba A-NR, Rios R (2009) Molecules 14:4747
Ogasawara M (2021) Chem Rec 21:3509
Schlögl K (1967) Stereochemistry of metallocenes. In: Allinger JL, Eliel EL (eds) Topics in stereochemistry, vol 1. Interscience Publishers, New York, pp 39–91
Schlögl K (1986) J Organomet Chem 300:219
Peluso P, Mamane V (2023) Electrophoresis 44:158–189
Rinehart KL, Curby RJ (1957) J Am Chem Soc 79:3920
Thomson JB (1959) Tetrahedron Lett 1:26
Schlögl K, Falk H (1974) Angew Chem 76:570
Slocum DW, Rockett BW, Hauser CR (1965) J Am Chem Soc 87:1241
The compounds is readily accessible by aminomethylation (Mannich reaction) from ferrocene: Hauser CR, Lindsay JK (1956) J Org Chem 21:382
Lindsay JK, Hauser CR (1957) J Org Chem 22:355
Aratani T, Gonda T, Nozaki H (1969) Tetrahedron Lett 10:2265
Gokel G, Hoffmann P, Kleimann H, Klusacek H, Marquarding D, Ugi I (1970) Tetrahedron Lett 1771:11
Marquarding D, Klusacek H, Gokel G, Hoffmann P, Ugi I (1970) J Am Chem Soc 92:5389
Gokel GW, Marquarding D, Ugi IK (1972) J Org Chem 37:3052
Gleiter R, Bleiholder C, Rominger F (2007) Organometallics 26:4850
Hayashi T, Yamamoto K, Kumada M (1974) Tetrahedron Lett:4405
Hayashi T, Mise T, Mitachi S, Yamamoto K, Kumada M (1976) Tetrahedron Lett:1133
Hayashi T, Mise T, Kumada M (1976) Tetrahedron Lett 4351:17
Hayashi T, Tamao K, Katsuro Y, Nakae I, Kumada M (1980) Tetrahedron Lett:1871 (Pd-catalysed hydrosilylation reported by the same group)
Hayashi T, Tajika M, Tamao K, Kumada M (1976) J Am Chem Soc 98:3718
Tamao K, Hayashi T, Matsumoto H, Yamamoto H, Kumada M (1979) Tetrahedron Lett:2155
Braunstein P, Naud F (2001) Angew Chem Int Ed 40:680. and references cited therein
For an illustrative example, see: Hayashi T, Mise T, Fukushima M, Kagotani M, Nagashima N, Hamada Y, Matsumoto A, Kawakami S, Konishi M, Yamamoto K, Kumada M (1980) Bull Chem Soc Jpn 53:1138
Hayashi T (1995) Asymmetric catalysis with chiral ferrocenylphosphine ligands (Ch. 2,) Togni A, Hayashi T (eds) Ferrocenes: homogeneous catalysis, organic synthesis materials science. Wiley-VCH, Weinheim, pp 105–142
Togni A, Breutel C, Schnyder A, Spindler F, Landert H, Tijani A (1994) J Am Chem Soc 116:4062
Blaser H-U, Brieden W, Pugin B, Spindler F, Studer M, Togni A (2002) Top Catal 19:3
Blaser HU, Pugin B, Spindler F (2021) Helv Chim Acta 104:e2000192
Clayden J (2003) Top Organomet Chem 5:251
Sammakia T, Latham HA, Schaad DR (1995) J Org Chem 60:10
Richards CJ, Damalidis T, Hibbs DE, Hursthouse MB (1995) Synlett:74
Nishibayashi Y, Uemura S (1995) Synlett:79
Rebière F, Riant O, Ricard L, Kagan HB (1993) Angew Chem Int Ed Engl 32:568
Ferber B, Kagan HB (2007) Adv Synth Catal 349:493
Riant O, Samuel O, Kagan HB (1993) J Am Chem Soc 115:5835
Mamane V (2010) Tetrahedron Asymm 21:1019
Sutcliffe OB, Bryce MR (2003) Tetrahedron Asymm 14:2297
Dai L, Xu D, Yang M-J (2023) J Organomet Chem 999:122831
Nishibayashi Y, Arikawa Y, Ohe K, Uemura S (1996) J Org Chem 61:1172
Tsukazaki M, Tinkl M, Roglans A, Chapell BJ, Taylor NJ, Snieckus V (1996) J Am Chem Soc 118:685
Laufer RS, Veith U, Taylor NJ, Snieckus V (2000) Org Lett 2:629
Jendralla U, Paulus E (1997) Synlett:471
Metallinos C, Szillat H, Taylor NJ, Snieckus V (2003) Adv Synth Catal 345:370
Sawamura M, Yamauchi A, Takegawa T, Ito Y (1991) J Chem Soc Chem Commun:874 (simple ortho-metalation)
Price D, Simpkins NS (1995) Tetrahedron Lett 36:6135 (enantioselective metalation)
For a recent example, see: Dayaker G, Erb W, Hedidi M, Chevallier F, Blot M, Gros PC, Hilmersson G, Roisnel T, Dorcet V, Bentabed-Ababsab G, Mongin F (2021) New J Chem 45:22579
López LA, López E (2015) Dalton Trans 44:10128
Sokolov VI, Troitskaya LL, Reutov OA (1977) J Organomet Chem 133:C28
Gaunt JC, Shaw BL (1975) J Organomet Chem 102:511
Sokolov VI, Troitskaya LL (1978) Chimia 32:122
Sokolov VI, Troitskaya LL, Reutov OA (1979) J Organomet Chem 182:537
Gao D-W, Shi Y-C, Gu Q, Zhao Z-L, You S-L (2013) J Am Chem Soc 135:86
Siegel S, Schmalz H-G (1997) Angew Chem Int Ed Engl 36:2456
Xia J-B, You S-L (2007) Organometallics 26:4869
Zhu D-Y, Cheng P, Xia J-B (2016) ChemCatChem 8:68
Gao D-W, Gu Q, Zheng C, You S-L (2017) Acc Chem Res 50:351
Liu C-X, Gu Q, You S-L (2020) Trends Chem 2:737
Zhang Z-Z, Huang D-Y, Shi B-F (2022) Org Biomol Chem 20:4061
Mou Q, Zhao R, Sun B (2022) Chem Asian J:e202200818
Li H, Luo H, Ban Y, Wang Y, Li D, Yang J (2022) Adv Synth Catal 364:2926
Cao F, Chen Q, Shan H, Ling L, Hu J, Zhang H (2022) J Org Chem 87:479
Cao F, Wang Y, Feng P, Hu J, Yang Y, Zhang H (2023) J Org Chem 88:5752
Čarný T, Šebesta R (2024) Synlett 35: 165 https://doi.org/10.1055/a-2089-4934
Gupta P, Madhavan S, Kapur M (2023) Angew Chem Int Ed 62:e202305278
For another recent example, making use of ferrocene amides, see: Erb W, Kadari L, Al-Mekhlafi K, Roisnel T, Dorcet V, Krishna PR, Mongin F (2020) Adv Synth Catal 362:832
Bernardo O, González-Pelayo S, Lópz LA (2022) Eur J Inorg Chem:e202100911
Fu GC (2000) Acc Chem Res 33:412
Fu GC (2004) Acc Chem Res 37:542
Fu GC (2006) Acc Chem Res 39:853
Ogasawara M, Wada S, Isshiki E, Kamimura T, Yanagisawa A, Takahashi T, Yoshida K (2015) Org Lett 17:2286
Yoshida K, Liu Q, Yasue R, Wada S, Kimura R, Konishi T, Ogasawara M (2020) ACS Catal 10:292
Siemeling U (2012) Eur J Inorg Chem:3523
Yoshida K, Yasue R (2018) Chem A Eur J 24:18575
Reshi NUD, Bera JK (2020) Coord Chem Rev 422:213334
Check CT, Jang KP, Schwamb CB, Wong AS, Wang MH, Scheidt KA (2015) Angew Chem Int Ed 54:4264
Fitzpatrick KP, Schwamb CB, Check CT, Jang K-P, Barsoum DN, Scheidt KA (2020) Organometallics 39:2705
John J, Wilson-Konderka C, Metallinos C (2015) Adv Synth Catal 357:2071
Forcher G, Silvanus A, de Frémont P, Jacques B, Pearson-Long MSM, Boeda F, Bertus P (2015) J Organomet Chem 797:1
Yasue R, Miyauchi M, Yoshida K (2017) Adv Synth Catal 359:255
Yasue R, Miyauchi M, Yoshida K (2017) Tetrahedron Asymm 28:824
Shikata Y, Yasue R, Yoshida K (2017) Chem A Eur J 23:16806
Yasue R, Yoshida K (2019) Organometallics 38:2211
van Staveren DR, Metzler-Nolte N (2004) Chem Rev 104:5931
Astruc D (2017) Eur J Inorg Chem:6
Ornelas C, Astruc D (2023) Pharmaceutics 15:2044. See also Refs. [2–4]
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Štěpnička, P. (2024). Planar Chiral Ferrocenes: A Concise Introduction. In: Hapke, M., Kotora, M. (eds) Metallocenes in Regio- and Stereoselective Synthesis. Topics in Organometallic Chemistry, vol 74. Springer, Cham. https://doi.org/10.1007/3418_2024_113
Download citation
DOI: https://doi.org/10.1007/3418_2024_113
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-57380-4
Online ISBN: 978-3-031-57381-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)