Abstract
This chapter focuses on the use of synthetic complexes for modeling iron sites in the iron-molybdenum nitrogenase enzyme, particularly on those with sulfur donors in the coordination sphere. This is an under-explored area that has promise to elucidate the way that Fe–S bonds contribute to N2 binding and activation. We review iron complexes with sulfide, thiolate, and thioether-containing supporting ligands and discuss the binding of N2 as well as reduced species such as hydrazine and diazene. The structures, spectroscopy, reactions, and other properties of key complexes are described, including recent results.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 N2 Reduction in Biological Systems
Nitrogen atoms are essential building blocks of biomacromolecules. Although atmospheric dinitrogen is a plentiful source of N atoms, N2 is relatively inert and only specialized organisms are capable of “fixing” N2 to a bioavailable form such as ammonia or nitrate [1]. In these nitrogen-processing (“diazotrophic”) bacteria and archaea, biological nitrogen fixation is catalyzed by nitrogenase enzymes according to the overall idealized reaction stoichiometry [2]:
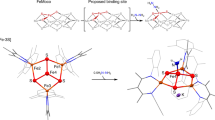
Nitrogenase enzymes are complicated multicomponent systems, and the reader may look elsewhere for detailed descriptions of their enzymology [3,4,5]. Here, we focus on the inorganic chemistry at the N2-binding cofactors, which are metalloclusters called the FeMoco (in molybdenum-dependent nitrogenases), the FeVco (in vanadium-dependent nitrogenases), or the FeFeco (in iron-only nitrogenases). Thus far, the only structurally characterized one of these cofactors is the FeMoco, which is an unusual [Fe7MoS9C] cluster (Fig. 1). In alternative nitrogenases, the molybdenum center is replaced by vanadium or iron, but the overall shape of the cofactor is thought to be similar [6, 7]. Based on the shared reactivity of all nitrogenase variants, as well as mutagenesis studies that show loss of N2 reduction ability upon mutation of His195 and Val70 (Fig. 1; numbering is for Azotobacter vinelandii FeMo-nitrogenase), the central iron atoms are most strongly implicated as the site of N2 binding [8].
Structure of the resting state of the FeMoco. (Top) Crystal structure of the FeMoco (PDB ID 4TKU) shown with key residues near the active site. (Bottom) The proposed oxidation state assignments for the iron and molybdenum ions based on spatially resolved anomalous dispersion refinement [9] and Mo K-edge X-ray absorption spectroscopy [10], respectively
Given the importance of nitrogenases in the global nitrogen cycle, and the prospect of unusual chemistry at the FeMoco, the mechanism of nitrogen reduction in the molybdenum-dependent nitrogenases has been a topic of great interest in the bioinorganic chemistry community. Substantial recent advances on the mechanism of N2 reduction by the FeMoco come from pulsed EPR studies on trapped intermediates from Seefeldt, Hoffman, Dean, and coworkers [5, 11,12,13,14]. They have constructed models that are based on the Lowe–Thorneley kinetic scheme [2, 15], which specifies that from the resting state (E0), four electrons and four protons are added to give the intermediate E4, which is the state that binds N2. Electron-nuclear double resonance (ENDOR) studies indicate that E4 has two hydrides bridging between iron centers, implying that the other two H atoms are probably protonated sulfides [16,17,18]. According to the Lowe–Thorneley model, the reversible reductive elimination of H2 is accompanied by N2 binding to iron [15]. Seefeldt and Hoffman propose that the bound N2 is immediately converted to a species at the diazene redox level, although the steps in this transformation and the structure of the resulting “N2H2” species are not clear. The sites of subsequent protonation are also unclear, but the possibilities are typically categorized as distal and alternating (see Fig. 2). In the distal pathway, proposed by analogy to small molecule molybdenum complexes [19, 20], the first molecule of ammonia is released following three protonations at the distal nitrogen. This results in the formation of a nitride that can accept three more protons and electrons to release a second equivalent of ammonia. In the alternating pathway, each N atom is protonated in turn to eventually yield a hydrazine species that undergoes N–N bond cleavage. The alternating mechanism has generally been favored for the FeMoco on the basis that diazene and hydrazine (which are intermediates unique to the alternating pathway) are also nitrogenase substrates, and that hydrazine is released from the enzyme during turnover under some conditions [5]. Although species following N–N cleavage (which are common to both the alternating and distal pathways) have been trapped and characterized by pulsed EPR [5], no intermediate that is unique to the distal or alternating pathway has been observed in the FeMoco. Furthermore, a recent study of a model complex suggests that crossover between intermediates in the distal and alternating pathways should also be considered [21]. Within each limiting mechanism, there are additional ambiguities to be resolved because the intermediates may be terminally bound or bridging between metal centers, and their coordination modes could change throughout the catalytic cycle. Thus, many questions remain about the mechanism of NH3 formation, even in the best-understood nitrogenase.
Only the resting state of the FeMoco has been structurally characterized. Rearrangement of synthetic iron–sulfur clusters upon ligand binding or redox changes is known [22,23,24], and therefore it is reasonable to predict that internal bond cleavage might occur upon reduction and N2 binding in the FeMoco. Additional evidence for this hypothesis comes from experimental studies of nitrogenase. For example, in the crystal structure of the CO-inhibited form of nitrogenase, one of the central bridging sulfides (S2B; see Fig. 1) is replaced by CO [25]. When CO is removed and the enzyme is exposed to catalytic conditions, the bridging sulfide is reincorporated and the enzyme regains full activity. In another example, Rees and coworkers replaced S2B with a selenide. Crystallographic studies demonstrated that the selenide migrates to the other central “belt” sites and is eventually extruded from the active site (with reincorporation of a sulfide) during acetylene reduction [26]. The fate of the released S2− or Se2− and the mechanism of the exchange processes in these experiments are not yet clear. Nevertheless, these studies demonstrate that sulfide dissociation and cluster rearrangement certainly can occur during turnover. However, 13C and 14C labeling studies confirm that the central carbide does not exchange during reduction of C2H2, CO, or N2 [27].
Given the uncertainty surrounding the mechanism of nitrogenase and its structure during turnover, many different intermediates could be postulated. A wide variety of mechanistic steps have been proposed based on DFT calculations [28,29,30,31,32,33,34,35,36,37,38,39]. Synthetic model compounds have been used to test the feasibility of some of these proposed chemical transformations. Iron complexes that cleave N2 [40,41,42,43] as well as iron complexes that catalytically reduce N2 to ammonia and/or silylamine [44,45,46,47,48,49] have been reported. However, these compounds and others that bind N2 and/or partially reduced N2 species (N x H y ; x = 1–2, y = 1–4) typically contain phosphorus, nitrogen, and/or carbon donors and it is unclear what effects these abiological ligands have on catalysis. Nitrogenase-relevant compounds with these types of ligand frameworks have been reviewed elsewhere [20, 50,51,52,53,54,55,56].
Iron complexes with sulfur supporting ligands that bind N x H y fragments can give distinctive insight into the reactivity patterns that can be expected in the FeMoco [57]. As shown in the examples below, the sulfur donors may serve as functional models for sulfides, for example, by facilitating proton delivery or by stabilizing N x H y species through hydrogen bonding interactions. Furthermore, these complexes can replicate the weak ligand field, the low coordination number, and the high-spin electronic configuration of the iron sites in the FeMoco. Complexes containing sulfides are the most relevant to nitrogenase, but more often thiolates are used as anionic donors. A number of iron complexes with thioether ligands, which model protonated sulfides in nitrogenases, are also known. Here, we discuss these iron complexes with sulfur-containing ligands that bind N2 and N x H y fragments in the context of nitrogenase modeling.
2 Fe–N2 Complexes with S Ligands
A variety of coordination modes have been proposed for N2 in the FeMoco [30, 33, 58]. These include bridging, terminal, and side-on coordination, in some cases involving rearrangement of the cofactor. Model complexes can illustrate the plausibility of N2 binding in these coordination modes in a sulfur-rich coordination environment.
The interaction of N2 with transition metals consists primarily of π-backbonding from filled iron d-orbitals into the empty π* orbitals of N2, which results in a weakening of the N–N triple bond [50, 59]. The deviation of the N–N bond length and/or N–N stretching frequency from the values for free N2 can be used as measures of the degree of activation of the N–N bond. The parameters for structurally characterized N2 complexes with sulfur supporting ligands are given in Table 1. These complexes, along with other species that can be shown by reactivity or spectroscopic studies to bind N2, are discussed below.
2.1 N2 Complexes with Thioether-Containing Ligands
The first structurally characterized iron–N2 complex with any type of sulfur donor was the tetrahydrothiophene (THT) adduct of the complex [Fe(iPrPDI)(N2)] (1) [61] (Fig. 3). Peters and coworkers later reported a set of thioether derivatives of the complex [Fe(SiPiPr)3(N2)]+ (2) [62, 63]. When a thioether was incorporated in place of one of the phosphine ligands in 2, the complex was still able to bind N2, although the replacement of the phosphine with thioether made the N2 more labile and slightly less activated, as demonstrated by the increase in ν NN from 2,143 cm−1 in the parent complex 2 to 2,156 cm−1 in 3. When a second phosphine was replaced with a thioether, N2 binding was no longer observed. Addition of a hydride led to N2 binding to both the mono and bis(thioether) complexes and a more activated N2 ligand as compared to analogous complexes without a hydride ligand, as shown by the 101 cm−1 decrease in ν NN in 4 (ν NN = 2,055 cm−1) as compared to 3. The formation of hydrides could play a similar role in promoting N2 binding and activation in the FeMoco. Although it could not be crystallographically characterized, the mixed-valence iron(I)/iron(II) bridging N2 complex 6 was also accessible via treatment of the solvent adduct [Fe(SiPiPrSAd 2)(Et2O)]+ with 0.5 equivalents of CrCp2 or CoCp2. Complex 6 exhibits an N–N stretching vibration at 1,881 cm−1, significantly lower than any of the monometallic complexes with this ligand, which illustrates how N2 binding and activation could be facilitated by multimetallic cooperativity [66].
2.2 N2 Complexes with Thiolate Ligands
There are also examples of thiolate complexes with N2 ligands. Peters and coworkers generated a series of thiolate-bridged terminal N2 complexes in three different oxidation states (7–9) [64] (Fig. 4). Terminal hydride analogs of complexes 8 and 9 (10 and 11) were also reported. Complex 9 catalyzed low turnovers of NH3 formation from N2 but was much more effective for hydrazine disproportionation into NH3 and N2. Interestingly, under the same conditions the analogous monometallic complex 3 did not perform either of these reactions efficiently, suggesting that the thiolate plays an important role in catalysis, perhaps by allowing cooperativity between the iron centers and/or acting as a proton shuttle during turnover.
In complexes 3–11, N2 binding is in part promoted by the presence of phosphine ligands. More recently, it has been possible to observe N2 binding using a supporting ligand that contains only sulfur and carbon donors [65]. Reduction of the tris(thiolate) complex 12 yielded the terminal N2 complex 13, in which the iron center is coordinated to two thiolates and has an η2 interaction with the central arene ring (Fig. 5), which models a potential S2C donor set in the FeMoco. The observation of Fe–S bond cleavage upon reduction and N2 binding shows that dissociation of a sulfide is a chemically reasonable step for N2 binding and activation in the FeMoco. The relatively low ν NN of this complex (1,880 cm−1) is indicative of an N2 unit that is quite weakened, despite the high-spin electronic configuration of the complex. This demonstrates that a low-coordinate iron center with weak-field ligands can lead to substantial weakening of N2 and suggests that the electron-rich thiolates lead to strong backbonding.
2.3 Interaction of N2 with Iron–Sulfide Clusters
The study of synthetic iron–sulfur clusters in the context of nitrogenase has also been the topic of a significant body of work [22,23,24, 67, 68]. Although there are no structurally characterized iron–sulfur clusters with bound N2 ligands, catalytic and spectroscopic studies have demonstrated that N2 can bind to synthetic iron–sulfur clusters. Electrochemical reduction of N2 to NH3 is catalyzed by [Fe4S4(SPh)4]2− and [Mo2Fe6S8(SPh)9]3− clusters, albeit with very low Faradaic efficiency [69]. More recently, the photochemical conversion of N2 to NH3 by chalcogenide aerogels (chalcogels) containing Mo2Fe6S8(SPh)3 and Fe4S4 clusters has been reported [70, 71]. Using infrared spectroscopy, N–N stretching bands were observed at 1,746 and 1,753 cm−1 upon irradiation of these chalcogels with visible light under N2 atmosphere. The N–N stretching frequencies are even lower than the S2C supported complex above and are indicative of some form of reduced N2 species, but the redox and protonation state of this species are not known. Nevertheless, these data provide evidence for the formation of a cluster-bound N x H y species during turnover. N2 binding to Fe2S2 +, Fe3S3 +, and Fe4S4 + clusters in the gas phase has also been observed by mass spectrometry in ion-trapping experiments [72]. The structures of the N2 adducts are not known experimentally but were suggested from DFT calculations.
Finally, in a recent study, a diferrous iron sulfide hydride complex with a β-diketiminate coligand was reduced under N2 to give a diiron(0) N2 complex in 24% spectroscopic yield [73]. Gas chromatography indicates that H2 is produced during this process. The reactivity of this complex thus models the E4 state of the FeMoco, in which H2 loss from an iron hydride sulfide core results in N2 binding [14, 74,75,76]. However, in the model complex the H2 production results from a bimolecular reaction, the N2-containing product did not also contain a sulfide, and mechanistic studies of the reaction leading to sulfide extrusion and N2 binding were precluded by the presence of a significant number of unidentified byproducts in the reaction mixture.
3 Fe Complexes with N x H y Ligands
There are no examples of N2 functionalization giving a well-defined N x H y complex for iron complexes with sulfur supporting ligands. However, a number of diazene (HN=NH) and hydrazine (NH2–NH2) complexes are known with sulfur-based supporting ligands, as are alkyl- or aryl-substituted diazene and hydrazine derivatives. Generation of the hydrazine species typically proceeds in a straightforward manner by addition of hydrazine to precursor complexes. In some cases, hydrazine addition instead results in isolable diazene complexes accompanied by formation of amines, implying that the diazene was generated via hydrazine disproportionation. The generation of diazene complexes via direct addition of diazene to a precursor is more problematic because diazene is unstable in solution [77,78,79], but there are examples where diazene is generated and trapped in situ by iron complexes. Isolated diazene complexes are most commonly synthesized by hydrazine disproportionation or by oxidation of corresponding hydrazine species. In this section, we discuss the generation, structural characterization, and reactivity of these N x H y compounds.
3.1 N x H y Complexes with Iron–Sulfide Clusters
An early report demonstrated that the electrochemical reduction of hydrazine is catalyzed with high Faradaic efficiency by [Fe4S4(SPh)4]2− and [Mo2Fe6S8(SPh)9]3− clusters, but the mechanism of NH3 formation was not examined [80]. Chemical reduction of vanadium- and molybdenum-containing iron–sulfur cubanes also generates ammonia from hydrazine, but in all of these cases the V or Mo centers were implicated as the sites of hydrazine binding and reduction [81,82,83,84,85,86,87,88,89].
The only examples of structurally characterized iron N x H y complexes incorporating sulfides are the β-diketiminate supported complexes 14–16 [90, 91] (Fig. 6). Reaction of the diferrous monosulfide-bridged precursor with ammonia, methylhydrazine, or 1,1-dimethylhydrazine yielded 2:1 complexes in which one of the N-donors is bound to each iron center (14). In contrast, reaction with the parent hydrazine yielded complex 15 in which an N2H4 ligand bridges between the iron centers. Treatment of the diferrous precursor with 1.5 equivalents of phenylhydrazine resulted in the formation of the mixed-valence iron(II)/iron(III) phenylhydrazido-bridged complex 16 via the overall reaction:
This reactivity demonstrates that sulfide-bridged iron centers are capable of N–N bond cleavage. The phenylhydrazido species 16 was also the subject of a detailed ENDOR study, which enabled the first determination of the hyperfine coupling parameters of a well-defined bridging hydrazido species that could be compared to the ENDOR parameters of nitrogenase intermediates [92].
3.2 N x H y Complexes with Thioether/Thiolate Ligands
Sellmann and coworkers reported a series of complexes with supporting ligands containing two thiolate donors with additional thioether, amine, and/or pyridine groups [93,94,95]. Of particular note are several trans diazene complexes (two representative examples are shown in Fig. 7), which were generated either by air oxidation of a corresponding hydrazine species [96, 97] or by trapping of diazene generated in situ from potassium azodicarboxylate or benzenesulfonic acid hydrazide [98, 99]. In these complexes, the diazene ligand bridges between two iron centers, with the protons of the diazene forming one long (~2.8 Å) and one short (~2.2 Å) hydrogen bond to the thiolate moieties of the supporting ligand [96, 97, 99]. DFT calculations suggest that the strong hydrogen-bonding interaction contributes significantly to the overall stability of the complex [100, 101]. The sulfides in the FeMoco, as well as adjacent amino acids, may play a similar role in stabilizing intermediates during N2 reduction. A further contribution to the stability of these complexes arises from the strong π-backbonding interaction between the iron center and the diazene ligand, as demonstrated by their intense blue color that arises from an iron to diazene charge transfer transition [102, 103]. This interaction leads to slight weakening of the N–N bond as compared to free diazene: for example, in complex 17, the N–N bond length [96] is 1.301(5) Å compared to 1.252 Å in free diazene [104]. The N–N stretching frequency is 1,382 cm−1 [103], which falls between the values for free diazene (ν NN = 1,529 cm−1 [105]) and free hydrazine (ν NN = 876 cm−1 [106]). Using normal coordinate analysis, Lehnert and coworkers determined an N–N bond order of 1.6 for this complex indicating partial reduction to a hydrazido(2−) species [103]. SCF-Xα-SW calculations indicated that the LUMO of this complex consists primarily of a diazene π* orbital, which implies that reduction of the complex should further weaken the N–N bond of the diazene moiety [102]. However, in practice the reduction occurred at an extremely negative potential and led instead to decomposition of the complex.
Although structurally characterized monometallic iron complexes that bind hydrazine [107,108,109] and ammonia [107] are also known in these systems, a corresponding N2 species could not be generated. Interestingly, however, oxidation of the diazene complex 18 with two equivalents of ferrocenium at −78 °C caused a color change from blue to purple [110, 111]. Since the HOMO of complex 18 is primarily an iron orbital [102], this oxidation was expected to result in the formation of a diferric diazene species. Warming the purple oxidized species above −40 °C resulted in N2 evolution and formation of a green ferrous product. This implies that the purple species may be a ferrous N2 complex, which is a valence tautomer of the expected diferric diazene complex (Fig. 8). This process would model the reverse of N2 binding in the FeMoco and illustrate how sulfides could facilitate proton transfer. Unfortunately, the instability of the purple oxidized species prevented further characterization and its identity remains unclear.
3.3 N x H y Complexes with Thiolate Ligands
Other complexes containing thiolate donors are also known to bind N2H4 and NH3. In these systems, like those described above, the hydrazine ligands often form extensive hydrogen-bonding networks with the thiolates and/or solvents of crystallization. For example, Sellmann and coworkers crystallized iron bis(benzenedithiolate) complexes with hydrazine bound in bridging [112] and terminal [113] coordination modes. The terminal complex in particular contains an extensive hydrogen-bonding network between the metal-bound hydrazine and N2H4 and N2H5 molecules in the crystal lattice.
Upon reaction of the sterically hindered thiolate-bridged dimer Fe2(μ-STriph)2(STriph)2 (STriph = 2,4,6-triphenylbenzenethiolate) with hydrazine, rearrangement to the hydrazine-bridged dimer Fe2(μ-N2H2)2(N2H2)2(STriph)4 (19) was observed [114] (Fig. 9). The precursor complex Fe2(μ-STriph)2(STriph)2 also reacted with amines to yield mono- and bimetallic coordination compounds and was observed to catalyze the disproportionation of 1,2-diphenylhydrazine to aniline and azobenzene (PhN=NPh). Complex 20 also coordinates ammonia and hydrazine [115]. The NH3 and N2H4 ligands in 20 are labile in solution but are stabilized in the solid state by hydrogen-bonding interactions to solvent molecules. This complex catalyzes hydrazine reduction, but the number of turnovers is low and no intermediates could be observed during the reaction.
Hydrazine cleavage at iron sites has also been observed in other systems. A series of ferric iron-imide heterocubanes (21) were generated by the reaction of ferrous complexes containing sterically hindered thiolate ligands with 1,2-diarylhydrazines [116, 117]. The mechanism of the N–N bond cleavage step leading to imide formation is not clear but was proposed to occur through formation of a diferrous complex containing an arylhydrazine bound in a μ-η2:η2 fashion, followed by electron transfer from the ferrous centers to the hydrazine resulting in N–N bond cleavage. A similar reaction leading to N–N bond cleavage and formation of a bridging imide could be envisioned in the FeMoco. Note that these cubane structures were obtained only with sterically hindered thiolates and 1,2-diarylhydrazines; all other substrates gave mixtures of products. In a different set of studies, iron-imide-sulfide heterocubanes were also structurally and spectroscopically characterized [118, 119].
3.4 N x H y Complexes with Thiolate and Cp Ligands
Several complexes incorporating thiolate and Cp donors are also known to catalyze the N–N bond cleavage of hydrazines. Qu and coworkers reported that alkylthiolate-bridged iron complexes react with hydrazines to form cis diazene complexes (22) [120, 121]:
In the presence of reductant and acid, these compounds catalytically cleave the N–N bond of various alkyl- and aryl-substituted hydrazines. No intermediates could be detected in this reaction, but DFT calculations suggested that protonation and reduction of diazene involves isomerization to a (μ-NH)-NH2 species [122]. The strong donation of the thiolates and their ability to shift coordination mode are thought to be important in allowing this isomerization to occur. Nishibayashi and coworkers reported a similar system containing an ortho-substituted aryl thiolate in which a methyldiazene (CH3–N=NH) or methyldiazenido (N=NCH3 −) moiety is bound side-on between two iron centers (23–24) [123] (Fig. 10). In this case, the bridging structure was important for promoting selective hydrazine cleavage; a corresponding monomeric complex promoted H2 formation rather than NH3 production. Again, however, the mechanism of N–N bond cleavage was not clear and the key hydrazido (R2N-NR−) intermediate could not be detected.
In another system, Qu and coworkers studied the generation of ammonia from treatment of a benzenedithiolate-bridged diiron complex with hydrazine [124]. Several intermediates along the pathway to NH3 release were accessible via stepwise protonation and/or reduction. Starting from a diferrous complex with cis-diazene bound μ-η1:η1 between the iron centers (25), protonation led to electron transfer from the iron centers to the diazene ligand and rearrangement to afford a diferric complex in which a hydrazido (N2H3 −) ligand is bound asymmetrically in a μ-η1:η2 fashion (26). The bridging benzenedithiolate ligand also rearranged to a μ-η1:η2 coordination mode, which resembles the hypothesized rearrangement of sulfides in the FeMoco during turnover. Subsequent two-electron reduction and protonation of 26 resulted in ammonia release and formation of an amido-bridged diferrous complex (27). DFT calculations suggest that the ammonia comes from the non-bridging NH2 of the hydrazido species via the mechanism shown in Fig. 11. Note that this process involves an NH–NH3 intermediate that is not part of the traditional distal or alternating pathways but has been proposed for the FeMoco based on computational results [34]. The amido complex 27 also produces NH3 upon further reduction and protonation, although the yield is low due to competitive proton reduction.
Proposed mechanism of NH3 release leading to the formation of 27 upon protonation and reduction of 25 via 26. Of the complexes shown in this figure, only 26 and 27 are structurally characterized, although the structure of 25 (which is an isolable species) can be inferred based on the structure of the analogous one-electron oxidized species. The sequence of protonation and electron transfer steps is proposed based on DFT calculations
4 Conclusions
The results presented above show the variety of synthetic strategies that have yielded N2 and N x H y complexes of iron with sulfur-containing supporting ligands. It is becoming clear that the coordinative flexibility, electron-rich character, and hydrogen-bonding capability of sulfur atoms can influence the behavior of bound N2 and N x H y coligands. Understanding the electronic structure and reactivity of these compounds has begun to provide insight into the possible role(s) of the sulfides in the FeMoco of nitrogenase.
References
Ribbe M (ed) (2011) Nitrogen fixation. Humana, New York, NY
Burgess BK, Lowe DJ (1996) Chem Rev 96:2983
Holland PL (2004) Nitrogen fixation. In: McCleverty J, Meyer TJ (eds) Comprehensive Coordination Chemistry II. Elsevier, Oxford, Vol. 8, pp 569–599
Hu Y, Ribbe MW (2015) J Biol Inorg Chem 20:435
Hoffman BM, Lukoyanov D, Yang Z-Y, Dean DR, Seefeldt LC (2014) Chem Rev 114:4041
Eady RR (1996) Chem Rev 96:3013
Krahn E, Weiss BJR, Kröckel M, Groppe J, Henkel G, Cramer SP, Trautwein AX, Schneider K, Müller A (2002) J Biol Inorg Chem 7:37
Seefeldt LC, Dance IG, Dean DR (2004) Biochemistry 43:1401
Spatzal T, Schlesier J, Burger E-M, Sippel D, Zhang L, Andrade SLA, Rees DC, Einsle O (2016) Nat Commun 7:10902
Bjornsson R, Lima FA, Spatzal T, Weyhermüller T, Glatzel P, Bill E, Einsle O, Neese F, DeBeer S (2014) Chem Sci 5:3096
Hoffman BM, Dean DR, Seefeldt LC (2009) Acc Chem Res 42:609
Seefeldt LC, Hoffman BM, Dean DR (2009) Annu Rev Biochem 78:701
Hoffman BM, Lukoyanov D, Dean DR, Seefeldt LC (2013) Acc Chem Res 46:587
Lukoyanov D, Yang Z-Y, Khadka N, Dean DR, Seefeldt LC, Hoffman BM (2015) J Am Chem Soc 137:3610
Thorneley RNF, Lowe DJ (1985) Met Ions Biol 7:221
Igarashi RY, Laryukhin M, Dos Santos PC, Lee H-I, Dean DR, Seefeldt LC, Hoffman BM (2005) J Am Chem Soc 127:6231
Lukoyanov D, Yang Z-Y, Dean DR, Seefeldt LC, Hoffman BM (2010) J Am Chem Soc 132:2526
Doan PE, Telser J, Barney BM, Igarashi RY, Dean DR, Seefeldt LC, Hoffman BM (2011) J Am Chem Soc 133:17329
Schrock RR (2005) Acc Chem Res 38:955
Nishibayashi Y (2015) Inorg Chem 54:9234
Rittle J, Peters JC (2016) J Am Chem Soc 138:4243
Lee SC, Holm RH (2003) Proc Natl Acad Sci U S A 100:3595
Lee SC, Holm RH (2004) Chem Rev 104:1135
Lee SC, Lo W, Holm RH (2014) Chem Rev 114:3579
Spatzal T, Perez KA, Einsle O, Howard JB, Rees DC (2014) Science 345:1620
Spatzal T, Perez KA, Howard JB, Rees DC (2015) Elife 4:e11620
Wiig JA, Lee CC, Hu Y, Ribbe MW (2013) J Am Chem Soc 135:4982
Dance I (2008) Dalton Trans 5977
Dance I (2008) Dalton Trans 5992
Dance I (2012) Dalton Trans 41:4859
Dance I (2014) A unified chemical mechanism for hydrogenation reactions catalyzed by nitrogenase. In: Weigand W, Schollhammer P (eds) Bioinspired catalysis. Wiley, Weinheim, pp 249–288
Huniar U, Ahlrichs R, Coucouvanis D (2004) J Am Chem Soc 126:2588
Schimpl J, Petrilli HM, Blöchl PE (2003) J Am Chem Soc 125:15772
Kästner J, Blöchl PE (2007) J Am Chem Soc 129:2998
Hinnemann B, Nørskov JK (2004) J Am Chem Soc 126:3920
Varley JB, Wang Y, Chan K, Studt F, Nørskov JK (2015) Phys Chem Chem Phys 17:29541
Rao L, Xu X, Adamo C (2016) ACS Catalysis 6:1567
McKee ML (2016) J Phys Chem A 120:754
Siegbahn PEM (2016) J Am Chem Soc 138:10485
Rodriguez MM, Bill E, Brennessel WW, Holland PL (2011) Science 334:780
Grubel K, Brennessel WW, Mercado BQ, Holland PL (2014) J Am Chem Soc 136:16807
MacLeod KC, McWilliams SF, Mercado BQ, Holland PL (2016) Chem Sci 7:5736
Lee Y, Sloane FT, Blondin G, Abboud KA, García-Serres R, Murray LJ (2015) Angew Chem Int Ed Engl 54:1499
Anderson JS, Rittle J, Peters JC (2013) Nature 501:84
Creutz SE, Peters JC (2014) J Am Chem Soc 136:1105
Ung G, Peters JC (2015) Angew Chem Int Ed Engl 54:532
Del Castillo TJ, Thompson NB, Peters JC (2016) J Am Chem Soc 138:5341
Yuki M, Tanaka H, Sasaki K, Miyake Y, Yoshizawa K, Nishibayashi Y (2012) Nat Commun 3:1254
Kuriyama S, Arashiba K, Nakajima K, Matsuo Y, Tanaka H, Ishii K, Yoshizawa K, Nishibayashi Y (2016) Nat Commun 7:12181
MacKay BA, Fryzuk MD (2004) Chem Rev 104:385
Crossland JL, Tyler DR (2010) Coord Chem Rev 254:1883
Hazari N (2010) Chem Soc Rev 39:4044
MacLeod KC, Holland PL (2013) Nat Chem 5:559
Köthe C, Limberg C (2015) Z Anorg Allg Chem 641:18
Tanabe Y, Nishibayashi Y (2016) Chem Rec 16:1549
Ohki Y, Seino H (2016) Dalton Trans 45:874
Čorić I, Holland PL (2016) J Am Chem Soc 138:7200
Hallmen PP, Kästner J (2015) Z Anorg Allg Chem 641:118
Bazhenova TA, Shilov AE (1995) Coord Chem Rev 144:69
NIST Computational Chemistry Comparison and Benchmark Database (2015) National Institute of Standards and Technology. http://cccbdb.nist.gov/. Accessed 21 Sept 2015
Bart SC, Lobkovsky E, Bill E, Wieghardt K, Chirik PJ (2007) Inorg Chem 46:7055
Lee Y, Mankad NP, Peters JC (2010) Nat Chem 2:558
Takaoka A, Mankad NP, Peters JC (2011) J Am Chem Soc 133:8440
Creutz SE, Peters JC (2015) J Am Chem Soc 137:7310
Čorić I, Mercado BQ, Bill E, Vinyard DJ, Holland PL (2015) Nature 526:96
McWilliams SF, Holland PL (2015) Acc Chem Res 48:2059
Venkateswara Rao P, Holm RH (2004) Chem Rev 104:527
Henderson RA (2014) Binding substrates to synthetic Fe–S-based clusters and the possible relevance to nitrogenases. In: Weigand W, Schollhammer P (eds) Bioinspired catalysis. Wiley, Weinheim, pp 289–324
Tanaka K, Hozumi Y, Tanaka T (1982) Chem Lett 11:1203
Banerjee A, Yuhas BD, Margulies EA, Zhang Y, Shim Y, Wasielewski MR, Kanatzidis MG (2015) J Am Chem Soc 137:2030
Liu J, Kelley MS, Wu W, Banerjee A, Douvalis AP, Wu J, Zhang Y, Schatz GC, Kanatzidis MG (2016) Proc Natl Acad Sci U S A 113:5530
Heim HC, Bernhardt TM, Lang SM, Barnett RN, Landman U (2016) J Phys Chem C 120:12549
Arnet NA, Dugan TR, Menges FS, Mercado BQ, Brennessel WW, Bill E, Johnson MA, Holland PL (2015) J Am Chem Soc 137:13220
Yang Z-Y, Khadka N, Lukoyanov D, Hoffman BM, Dean DR, Seefeldt LC (2013) Proc Natl Acad Sci U S A 110:16327
Lukoyanov D, Khadka N, Yang Z-Y, Dean DR, Seefeldt LC, Hoffman BM (2016) J Am Chem Soc 138:10674
Lukoyanov D, Khadka N, Yang Z-Y, Dean DR, Seefeldt LC, Hoffman BM (2016) J Am Chem Soc 138:1320
Stanbury DM (1991) Inorg Chem 30:1293
Liao G-L, Palmer G (1998) Biochemistry 37:15583
Barney BM, McClead J, Lukoyanov D, Laryukhin M, Yang T-C, Dean DR, Hoffman BM, Seefeldt LC (2007) Biochemistry 46:6784
Hozumi Y, Imasaka Y, Tanaka K, Tanaka T (1983) Chem Lett 12:897
Coucouvanis D, Mosier PE, Demadis KD, Patton S, Malinak SM, Kim CG, Tyson MA (1993) J Am Chem Soc 115:12193
Malinak SM, Demadis KD, Coucouvanis D (1995) J Am Chem Soc 117:3126
Demadis KD, Malinak SM, Coucouvanis D (1996) Inorg Chem 35:4038
Malinak SM, Simeonov AM, Mosier PE, McKenna CE, Coucouvanis D (1997) J Am Chem Soc 119:1662
Palermo RE, Singh R, Bashkin JK, Holm RH (1984) J Am Chem Soc 106:2600
Coucouvanis D (1996) J Biol Inorg Chem 1:594
Demadis KD, Coucouvanis D (1994) Inorg Chem 33:4195
Demadis KD, Coucouvanis D (1995) Inorg Chem 34:3658
Coucouvanis D, Demadis KD, Malinak SM, Mosier PE, Tyson MA, Laughlin LJ (1996) J Mol Catal A Chem 107:123
Vela J, Stoian S, Flaschenriem CJ, Münck E, Holland PL (2004) J Am Chem Soc 126:4522
Stubbert BD, Vela J, Brennessel WW, Holland PL (2013) Z Anorg Allg Chem 639:1351
Lees NS, McNaughton RL, Vargas Gregory W, Holland PL, Hoffman BM (2008) J Am Chem Soc 130:546
Sellmann D, Sutter J (1997) Acc Chem Res 30:460
Sellmann D, Utz J, Blum N, Heinemann FW (1999) Coord Chem Rev 190–192:607
Sellmann D, Sutter J (1996) J Biol Inorg Chem 1:587
Sellmann D, Soglowek W, Knoch F, Moll M (1989) Angew Chem Int Ed Engl 28:1271
Sellmann D, Friedrich H, Knoch F, Moll M (1994) Z Naturforsch B 49:76
Sellmann D, Hennige A (1997) Angew Chem Int Ed Engl 36:276
Sellmann D, Blum DCF, Heinemann FW (2002) Inorg Chim Acta 337:1
Reiher M, Sellmann D, Hess AB (2001) Theor Chem Acc 106:379
Reiher M, Salomon O, Sellmann D, Hess BA (2001) Chem Eur J 7:5195
Lehnert N, Wiesler BE, Tuczek F, Hennige A, Sellmann D (1997) J Am Chem Soc 119:8869
Lehnert N, Wiesler BE, Tuczek F, Hennige A, Sellmann D (1997) J Am Chem Soc 119:8879
Carlotti M, Johns JWC, Trombetti A (1974) Can J Phys 52:340
Bondybey VE, Nibler JW (1973) J Chem Phys 58:2125
Giguère PA, Liu ID (1952) J Chem Phys 20:136
Sellmann D, Soglowek W, Knoch F, Ritter G, Dengler J (1992) Inorg Chem 31:3711
Sellmann D, Shaban S, Heinemann F (2004) Eur J Inorg Chem 2004:4591
Sellmann D, Blum N, Heinemann F (2001) Z Naturforsch B 56:581
Sellmann D, Hennige A, Heinemann FW (1998) Inorg Chim Acta 280:39
Sellmann D, Hofmann T, Knoch F (1994) Inorg Chim Acta 224:61
Sellmann D, Kreutzer P, Huttner G, Frank A (1978) Z Naturforsch B 33:1341
Sellmann D, Friedrich H, Knoch F (1994) Z Naturforsch B 49:660
Zdilla MJ, Verma AK, Lee SC (2008) Inorg Chem 47:11382
Chang Y-H, Chan P-M, Tsai Y-F, Lee G-H, Hsu H-F (2014) Inorg Chem 53:664
Verma AK, Lee SC (1999) J Am Chem Soc 121:10838
Zdilla MJ, Verma AK, Lee SC (2011) Inorg Chem 50:1551
Chen X-D, Duncan JS, Verma AK, Lee SC (2010) J Am Chem Soc 132:15884
Chen X-D, Zhang W, Duncan JS, Lee SC (2012) Inorg Chem 51:12891
Chen Y, Zhou Y, Chen P, Tao Y, Li Y, Qu J (2008) J Am Chem Soc 130:15250
Chen Y, Liu L, Peng Y, Chen P, Luo Y, Qu J (2011) J Am Chem Soc 133:1147
Luo Y, Li Y, Yu H, Zhao J, Chen Y, Hou Z, Qu J (2012) Organometallics 31:335
Yuki M, Miyake Y, Nishibayashi Y (2012) Organometallics 31:2953
Li Y, Li Y, Wang B, Luo Y, Yang D, Tong P, Zhao J, Luo L, Zhou Y, Chen S, Cheng F, Qu J (2013) Nat Chem 5:320
Acknowledgement
The authors thank the National Institutes of Health (GM065313) for funding.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing AG
About this chapter
Cite this chapter
Speelman, A.L., Holland, P.L. (2016). Sulfur-Supported Iron Complexes for Understanding N2 Reduction. In: Nishibayashi, Y. (eds) Nitrogen Fixation. Topics in Organometallic Chemistry, vol 60. Springer, Cham. https://doi.org/10.1007/3418_2016_4
Download citation
DOI: https://doi.org/10.1007/3418_2016_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57713-5
Online ISBN: 978-3-319-57714-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)