Abstract
Comparative approaches to the auditory system have yielded great insight into the evolution of sound localization circuits, particularly within the nonmammalian tetrapods. The fossil record demonstrates multiple appearances of tympanic hearing, and examination of the auditory brain stem of various groups can reveal the organizing effects of the ear across taxa. If the peripheral structures have a strongly organizing influence on the neural structures, then homologous neural structures should be observed only in groups with a homologous tympanic ear. Therefore, the central auditory systems of anurans (frogs), reptiles (including birds), and mammals should all be more similar within each group than among the groups. Although there is large variation in the peripheral auditory system, there is evidence that auditory brain stem nuclei in tetrapods are homologous and have similar functions among and within these groups. It appears that the more pronounced changes in processing are related to detecting airborne sound, the addition of high-frequency hearing, and the extent of acoustic coupling of the ears. This chapter focuses on the similarities and differences in peripheral structures as well as the anatomy and physiology of auditory brain stem nuclei.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
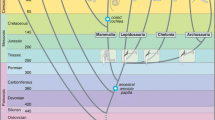
Two developments characterize the adaption of the tetrapod auditory system to life in air. These are the development of the tympanum, or eardrum, which allows sensitive responses to airborne sound (Clack, 2002), and the separation of the mouth cavity from the middle ear (Manley, 2010; chapter by Christensen-Dalsgaard & Manley, this volume). The ancestors of tetrapods moved onto land in the Devonian, and paleontologists estimate that tympanic hearing emerged about 100 million years later, after the major tetrapod lineages emerged (Clack, 2002) (Fig. 1). Tympanic hearing appears to have developed independently in at least five major tetrapod groups—the anurans (frogs and toads), testudines (turtles and tortoises), lepidosaurs (lizards and snakes), archosaurs (birds and crocodilians), and mammals (Fig. 1). The emergence of a tympanic ear would have increased the frequency range and sensitivity of hearing (Clack, 2002; Christensen-Dalsgaard & Carr, 2008). Further, tympana were in many cases acoustically coupled through the mouth cavity and therefore inherently directional, acting as pressure difference receivers. The later closure of the middle ear cavity, to varying degrees, in turtles and archosaurs is a derived condition, and would have profoundly changed the operation of the ear by decoupling the tympana.
This chapter addresses how these changes in the auditory periphery, that is, the evolution of eardrums and coupled ears, could have influenced the organization of the central auditory system. Wilczynski and Capranica (1984) discussed the effect of peripheral changes, and argued that, because new peripheral structures are specializations of preexisting structures, their central systems must also be specializations of (and homologous to) preexisting areas. Thus, a major evolutionary change in the periphery need not initially require a parallel genetic change in brain architecture because the new inputs in the periphery should cause epigenetic rearrangements at other levels of a functional system.
One way to examine the influence of the tympanic ear on the central auditory system is to compare the organization of the auditory system of groups that share a common origin of tympanic hearing. A strongly organizing influence of the tympanic ear would result in homologous neural structures only in groups that share a homologous tympanic ear. Therefore, anurans, reptiles, and mammals should have central auditory systems that are more similar within each group than among the groups. This idea is supported by the existence of morphotypes, or conserved patterns of organization, observed within each group (Northcutt & Kaas, 1995).
Evolution of tympanic ears in vertebrates. The appearances of tympanic ears within the fossil record (orange; Clack & Allin, 2004). These ears exhibit varying degrees of coupling (gray). (from Schnupp & Carr, 2009, used wtih permission)
1.1 Basal Patterns Among the Tetrapods
The overall structure of the central auditory system is similar among the vertebrates, and has been reviewed recently (Carr & Edds-Walton, 2008). Similarities between different vertebrate groups cannot be used as evidence for or against an early origin of the auditory system, as opposed to multiple origins, because the similarities could simply reflect the overall organization of the octavo-lateral system (Grothe et al., 2004).
With this caveat, in the major vertebrate taxa, the auditory midbrain receives ascending inputs from both monaural and binaural hindbrain nuclei, and physiological recordings have revealed the emergence of complex response properties there (reviews in McCormick, 1999; Grothe, 2003; Carr & Edds-Walton, 2008). The midbrain projects to the thalamus, which in turn projects to auditory stations in the telencephalon. The organization of the telencephalon is structurally and functionally diverse. In all vertebrate clades, the telencephalon exhibits large changes associated with the increased use of sound for communication (Wilczynski & Capranica, 1984).
The changes in the auditory periphery outlined above, that is, the evolution of eardrums and coupled ears, may have influenced the organization of the central auditory system in the anurans (frogs and toads), testudines (turtles and tortoises), lepidosaurs (lizards and snakes), and archosaurs (birds and crocodilians). In general, mammalian middle ears are not coupled, but connected to the mouth via narrow Eustachian tubes (chapter by Christensen-Dalsgaard & Manley, this volume).
1.2 Amphibians
Amphibians show great variety in the degree of specialization of the auditory periphery (chapter by Narins & Mann, this volume). Most anurans possess a tympanic middle ear sensitive to airborne sound that is processed by the amphibian and basilar papillae (Christensen-Dalsgaard, 2009). Associated with responses to airborne sound, a dorsal medullary auditory nucleus appears in anurans. Its origins and possible homology to the amniote cochlear nuclei are still unclear (Wilczynski & Capranica, 1984). The dorsal medullary nucleus receives tonotopic projections from the amphibian and basilar papillae, as well as inputs from the lagena and possibly the saccule (review in McCormick, 1999). Interestingly, it also receives extensive commissural projections from the contralateral dorsal medullary nucleus, indicating significant binaural interaction at this early stage (reviews in Zakon & Wilczynski, 1988; Christensen-Dalsgaard, 2005).
1.3 Sauropsids or Reptilia
The sauropsids (Reptilia) are a clade that includes testudines (turtles and tortoises), lepidosaurs (snakes and lizards), and archosaurs (crocodilians and birds), as well as a number of extinct groups. The archosaurs are more closely related to each other than they are to the lepidosaurs, while the position of testudines is controversial (Hedges & Poling 1999). Recent molecular phylogenies of testudines support an archosaur affinity (Zardoya & Meyer, 2001; Shen et al., 2011; Crawford et al., 2012). However, other recent analyses support the traditional position of testudines as parareptiles, sister to the entire diapsid (lepidosaurs and archosaurs) clade (Lyson et al., 2010). Despite the great diversity of sauropsids, their central auditory systems show a common plan, suggesting an origin of tympanic hearing before the lepidosaur–archosaur split, especially because the evidence for an independent origin of the tympanic ear in lepidosaurs and archosaurs is weak (Clack & Allin 2004). Most major differences among sauropsid auditory systems are associated with changes in the periphery. The following sections focus on the effects of these changes in the auditory periphery, such as coupled middle ears and the presence of a tympanum.
Reptiles have a tympanum, and may or may not have an external ear. In snakes and some lizards, loss of the tympanum is secondary. All have a single middle ear bone, the columella–extracolumella, which either connects the tympanum and the oval window, or, in snakes, connects the quadrate and oval window. Movement of the columella activates the hair cells of the basilar papilla (Miller, 1980; Manley, 2000). Sensory hair cells of the basilar papilla are innervated by the auditory nerve, which projects to two nuclei in the dorsal medulla, the nucleus magnocellularis (NM) and the nucleus angularis (NA). NM projects to the second order nucleus laminaris (NL), which in turn projects to the superior olive (SO), to the lemniscal nuclei and to the central nucleus of the auditory midbrain (see Fig. 4). Nucleus angularis projects to the superior olive, to the lemniscal nuclei and to the central nucleus of the auditory midbrain. The lemniscal nuclei project to midbrain, thalamic, and forebrain targets. There have been many independent developments superimposed upon this conserved pattern, the details of which have been recently reviewed (Grothe et al., 2004; Carr & Edds-Walton, 2008).
2 Anurans
Of the three orders of amphibians—anurans, urodeles, and caecilians—only the anurans have developed a tympanic ear, which correlates with a comparatively extended frequency range and sensitivity of hearing (review in Christensen-Dalsgaard & Carr, 2008). Also, almost all anurans use vocal communication, and in many species the female’s identification of the conspecific call and localization of calling males is a prerequisite for successful reproduction (review in Kelley, 2004). Behavioral experiments have exploited the robust phonotaxic behavior of gravid females toward the mating call (review in Kelley, 2004). Because non-communication hearing has proved difficult to test behaviorally in anurans, understanding of anuran hearing is somewhat biased toward communication signals (review in Wilczynski & Ryan, 2010). From physiological studies it is clear, however, that the frog auditory system responds to a variety of non-communication sounds, and it is likely that anurans use their hearing for universal purposes, such as predator avoidance or general information about the surroundings (auditory scene analysis) (review in Wilczynski & Ryan, 2010). The present review describes the auditory pathway of anurans and then concentrates on the two aspects of anuran hearing for which there are behavioral data, that is, sound localization and communication.
2.1 The Anuran Auditory Pathway
Sound can enter the anuran ear by a variety of pathways. The most obvious is via the large tympana situated on the surface of the skull. The tympanum is usually rather undifferentiated skin, but in some species, notably the ultrasonic-sensitive concave-eared torrent frog (Odorrana tormota: Feng et al., 2006) and hole-in-the-head frog (Huia cavitympanum: Arch et al., 2011), it is very thin and transparent. Also, in these species the eardrum is recessed, which shortens the middle ear bone and reduces its mass, improving the high-frequency response (chapter by Narins and Mann, this volume). The eardrum is connected to the middle ear bone, the columella (homologous to the mammalian stapes) by a cartilaginous extracolumella. The extracolumella creates a movable joint in the anuran middle ear apparatus and may improve the sensitivity of the eardrum. Vibrations of the columella are transmitted to the inner ear via the oval window. Other pathways for sound are through the lungs and middle ear cavity, through the mouth cavity and through extratympanic pathways (review in Christensen-Dalsgaard, 2005).
Importantly for directional hearing, the two tympana are coupled together and to the lungs through the wide Eustachian tubes and mouth cavity. The coupling is not as strong as in lizards, but it does generate an effective directional difference of up to 10 dB (reviews in (Christensen-Dalsgaard, 2005, 2009) with a strongly lateralized directivity pattern. Part of the low-frequency (below 400 Hz) directionality, resulting in a figure-eight pattern with reduced responses from frontal and caudal directions, may be generated by extratympanic pathways (Jørgensen & Christensen-Dalsgaard, 1997a,b) (Fig. 3). It should be noted that a number of frog species, the so-called earless frogs, have secondarily lost part of the middle ear apparatus (Jaslow et al., 1988). Most of these species communicate by sound and, therefore, must hear solely through extratympanic pathways.
2.2 Processing of Directional Information
The first-order and olivary nuclei project predominantly to the contralateral midbrain torus, where most of the cells show directional responses, such as direction-dependent firing rates or latencies, with free field stimulation (Feng & Shofner, 1981). The directional information apparently is distributed over most of the torus (principal, magnocellular, and laminar nuclei) (Fig. 3) and shows either strongly lateralized or figure-eight shaped (low responses from frontal and caudal directions), irrespective of frequency. With closed-field dichotic stimulation in grass frog (Rana temporaria) almost all torus units were binaural (Melssen et al., 1990). Most response types were excitatory–inhibitory (EI) with best frequencies uniformly distributed between 100 and 3000 Hz and most sensitive to interaural level differences from –4 to 4 dB. Forty percent of the units showed intensity-invariant responses, which again reflects additional processing by the central nervous system. With free-field stimulation, tuning curves of single neurons in the torus varied systematically with sound direction (Gooler et al., 1996), and the tuning curves were broader with contralateral than with ipsilateral stimulation. This effect is probably caused by neural interactions, especially ipsilateral inhibition (Zhang et al., 1999). However, it should be noted also that directional responses are already present in the auditory nerve. Although most of these studies used interaural level difference (ILD) stimuli, at the level of the midbrain there is no evidence for separate processing of interaural time differences (ITDs). Also, there is no evidence for a spatial map in the torus. Rather, it appears that the torus computes lateralized differences mediated by hemispheric inhibition.
The processing of directional information in the frogs probably differs from the processing in mammals and birds. There is no indication of a separate ITD pathway in the frogs. Binaural neurons are present in the first-order dorsal medullary nucleus and generally respond to specific combinations of ITD and interaural level differences when stimulated dichotically (Christensen-Dalsgaard & Kanneworff, 2005). Binaural responses are mainly excitatory–inhibitory, although both ipsi- and contralateral inputs are excitatory when presented monaurally. With a pressure difference receiver ear a specific combination of ITD and interaural level differences corresponds to a specific location cone in space, and with free-field stimulation the responses of the neurons seem to be a simple sharpening of the directional response (Christensen-Dalsgaard & Kanneworff, 2005) (Fig. 3). For the high-frequency information, excitatory–inhibitory processing would be mostly based on spike rate because the ability of frog nerve fibers to phase lock is severely degraded above 700 Hz (Hillery & Narins, 1987). Excitatory–inhibitory neurons are also found in the superior olive, the subsequent nucleus in the auditory pathway (Feng & Capranica, 1978). Generally, the response patterns reported in this single study are very similar to the processing in the dorsal medullary nucleus, but more information is obviously needed.
Acoustic circuits in ranid frogs. Sagittal view (rostral is left, dorsal is up) of ascending auditory connections of the torus semicircularis and lower auditory nuclei (green), the torus in yellow, and projections from thalamus and ventral striatum (STv) in blue. Descending projections from the torus are shown in red. The midline dorsolateral nucleus (Ndl), also known as the dorsal medullary nucleus, projects to both the torus and the superior olive (Os). A, anterior thalamic nucleus; C, central thalamic nucleus; Cer, cerebellum; DP, dorsal pallium; Hd, dorsal habenular nucleus; Hv, ventral habenular nucleus; LA, lateral amygdala; LC, locus coeruleus; MA, medial amygdala; MP, medial pallium; MS, medial septum; Ndl, dorsolateral nucleus; NI, isthmal nucleus; P, posterior thalamic nucleus; PA, preoptic area; PT, posterior tuberculum; Ptg, pretectal grey; SC, suprachiasmatic nucleus; Stv, ventral striatum; Tec, optic tectum; Teg, tegmentum; Tl, laminar nucleus of the torus semicircularis; Tm, magnocellular nucleus of the torus semicircularis; Tp, principal nucleus of the torus semicircularis; VH, ventral hypothalamic nucleus; VM, ventromedial thalamic nucleus. Roman numerals are cranial nerves. (From Endepols et al., 2000, used with permission.)
Directional sensitivity in frog auditory nerve and brain stem. Polar plots of single-unit responses from the dorsal medullary nucleus show the response of a representative low-frequency (a) and high-frequency (b) cell. For comparison, the response of grassfrog auditory nerve fibers with similar best frequencies is plotted in the polar plots (dotted lines). Note the sharpened directionality in the dorsal medullary neurons. (From Christensen-Dalsgaard & Kanneworff, 2005, used with permission.)
2.3 Processing of Communication Sounds
Much of the early interest in frog auditory neuroethology was stimulated by the ease of combining behavioral studies of evoked calling and phonotaxis with neurophysiology (Capranica, 1966). The unresponsiveness of frogs to non-communication sounds led to the view that frog hearing is not only specialized for communication sounds, but also selective. The auditory periphery was viewed as a matched filter (Ehret et al., 1983), where nonlinear processes (two-tone suppression) in the inner ear would already quench the responses to other sounds. The matched filter hypothesis is probably too great a simplification. Even if the main selective drive for the evolution of the anuran tympanic ear were sound communication, the tetrapod ancestors still had a sense of hearing, demonstrated in the similar sensitivities of tetrapods to low-frequency sounds, and the very similar sensitivities to low-frequency sounds of frogs irrespective of their calling frequencies. Generally, sounds in the immediate environment are a sign of mechanical disturbances that can be advantageous to pay attention to, even though it seems that the predominant response of frogs to unknown sounds is to keep quiet.
Nevertheless, the auditory pathways of frogs show many clear specializations for communication sounds, which may be caused in large part by sensory exploitation—that females select calls that are maximally audible and localizable (Wilczynski et al., 2001). It is hypothesized that the basilar papilla will usually be tuned to call frequency components in the high-frequency range. Temporal properties of the call are represented in the neural discharges, while inner ear properties such as two-tone suppression and postexcitatory suppression (Christensen-Dalsgaard et al., 1998) likely sharpen the temporal representation. As the information ascends the auditory pathway, the time representation in the periphery is gradually transformed to a rate code (Elliott et al., 2011). Also, the representation of specific features, such as cells responding to certain spectral or temporal properties, emerges, usually in the torus. The processing of temporal parameters is by cells selective for specific amplitude modulation rates (Rose & Capranica, 1985) or pulse rates (Elliott et al., 2011). Also, in the Californian tree frog (Hyla regilla), in which the female frogs are selective for a certain number of pulses in the call, cells in the torus respond selectively by counting the pulse intervals (Edwards et al., 2002).
It is evident from these studies that the complexity of the neural responses increases from the periphery to the central nervous system. However, it is unlikely that the processing is a simple hierarchy leading to more and more complex cells (“mating call detectors”) in a well-defined region of torus or pallium, and the search for such complex neurons has so far proved elusive. There is instead evidence that processing is distributed over a large part of the torus, as suggested by the finding that the latency for antiphonal calling in the genus Bombina is too short to involve forebrain structures (Walkowiak, 1992), so all processing of this type of call takes place at or below the torus. Because most toral cells have relatively broad specificities, this suggests that sound stimuli are encoded in the distributed activity of many neurons. This hypothesis has not yet been corroborated by simultaneous recordings from many cells in torus, but a recent study of egr-1 early gene expression (correlated with recent synaptic activity) in the torus of the tungara frog (Physalaemus pustulosus: Hoke et al., 2004) shows a highly distributed activity pattern in response to stimulation with different conspecific call types. The activity cannot be explained by any simple acoustic parameters, and the collected activity of four toral subnuclei is the best predictor of the call type. Whether this distributed activity directly communicates with premotor centers, or there are additional decision-making neurons or groups of neurons, is an exciting question to address in the future.
3 Lizards
Lizard ears are highly directional, with middle ears connected through the mouth cavity (Christensen-Dalsgaard & Manley, 2008; chapter by Manley, Kraus, Köppl, & Sienknecht, this volume) (Fig. 3a). This connection enhances the directionality of the ear by allowing sound access to both sides of each tympanic membrane. The acoustically coupled ears create directional responses from the tympanum (Christensen-Dalsgaard, 2005; Christensen-Dalsgaard & Manley, 2008). Thus, all neurons in the central auditory system should show directional responses, with the possible exception of very low best frequency (below 300 Hz) responses (Christensen-Dalsgaard et al., 2011). In addition, lizards have evolved micromechanical hair cell tuning (Eatock et al., 1981), permitting emergence of sensitive high-frequency hearing in a specialized region of the papilla (Manley, 2002). Thus all lizard auditory responses should be directional, and should include responses to high frequency stimulation.
3.1 Ascending Auditory Pathways
As discussed in Section 1, major differences among central auditory structures appear seldom; these differences are mostly found in the hindbrain and forebrain, with hindbrain changes driven by the development of new end organs in the auditory periphery (Wilczynski & Capranica, 1984). The formation of a new division of the cochlear nucleus angularis (NA) in lizards coincident with the development of a new population of high-frequency hair cells (Szpir et al., 1990) is an example of a central change that appears to result from changes in the periphery.
Lizard inner ears are highly specialized, with sensitive high-frequency hearing originating from a specialized region of the papilla (Manley, 2002). Projections from the high and low best frequency regions of the papilla form parallel low and high best frequency projections into the nucleus magnocellularis (NM) and the NA (Szpir et al., 1995). Data from tokay geckos (Gekko gecko: Tang et al., 2012) and monitor lizards (Barbas-Henry & Lohman, 1988) show similar patterns of auditory nerve fiber projections, with distinct projections of low and high best frequency fibers to NM and NA. Despite the emergence of these parallel channels, there are otherwise few differences between lizards and other sauropsids in the organization of the auditory brain stem circuits, suggesting homology of the brain stem nuclei (Tang et al., 2012).
3.1.1 First-Order Nuclei and the Nucleus Laminaris
In birds, the auditory nerve tends to form large axosomatic synapses in nucleus magnocellularis, associated with phase locking and the preservation of temporal information, while the projection to nucleus angularis generally forms bouton terminals (Carr & Boudreau, 1991; Ryugo & Parks, 2003). Recent work in tokay geckos showed that auditory nerve terminals in NM form larger boutons than those in NA. This is also the case in the alligator lizard, where the fibers terminating in medial NM can form axosomatic endings that resemble mammalian and avian endbulbs of Held (Szpir et al., 1990). NM neurons have large ovoid cell bodies, whereas NA contains more heterogenous cell types (Szpir et al., 1995).
NM projects bilaterally to NL in geckos (Tang et al., 2012) (Fig. 5d). Like birds, lizards have an NL, composed of a lamina of bitufted cells that partially overlaps NM (Yan et al., 2010). A clear NL had not been previously observed in other lizard species, but controversy over its presence or absence appears to depend on its correct identification using modern anatomical techniques (Yan et al., 2010). By means of modern techniques, NL is evident in geckos and consists of a distinct population of bitufted calretinin-immunoreactive neurons below the NM. NL receives projections from both ipsilateral and contralateral NM, such that the ipsilateral projection innervates the dorsal NL neuropil, while the contralateral projection crosses the midline and innervates the ventral dendrites of NL neurons. This connection pattern is similar to that found in birds, in which NL forms a circuit for computing interaural time difference (Young & Rubel, 1983; Carr & Konishi, 1990; Krützfeldt et al., 2010). Because lizard ears are coupled, all lizard auditory responses should be directional (Christensen-Dalsgaard et al., 2011), begging the question of how NL responds to bilateral inputs. Binaural comparisons are still necessary because monaural directional responses are ambiguous with respect to level and location. Lateralized responses could be generated by rate-based binaural comparisons, similar to the rate-based comparisons in two lateralized channels proposed for the gerbil and guinea pig by McAlpine et al. (2001) and for the cat auditory cortex by Stecker et al. (2005). In the lizard, however, these responses could be generated as early as the lower brain stem. Binaural excitatory–inhibitory comparisons can produce an effective steering toward the sound source (Christensen-Dalsgaard et al., 2011).
In geckos, NL projects to the contralateral torus semicircularis, and to the contralateral ventral SO (Tang et al., 2012). NL projects to ipsilateral SO, sends a major projection to the contralateral ventral SO, and projects to torus semicircularis (Fig. 4b). The SO projects to the contralateral ventral SO, which projects back to the ipsilateral NM, NL, and NA. These results suggest homologous patterns of auditory connections in lizards and archosaurs, and also different processing of low- and high-frequency information in the brain stem.
The evolution of specialized high best frequency region(s) of the lizard papilla may have driven the development of parallel low- and high-frequency streams in the brain stem. In ancestral atympanate tetrapods, low-frequency sound may have been processed by non-tympanic mechanisms like those in extant amphibians. The subsequent emergence of tympanic hearing would have led to increased sensitivity to higher frequency sounds and potentially to changes in the central auditory processing in all the tympanate vertebrates. The unitary nature of the auditory experience and the need for adequate auditory steering should, however, have led to mechanisms to link high and low best frequency responses to form single auditory objects by convergence of high- and low-frequency streams in higher stations of the auditory pathway.
3.1.2 Midbrain
The torus semicircularis is the homolog of the inferior colliculus in birds and mammals and is located caudal and ventral to the optic tectum. In lizards, the torus is composed of an auditory central nucleus, surrounded by laminar and superficial nuclei (Díaz et al., 2000). The central nucleus is the recipient of lemniscal fibers and projects to the auditory nucleus of the thalamus (Pritz, 1974; Foster & Hall, 1978; ten Donkelaar et al., 1987). The reported auditory responses in previous studies were probably recorded in the central nucleus (Kennedy, 1974; Kennedy & Browner, 1981; Manley, 1981).
The central nucleus is differentiated from the surrounding periventricular regions by distinct patterns of calcium binding protein expression (Yan et al., 2010). Immunohistochemical studies revealed three subdivisions, a lateral NL and NA recipient subdivision with large calretinin positive fibers and terminals, and a second ventral division delineated by parvalbumin immunoreactive neuropil, which appears to receive input from the olivary and lemniscal nuclei (Tang, unpublished observations). The largest division of the central nucleus is dorsomedial, and it does not appear to receive ascending auditory inputs. It is characterized by calbindin immunoreactivity (Yan et al., 2010).
Tract tracing studies, combined with electrophysiological experiments, are needed to determine whether the gecko torus, like the torus and inferior colliculus in other vertebrates, has divisions based on different afferent inputs (review in Covey & Carr, 2004). The data are consistent with the following hypothesis: there is a restricted lateral distribution of calretinin immunoreactive terminals in the central nucleus that is the recipient zone for input from NA and NL, while a ventral division receives projections from the olivary and lemniscal nuclei. Thus it appears that the central nucleus of the torus is subdivided on the basis of afferent inputs (Yan et al., 2010). This organization resembles the barn owl inferior colliculus, in which input from NL to the central nucleus core abuts an input region of a similar best frequency from the lemniscal nuclei and NA into the central nucleus shell (Takahashi & Konishi, 1988a,b; Wagner et al., 2002). Similar divisions characterize the chicken inferior colliculus (Wang & Karten, 2010).
3.2 Localization and Coupled Ears
Lizards have highly directional ears. The directionality is caused by strong acoustical coupling of the eardrums due to almost unattenuated interaural transmission of sound from the contralateral ear (Christensen-Dalsgaard & Manley, 2005, 2008; chapter by Manley, Kraus, Köppl, & Sienknecht, this volume). The coupled ears in geckos produce directionally sensitive responses in the auditory nerve (Christensen-Dalsgaard et al., 2011) that, in many respects, resemble computed binaural responses from the neural circuits in the avian NL and the mammalian SO nuclei (reviews in Grothe et al., 2010; Ashida & Carr, 2011) (Figs. 4 and 5). An important difference between gecko auditory nerve and the binaural responses recorded in birds and mammals is that gecko responses reflect the interaction of ipsilateral and contralateral inputs on the motion of the eardrum and therefore simultaneously encode interaural time and level differences. In free-field stimulation, auditory nerve responses simply reflect the strong directionality of the eardrum (Christensen-Dalsgaard & Carr, 2008).
Most or all neurons in the lizard central auditory pathway should be directional (Fig. 4c). This prediction is supported by results from free-field stimulation of the torus semicircularis of the tokay gecko (Manley, 1981), in which toral units exhibited directivity with activity almost completely suppressed at ipsilateral angles. The embedded directionality of all auditory responses begs the question of the function and origins of the NM–NL circuit, which resembles the circuit found in other reptiles. The most plausible role for NL is for binaural comparisons. These are still necessary, as any monaural directional response is ambiguous with respect to level and location. Further, the directional response of the eardrum is weaker at low frequencies, so neural processing of low-frequency information by the NM–NL circuit might be important. Eardrum directionality is strongly asymmetric across the midline, so it is possible that lateralized responses could be generated by rate-based binaural comparisons. These would be similar to the rate based comparisons in two lateralized channels proposed for the gerbil and guinea pig (McAlpine et al., 2001) and for the cat auditory cortex (Stecker et al., 2005).
Comparisons between geckos, birds and mammals may be instructive. In birds and crocodilians, ITD processing is mediated by a circuit in the NL, consistent with the Jeffress model, which assumes arrays of coincidence-detector neurons that respond maximally when phase-locked inputs converge (Carr & Konishi, 1990; Overholt et al., 1992; Köppl & Carr, 2008). In mammals, sound source location may instead be computed from the overall discharge rate within the broadly tuned ITD channel on one side of the brain, provided that comparisons with the other ear allow resolution of ambiguity (Lesica et al., 2010). Thus birds use delay lines to map sound location across an array or map of neurons, whereas mammals have no such requirement. The nature of delays in mammals appears multifaceted, whereas in geckos, a fixed acoustic delay across the mouth cancels tympanic motion when it exactly compensates for the ITD presented through earphones or when the delay across the mouth exactly compensates for the sound location in free field. Thus, there should be no range of ITDs in geckos and other lizards (Fig. 5d). Instead, there should be broadly tuned ITD channels on each side of the brain.
Pressure difference receiver ear, and connections of the cochlear nuclei and superior olive complex in gecko. (a) Sound stimulates the tympanum from both the exterior and interior of the head by traveling though the mouth cavity, resulting in a virtually expanded head. View through a Gecko head at the level of the caudal medulla. This section contains the tympanic membrane, a portion of the extracolumella (arrow), the external ear opening, open middle ear, and the buccal cavity. (From Christensen-Dalsgaard et al., 2011, used with permission.) (b) NA innervates the ipsilateral superior olive (SO), the contralateral ventral superior olive (SOv), and the contralateral torus semicircularis (TS). The olivary nuclei both project back to the ipsilateral first-order nuclei (dashed lines). (c) ITD-sensitive recordings from the gecko auditory nerve at 3 interaural level differences (±4 and 0 dB). (Data from Christensen-Dalsgaard et al., 2011.) (d) NM projects to the dorsal neuropil of the ipsilateral NL and across the midline to the ventral neuropil of the contralateral NL, while NL projects to the ipsilateral SO and to the auditory midbrain, through a fiber bundle that descended to run ventral to the contralateral SOv (gray line). SOv projects back to the ipsilateral NM and NL (dashed line). (Data in B, D from Tang et al., 2012.)
4 Snakes
Snakes are lepidosaurs that lack both an outer ear and a tympanic middle ear, which in most tetrapods provides impedance matching between the air and the inner ear and hence underlies sensitive hearing of airborne sound. In snakes, and in some earless lizards, the columella instead connects to the quadrate, which should confer acute sensitivity to substrate vibrations (review in Young, 2003). This turns out to be the case; Christensen and colleagues recently showed that detection of cranial vibrations, induced either by airborne sound or by substrate vibration, underlies the inner ear responses of the royal python (Python regius: Christensen et al., 2011). Thus, pythons, and possibly all snakes, have lost effective pressure hearing. Their acute vibration sensitivity may instead be used for communication and detection of predators and prey.
Unlike other lepidosaurs, snake ears are not coupled, because snakes do not have tympanic hearing. Nevertheless, effective coupling may occur via the columella’s connection to the quadrate. The columellae connect each oval window to the lower jaw, which can rest on the substrate. Prey movements can generate low propagation velocity Rayleigh waves that can vibrate each quadrate (Friedel et al., 2008). Thus, stereo responses to incoming vibrations and sound source localization via coupled quadrates are possible (Friedel et al., 2008).
4.1 Ascending Auditory Pathways
The loss of pressure hearing in snakes might have led to changes in the central circuitry. Miller described two cochlear nuclei, in the pattern of the other sauropsids (Miller, 1980), and used degeneration techniques to show that the auditory branch of the VIIIth nerve projected to NA and NM, whereas NM, in turn, projected bilaterally to NL. Other authors found no NL (Weston, 1936) or only one cochlear nucleus (Holmes, 1903), so analyses of central auditory and vestibular pathways are required to determine which octaval endorgans project to which octaval targets. Are vibration stimuli encoded by NM and NA, or is there cross-talk between central “auditory” and central vestibular targets? Some combination of the first-order nuclei project to torus semicircularis, but there are no detailed studies on this portion of the pathway (Young, 2003). Physiological data from the midbrain show responses to both auditory and vibration stimuli (Hartline & Campbell, 1969; Hartline, 1971a,b). Given the results of Christensen et al. (2011), one assumes that the midbrain auditory responses are derived from vibration stimuli.
5 Turtles and Tortoises (Testudines)
Turtles and tortoises (collectively known as Testudines) are a monophyletic group that occupies a wide range of ecological niches, from the desert to the ocean. With the introduction of molecular techniques to phylogenetics, the traditional position of testudines as the only extant member of parareptilia, as established by morphological studies, has been called into question. Analyses of mitochondrial DNA and nuclear genes suggest testudines are a sister group to the archosaurs (Hedges & Poling, 1999), supported by recent more compete analyses (Shen et al., 2011; Chiari et al., 2012; Crawford et al., 2012).
Testudines hear through stimulation of a cartilaginous tympanic disk, visible through the relatively undifferentiated skin behind the eye. The disk moves via a hinged connection to the bony capsule wall surrounding it (Wever & Vernon, 1956a; Christensen-Dalsgaard et al., 2012). Behind the tympanic disk is the middle ear cavity, which is connected to the mouth by a small Eustachian tube. Laser vibrometry measurements suggest that the air in the middle ear cavity of turtles resonates in the underwater sound field, driving the disk and making the ear more sensitive to sound under water than in air (Christensen-Dalsgaard et al., 2012 and Section 5.2). Testudines do not have acoustically coupled ears, possibly because they are adapted for underwater hearing (Willis et al., 2013). The turtle auditory papilla is small and, like all amniote papillae, organized tonotopically, such that higher frequency sounds excite the hair cells at the base and lower frequencies those at the apex (Crawford & Fettiplace, 1980).
5.1 Ascending Auditory Pathways
Compared to other members of Reptilia, including archosaurs, little is known about how the central nervous system of testudines processes sound. Some studies have been published, but, like the aforementioned data on snakes, they often conflict and have not been carried out using modern technology.
5.1.1 First-Order Nuclei and the Nucleus Laminaris
Testudines lack a high-frequency region of their papilla (review in Manley, 2010). Their auditory nerve projects to the cochlear nuclei, NM and NA (Marbey & Browner, 1985; Sneary, 1988). NM then likely projects to NL, although there is some question about this (Miller & Kasahara, 1979) because of the difficulty in distinguishing NM from NL. NL appears to grade into the NM at its caudal end, and it has been suggested that NL developed from NM (Glatt, 1975a,b). This hypothesis is supported by studies in birds that show that NM and NL are derived from partially overlapping rhombomeres (Marin & Puelles, 1995; Cramer et al., 2000). A common origin for NM and NL may also explain the presence of some auditory nerve input to NL (Barbas-Henry & Lohman, 1988; Carr & Soares, 2002).
In testudines, NL forms a cluster of neurons beneath NM (Glatt, 1975b; Willis et al., 2011). It has been suggested that the organization of NL in testudines represents the primitive, all low-frequency condition. The reason for describing their NL as primitive is that it does not have a monolayer arrangement of its neurons, although they do have the typical bitufted appearance of NL neurons in archosaurs and lizards (Willis et al., 2011). In the evolutionary line leading to the archosaurs, NL becomes larger and attains a monolayer structure, probably correlated with an extension of NM’s and thus also NL’s frequency range. Observations on crocodilians (Glatt, 1975a; Carr & Soares, 2002), as well as birds (Winter & Schwartzkopff, 1961; Köppl & Carr, 1997), show that the caudolateral low-frequency end of the archosaur NL also has no clear monolayer structure, and is closely associated with NM.
Testudine NA is fairly round and lies more dorsal and rostral in the brain stem (Miller & Kasahara, 1979). NM runs in a column rostrocaudally and lies ventral and caudal to NA, and consists of densely packed medium to large-sized round cells. NL is smaller and ventral to NM. The cells of NL are loosely distributed (Miller & Kasahara, 1979). The cells of NM, NA and NL react to calcium-binding proteins and cytochrome oxidase (Belekhova et al., 2008). This pattern of immunoreactivity is similar to that in geckos (Yan et al., 2010). Interestingly, NA and NM are larger in pond turtles than in tortoises (Belekhova et al., 2008). This difference should be investigated further, particularly as it pertains to neural processing of sound.
5.1.2 Midbrain
Beyond the hindbrain anatomy, other studies have focused on the midbrain, specifically the torus semicircularis (Belekhova et al., 1985). They also defined an SO with a dorsal and ventral portion, as found in lizards (Ariëns Kappers et al., 1936). Retrograde labeling was used to show that the torus receives input from the contralateral first-order nuclei, the ipsilateral SO, the dorsal and ventral nuclei of the lateral lemniscus, and the contralateral torus. This is the same pattern seen in other reptiles. The torus may project back to the first order nuclei, the ipsilateral SO, the lateral lemniscus, and the contralateral torus semicircularis (Belekhova et al., 1985).
Testudines, lizards, birds, and anuran amphibians also all display distinct distribution patterns of calcium binding protein expression in the torus, with a mostly complementary pattern of calretinin and calbindin in the African clawed frog (Xenopus laevis: Morona & González, 2009) and a mostly complementary pattern of calretinin and parvalbumin in turtles (Belekhova et al., 2002). Parvalbumin predominates in a restricted core region of the central nucleus, with calretinin and calbindin in the surrounding peripheral areas of the central nucleus. These complementary patterns of expression in amphibians and reptiles are similar to the patterns of calretinin and acetylcholinesterase expression observed in the core and shell regions of the central nucleus of the barn owl inferior colliculus (Takahashi & Konishi, 1988b; Adolphs, 1993). In the turtle, midbrain there is also a core-and-belt organization: a core that is a “relay” and a belt that has both auditory and somatic inputs (Belekhova et al., 2008, 2010 and Section 3).
Testudine torus neurons of the central nucleus are mostly small to medium in size and vary in shape (Belekohova et al., 2010). They have reciprocal connections with the contralateral intercollicular nucleus. The core and belt regions generally have different chemical characteristics and receive inputs from and send projections to different targets (Belekhova et al., 2010). The central nucleus projects bilaterally to the nucleus reuniens of the dorsal thalamus (Belekhova et al., 1985). These data suggest that the turtle auditory mesencephalon is very similar to that of birds and crocodiles (see Section 6). Determining whether or not these areas are homologous, as suggested by Belekhova et al. (2010), may require additional developmental, molecular, and genetic studies.
5.2 Localization and Specialization
As amphibious animals, many turtle species face the problem of hearing both in air and underwater. Because of different density and sound velocity in the two media, an ear’s impedance-matching mechanisms would be different, and trade-offs are made regarding sensitivity to in-air versus underwater sound (Hetherington, 2008). Further, testudines largely respond to low-frequency sounds (Wever & Vernon, 1956b,c; Patterson, 1966; Ridgway et al., 1969; Bartol et al., 1999). Combined with generally small head widths, this could result in difficulty in localizing sound.
Using laser vibrometry and auditory brain stem responses, Christensen-Dalsgaard and co-workers (2012) investigated the auditory sensitivity of the red-eared slider, an amphibious pond slider (Trachemys scripta elegans), using both sound and vibration stimuli. The laser vibrometry showed peak vibrations at 500–600 Hz (maximal transfer function of 300 μm/s/Pa). The auditory evoked potential audiogram from the same study was V-shaped with a best sensitivity to airborne sound of about 300–500 Hz. Underwater, the ear was about 10 dB less sensitive to sound; in terms of sound pressure level (SPL), however, the hearing thresholds underwater were about 20–30 dB lower than thresholds in air. One possible explanation for turtles’ increased sensitivity to sound under water is that the large middle ear cavity resonates in the underwater sound field. Examining the anatomy of the middle ear cavity and associated auditory structures, Christensen-Dalsgaard et al. (2012) found that the compliant tympanic disk was the critical sound-receiving structure, and attached to a columella/extracolumella that ran through the middle ear cavity.
The organization of the turtle ear resembles that of the aquatic African clawed frog in that both have an air space in the middle ear that can resonate in the underwater sound field, driving a cartilaginous tympanic disk. This similarity suggests similar selection pressures on the development of an effective aquatic ear. The proposed model for middle ear function in turtles and aquatic frogs resembles other examples of air-filled swim bladders coupled to the ear or lateral line system (Webb et al., 2008). In otophysine fish, for example, this complex can increase sound sensitivity by 40 dB or more (Popper & Fay, 2011; chapter by Ladich, this volume).
A recent study examining the morphology and allometry of middle ear cavities across extant and extinct species supports this hypothesis (Willis et al., 2013). This study demonstrated that allometry and morphology are unchanged across testudines. Further, regardless of phylogenetic position or ecological niche, the middle ear cavity has a volume and morphology that improves hearing thresholds under water by resonance. These data lend further support to an aquatic origin for testudines (Willis et al., 2013) and also raise the question of how testudines localize sound. Their low-frequency hearing, combined with relatively small heads and uncoupled ears, should make sound localization by detection of interaural time differences difficult.
6 Crocodilians
Crocodilians share an archosaur common ancestor with the birds, and the central auditory pathways of the two groups are very similar, although the hearing range of crocodilians is lower (up to 2.9 kHz for Caiman) (Manley, 1970a; Higgs et al., 2002). Like birds, crocodilian middle ears are coupled via sinuses (Witmer & Ridgely, 2009; Bierman et al., 2011). The inner ear is large, with a long basilar membrane and unidirectional population of hair cells covered by a tectorial membrane (Düring et al., 1974; Wever, 1978). Auditory nerve units are relatively sharply tuned, and phase-lock to frequencies up to 1.5 kHz (Manley, 1970a; Smolders & Klinke, 1986).
6.1 Ascending Auditory Pathways
The auditory nerve projects to NA and NM (Leake, 1974). NA lies anterior to the root of the auditory nerve and is composed of large and small ovoid cells, whereas NM contains characteristic large round principal cells and is larger than NA (Leake, 1974). NM has lateral and medial divisions, with the caudal part of the medial division capped by a small-celled component (Glatt, 1975a,b). There is a similar small-celled region of NM of the bird that receives low best frequency auditory nerve fibers (Köppl, 1994; Köppl & Carr, 1997).
Crocodilian cochlear nuclei are tonotopically organized in a similar fashion to birds (Konishi, 1970; Manley, 1970b). Recordings from the cochlear nucleus in the caiman produced primary-like responses that were very similar to those in the some lizards and all birds (Manley, 1970a, 1974), except that, curiously, one of the tonotopic axes is reversed compared to birds. The crocodilians have a well-developed NL, which forms a sheet of bipolar spindle-shaped cells that is very similar to that seen in the basal land birds (Carr, 1993) (Fig. 5a, b). NA and NL project bilaterally to the torus, as is also the case in other sauropsids.
ITD circuit and coding strategies across taxa. (a) Chicken’s ITD coding circuit. (Left) Schematic drawing of the chicken’s brain stem. Axons from the ipsilateral NM enter NL dorsally, while those from contralateral NM enter ventrally. NL neurons are aligned in a thin flat layer. (Center) Jeffress-type organization of the chicken’s NM-NL circuit. Axonal conduction times lead to a place map in NL. Neurons near the lateral border of NL (marked as C) response maximally to sounds coming from the far contralateral side, and cells located close to the medial edges of NL (marked as F) fires maximally to sounds originating from in front of the animal’s head. (Right) Example ITD-response curves of NL cells tuned at 1 kHz. As stated previously, the peak position of the tuning curve depends on the location of the neuron in the place map. Positive ITD values mean contralateral ear leading (i.e., sound arrives earlier at the contralateral ear than at the ipsilateral ear). (b) Owl’s ITD coding circuit. (Left) Schematic drawing of the owl’s brain stem. Similar to the chicken brainstem, axons from the ipsilateral NM enter NL dorsally, while those from contralateral NM enter ventrally. Owl NL neurons, however, are not aligned in a layered structure, but are distributed sparsely throughout the nucleus. (Center) Multiple Jeffress-type place maps of the owl’s NM-NL circuit. Gradual changes in axonal conduction times along the dorsoventral dimension result in multiple place maps of NL cells. Neurons near the dorsal border of NL (marked as C) response maximally to sounds coming from the far contralateral side, and cells located close to the ventral edges of NL (marked as F) fires maximally to sounds originating from in front of the animal’s head. (Right) Example ITD-response curves of NL cells tuned at 5 kHz. As in chickens’ place map, the peak position of the tuning curve depends on the location of the neuron in the place map. (c) Gerbil’s ITD coding circuit. (Left) Schematic drawing of the gerbil’s brainstem. Spherical bushy cells in the VCN provide excitatory inputs to the MSO, while LNTB and MNTB neurons, which receive outputs of the globular bushy cells in the ipsi- and contralateral VCN, respectively, send glycinergic inhibitory inputs to MSO. (Center) Schematic picture of a gerbil MSO neuron. The principal neuron of the MSO has bipolar dendrites segregating ipsi- and contralateral excitatory inputs from the VCN. Inhibitory inputs from LNTB and MNTB are confined to the cell body region. (Right) Example ITD-response curves of MSO cells tuned at 1 kHz. In contrast to chicken’s NL cells, the tuning curves of MSO neurons are very similar. Peak positions of the tuning curves can lie out of the physiological ITD range (i.e., ITDs encountered naturally) shown by the shaded area. (d) Gecko’s ITD coding. (Left) Schematic drawing of the gecko’s head. The inner ears of the gecko are interconnected through the mouth cavity. (Center) Gecko’s ear as a pressure gradient receiver. Sound wave arriving at one ear travels through the mouth cavity to reach the tympanic membrane (eardrum) of the other ear, resulting in binaural sound interactions. The motion amplitude of the eardrum changes with the phase difference between the two sounds from inside and outside the ear. (Right) Example ITD-response curves of auditory nerves tuned at 2 kHz. ITD-dependent changes in the motion amplitude of the tympanic membrane results in the spike rate modulation of the auditory nerve in an ITD-dependent manner. Note that the trough of the ITD-response curve at around 200–250 ms corresponds to the conduction delay of sound through the mouth cavity. AN, auditory nerve; NA, nucleus angularis; NM, nucleus magnocellularis; NL, nucleus laminaris; VCN, ventral cochlear nucleus; LNTB, lateral nucleus of the trapezoid body; MNTB, medial nucleus of the trapezoid body; MSO, medial superior olive. (From Ashida & Carr, 2011, used with permission.)
6.2 Localization
Crocodilians are successful predators that hunt on both land and in water. Experimental behavioral evidence of sound localization is lacking, but most crocodilians are nocturnal hunters, and can produce a loud roaring call (Todd, 2007). Further, females can localize the contact calls made by their young (Hunt & Watanabe, 1982; Passek & Gillingham, 1999, chapter by Young, Tang, and Mathevon, this volume), so sound source localization is assumed to be behaviorally relevant to this group.
Crocodilians have well-developed neural circuits for encoding ITD (Carr et al., 2009). Both avian and crocodilian auditory circuits appear to conform to the requirements of the Jeffress model (Jeffress, 1947; Joris et al., 1998; Ashida & Carr, 2011) (Fig. 5a). The auditory nerve and NM phase-lock to sound in birds and crocodilians (Köppl, 1997), while NM target neurons in NL act as coincidence detectors for both tones and noise. Internal delays, equal and opposite to interaural delays, characterize barn owls (Carr & Konishi, 1990; Peña et al., 2001), chickens (Overholt et al., 1992; Funabiki et al., 1998; Köppl and Carr, 2008), and alligators (Carr et al., 2009). Best delays in alligator NL are such that neurons respond maximally to sound sources in the contralateral hemifield. Similarly, response delays to contralateral clicks are longer than to ipsilateral clicks (Wagner, 2005; Köppl & Carr, 2008). Thus the axonal delays from NM appear sufficient to account for the range of observed ITDs in alligators.
An additional feature of the Jeffress model is a systematic representation of ITDs, creating a place code of azimuthal position. There is support for a place code in the barn owl in vivo (Carr & Konishi, 1990) and in the chicken, both in vitro (Overholt et al., 1992) and in vivo (Köppl & Carr, 2008). Data from the alligator also support a place code, in that the distribution of lesions made at a range of best ITDs exhibits a trend from medially located best ITDs near 0 to laterally located best ITDs in the contralateral hemifield. The range of best ITDs was very large, however, with median values of about 450 μs, as compared to about 90 μs in chicken (Köppl & Carr, 2008) and 173 μs in the gerbil (Pecka et al., 2008). These are consistent with increased interaural delays from ears coupled by sinuses (Calford & Piddington, 1988; Bierman et al., 2011).
7 Birds
Birds are a sister group to the crocodilians, and their central auditory pathways are very similar. These similarities may be general archosaur synapomorphies (Clack, 1997, 2002), allowing us to hypothesize that similar pathways characterized the auditory systems of the extinct dinosaurs (Witmer & Ridgely, 2009). Dinosaurs may have been both vocal and sensitive to low-frequency sound, since CT scans of the braincase region of tyrannosaurs show that the cochlea is short (Witmer & Ridgely, 2009). Analyses in living archosaurs of best audiogram frequency versus body mass also suggest that in the larger dinosaurs hearing may have been restricted to low frequencies (below 3 kHz) (Gleich et al., 2005).
For birds, the connections and physiology of the auditory system and avian communication have been recently reviewed (Grothe et al., 2004; Moss & Carr, 2012; see also the chapter by Hahnloser & Ondracek, this volume) and this chapter only summarizes them. This chapter focuses on evidence for the role of coupled ears (Köppl, 2009) (Fig. 6a). Archosaurs have sinuses or cranial air spaces that connect their ears. CT scans of the braincase region of tyrannosaurs and other dinosaurs (theropoda and ankylosauria) reveal the presence of extensive air filled spaces (Witmer & Ridgely, 2009). These sinuses are well developed in birds, which have pneumatized skulls (Fig. 6a). In particular, the rostral tympanic recess is large, with a broad contralateral communication ventral to the brain cavity. This connection is also termed the interaural canal, because it connects the two middle ears (Hill et al., 1980; Coles & Guppy, 1988). This coupling is far weaker in birds than lizards (Klump, 2000), but appears effective at low frequencies, where it leads to larger interaural time and level differences than would be predicted from head size (Calford & Piddington, 1988; Köppl, 2009; Christensen-Dalsgaard, 2011) (Fig. 6b).
Coupled ears in birds. (a) Reconstructed microMRI showing the extent of the middle ear cavity and associated sinuses in the barn owl, Tyto alba (ventral view). Image stacks were constructed in 3-D using Neurolucida (Microbrightfield) by tracing the cavities and measuring their volumes (Gans et al., 2012). The interaural canals on each side had a diameter of about 1.8 mm as they exited the middle ear cavity, and formed large ventrally directed tubes that were united in the expansive rostral tympanic recess. This recess was about 1.5 cm in rostrocaudal extent. These measurements were consistent with those from the great horned owl (Witmer et al., 2008). Yellow: skull, Orange: brain, Green: interaural canals. (b) Interaural delays measured between the cochlear microphonics of a grass owl as a function of stimulus, azimuth, and frequency. Generally, lower frequencies produce larger delays. At higher frequencies (4, 8 kHz) interaural delay is close to the delay based on the path length around a sphere with the same diameter as the head. (Redrawn from Calford & Piddington, 1988.)
7.1 Ascending Auditory Pathways
In birds, as in other reptiles, the auditory nerve projects to the NA and NM (Carr & Boudreau, 1991) (Fig. 5b). NM projects bilaterally to NL (Young & Rubel, 1983), which in turn projects to the SO and the inferior colliculus (Takahashi & Konishi, 1988b). NL computes the time difference between the two ears, while NA receives sound intensity information from the auditory nerve and projects directly to the SO, the dorsal nucleus of the lateral lemniscus and the inferior colliculus (Takahashi & Konishi, 1988b; Euston & Takahashi, 2002). The superior olive provides γ-aminobutyric acid (GABAergic) feedback projections to NA, NM, and NL (Carr et al., 1989; Burger & Rubel, 2008; Wild et al., 2010).
In the owl, NM is the origin of a neural pathway that encodes timing information, while a parallel pathway for encoding sound level originates with NA (Takahashi, 1989) (Fig. 7). Recordings in the chicken cochlear nuclei have found a similar but less clear segregation of function. The similarities between the owl and the chicken suggest that the functional separation of time and level coding may be an emerging feature of the avian auditory system. Separation of time and intensity information should not characterize animals with strongly coupled ears, however, or characterize low best frequency processing in birds, as ITD and ILD co-vary when ears are coupled.
Auditory pathways in the barn owl. (a) Diagram of the dorsal brain stem shows the projection from the eighth nerve to the nucleus magnocellularis (NM), and from NM to the nucleus laminaris. Neurons and eighth nerve axon terminals in NM, and NL neurons, are all shown larger than life. Box shows area enlarged in B. (b) Low best frequency neurons in NL, scale bar 100 μm. (From Köppl & Carr, 1997, used with permission.)
The pathway for coding sound level begins with the cochlear NA, which responds to changing sound level over about a 30-dB range (review in Carr & Code, 2000). Each NA projects to contralateral dorsal nucleus of the lateral lemniscus (Krützfeldt et al., 2010; Wild et al., 2010). In barn owls, processing of level differences between the two ears begins in the lemniscal nuclei (review in Carr & Code, 2000). These level differences are produced by the shadowing effect of the head when a sound source originates from off the midline (Klump, 2000), and at low frequencies are intensified by coupling through the interaural canal (Larsen et al., 2006). Neurons of the dorsal nucleus of the lateral lemniscus do not encode elevation unambiguously, and may be described as sensitive to interaural level difference, but not selective because they are not immune to changes in sound level. The encoding of elevation improves in the auditory midbrain (reviews in Tollin, 2008; Grothe et al., 2010).
7.2 Localization
The coupling between the two ears is most effective at low frequencies (Hill et al., 1980; Coles & Guppy, 1988; Hyson, 2005; Köppl, 2009) (Figs. 6 and 8). Physiological recordings in brain stem and cochlear microphonics reveal much larger interaural time differences for low frequency sound stimulation than would be predicted from head size (Figs. 6 and 8). For sounds above about 2–3 kHz, however, the interaural pathway acts as an acoustical low-pass filter, and sound is attenuated too much for a pressure gradient mechanism to be effective (Moiseff & Konishi, 1981; Calford & Piddington, 1988; reviews in Klump, 2000; Christensen-Dalsgaard, 2011). Cochlea microphonic and neurophysiological recordings from a number of birds reveal interaural delays at high frequencies that are close to those expected from path length around the head, while delays measured at low frequencies are more than three times this expectation (Calford & Piddington, 1988; Köppl, 2009; Wagner et al., 2009) (Fig. 6b). These large delays provide even small birds with a useful range of interaural time differences for localization in azimuth. For best frequencies above about 1.5 kHz, however, interaural delays are a function of head size (Section 6.2) (Fig. 8a).
ITD responses to low best frequency sounds in birds. (a) Distribution of ITD peaks as a function of best frequency in tone responses in the central nucleus of the inferior colliculus of the barn owl. This figure focuses on low best frequencies, so only shows data from neurons with best frequencies of up to 3 KHz. The two lines indicate the ITD at 0.5 cycles, also known as the pi limit. For the y-axis, positive values indicate right ear leading, negative values left ear leading. Best ITDs recorded on both sides of the brain were pooled, explaining the symmetrical distribution. (Data from Wagner et al., 2007.) (b) Interaural delay curves plot the response of low best frequency nucleus laminaris neurons against changing ITDs. Note the wide range of ITDs at low best frequencies, consistent with the pressure difference effects observed in interaurally coupled ears. (One unit taken from Carr & Köppl, 2004; two are unpublished data.)
8 Summary and Conclusions
A great deal of the variation in tetrapod auditory systems is peripheral (Wilczynski & Capranica, 1984). Indeed, the tetrapod tympanic ear is a homoplaseous novelty, which has been modified and adapted to various lifestyles over the course of evolution (Manley & Köppl, 1998). In contrast, central processing may be largely conserved. The nuclei not only appear to be homologous (Tang et al., 2012), but also retain similar functions in the different groups. The changes in the central nervous system caused by the emergence of the tympanic ear may include the addition of high-frequency responses in existing nuclei and a requirement for additional binaural processing in those groups that have developed uncoupled or partially uncoupled ears, such as turtles and birds. There are otherwise few differences in the organization of the auditory brain stem circuits of all the groups discussed.
Abbreviations
- CT:
-
Computed tomography
- EI:
-
Excitatory–inhibitory
- ILD:
-
Interaural level difference
- ITD:
-
Interaural time difference
- MRI:
-
Magnetic resonance imaging
- NA:
-
Nucleus angularis
- NL:
-
Nucleus laminaris
- NM:
-
Nucleus magnocellularis
- SO:
-
Superior olive
- SPL:
-
Sound pressure level
- TS:
-
Torus semicircularis
References
Adolphs, R. (1993). Bilateral inhibition generates neuronal responses tuned to interaural level differences in the auditory brainstem of the barn owl. Journal of Neuroscience, 13(9), 3647–3668.
Arch, V. S., Burmeister, S. S., Feng, A. S., Shen, J.-X., & Narins, P. M. (2011). Ultrasound-evoked immediate early gene expression in the brainstem of the Chinese torrent frog, Odorrana tormota. Journal of Comparative Physiology A, 197(6), 667–675.
Ariëns Kappers, C. U., Huber, G. C., & Crosby, E. C. (1936). The comparative anatomy of the nervous system of vertebrates, including man. New York: Macmillan.
Ashida, G., & Carr, C. E. (2011). Sound localization: Jeffress and beyond. Current Opinion in Neurobiology, 21(5), 745–751.
Barbas-Henry, H. A., & Lohman, A. H. (1988). Primary projections and efferent cells of the VIIIth cranial nerve in the monitor lizard, Varanus exanthematicus. Journal of Comparative Neurology, 277(2), 234–249.
Bartol, S. M., Musick, J. A., & Lendhardt, M. L. (1999). Auditory evoked potentials of the loggerhead sea turtle (Caretta caretta). Copeia, 1999(3), 836–840.
Belekhova, M. G., Zharskaja, V. D., Khachunts, A. S., Gaidanenko, G. V., & Tumanova, N. L. (1985). Connections of the mesencephalic, thalamic and telencephalic auditory centers in turtles: Some structural bases for audiosomatic interrelations. Journal für Hirnforschung, 26(2), 127–152.
Belekhova, M. G., Kenigfest-Rio, N. B., Vesslkin, N. P., Rio, J.-P., Reperant, J., & Ward, R. (2002). Evolutionary significance of different neurochemical organisation of the internal and external regions of auditory centres in the reptilian brain: An immunocytochemical and reduced NADPH-diaphorase histochemical study in turtles. Brain Research, 925, 100–106.
Belekhova, M. G., Chudinova, T. V., Kenigfest, N. B., & Krasnoshchekova, E. I. (2008). Distribution of metabolic activity (cytochrome oxidase) and immunoreactivity to calcium-binding proteins in the turtle brainstem auditory nuclei. Journal of Evolutionary Biochemistry and Physiology, 44(3), 354–364.
Belekhova, M. G., Chudinova, T. V., Repérant, J., Ward, R., Jay, B., Vesselkin, N. P., & Kenigfest, N. B. (2010). Core-and-belt organisation of the mesencephalic and forebrain auditory centres in turtles: Expression of calcium-binding proteins and metabolic activity. Brain Research, 1345, 84–102.
Bierman, H. S., Carr, C. E., Brandt, C., Young, B. A., & Christensen-Dalsgaard, J. (2011). Evidence for low-frequency sound localization in the American alligator (Alligator mississippiensis). Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience. http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=8e1b3d78-7471-4315-a0c5-fd54ff3be697&cKey=1a0ea064-1637-4e73-a3fe-3b8b347637df&mKey={8334BE29-8911-4991-8C31-32B32DD5E6C8}
Burger, R. M., & Rubel, E. W (2008). Encoding of interaural timing for binaural hearing. In A. I. Basbaum, G. M. Shepherd, & G. Westheimer (Eds.), The senses: A comprehensive reference (Vol. 3, pp. 613–630). San Diego: Academic Press.
Calford, M. B., & Piddington, R. B. (1988). Avian interaural canal enhances interaural delay. Journal of Comparative Physiology A, 162, 503–510.
Capranica, R. R. (1966). Vocal response of the bullfrog to natural and synthetic mating Calls. Journal of the Acoustical Society of America, 40, 1131–1139.
Carr, C. E. (1993). Processing of temporal information in the brain. Annual Review of Neuroscience, 16, 223–243.
Carr, C. E., & Konishi, M. (1990). A circuit for detection of interaural time differences in the brain stem of the barn owl. Journal of Neuroscience, 10(10), 3227–3246.
Carr, C. E., & Boudreau, R. E. (1991). Central projections of auditory nerve fibers in the barn owl. Journal of Comparative Neurology, 314, 306–318.
Carr, C. E., & Code, R. A. (2000). Anatomy and physiology of the central auditory system of birds and reptiles. In A. N. Popper, R. R. Fay, & R. J. Dooling (Eds.), Comparative hearing: Birds and reptiles. New York: Springer Science+Business Media.
Carr, C. E., & Soares, D. (2002). Evolutionary convergence and the shared computational principles in the auditory system. Brain, Behavior and Evolution, 59, 294–311.
Carr, C. E., & Koppl, C. (2004). Coding interaural time differences at low best frequencies in the barn owl. Journal of Physiology, Paris, 98(1–3), 99–112.
Carr, C. E., & Edds-Walton, P. (2008). Vertebrate auditory pathways. In R. Hoy, P. Dallos, & D. Oertel (Eds.), Handbook of the senses, Vol. 1: Audition. Oxford: Elsevier.
Carr, C. E., Fujita, I., & Konishi, M. (1989). Distribution of GABAergic neurons and terminals in the auditory system of the barn owl. Journal of Comparative Neurology, 286, 190–207.
Carr, C. E., Soares, D., Smolders, J., & Simon, J. Z. (2009). Detection of interaural time differences in the alligator. Journal of Neuroscience, 29(25), 7978–7990.
Chiari, Y., Cahais, V., Galtier, N., & Delsuc, F. (2012). Phylogenomic analyses support the position of turtles as the sister group of birds and crocodiles (Archosauria). BMC Biology, 10(1), 65–79.
Christensen, C. B., Christensen-Dalsgaard, J., Brandt, C., & Madsen, P. T. (2011). Hearing with an atympanic ear: Good vibration and poor sound-pressure detection in the royal python, Python regius. Journal of Experimental Biology, 215(2), 331–342.
Christensen-Dalsgaard, J. (2005). Directional hearing in nonmammalian tetrapods. In R. R. Fay & A. N. Popper (Eds.), Sound source localization. (pp. 67–123). New York: Springer Science+Business Media.
Christensen-Dalsgaard, J. (2009). Amphibian bioacoustics. In S. Kuwano & M. Vorlander (Eds.), Handbook of signal processing in acoustics (pp. 1861–1885). New York: Springer.
Christensen-Dalsgaard, J. (2011). Vertebrate pressure-gradient receivers. Hearing Research, 273(1–2), 37–45.
Christensen-Dalsgaard, J., & Kanneworff, M. (2005). Binaural interaction in the frog dorsal medullary nucleus. Brain Research Bulletin, 66(4–6), 522–525.
Christensen-Dalsgaard, J., & Manley, G. A. (2005). Directionality of the lizard ear. Journal of Experimental Biology, 208(6), 1209–1217.
Christensen-Dalsgaard, J., & Carr, C. E. (2008). Evolution of a sensory novelty: Tympanic ears and the associated neural processing. Brain Research Bulletin, 75(2–4), 365–370.
Christensen-Dalsgaard, J., & Manley, G. A. (2008). Acoustical coupling of lizard eardrums. Journal of the Association for Research in Otolaryngology, 9(4), 407–416.
Christensen-Dalsgaard, J., Jørgensen, M. B., & Kanneworff, M. (1998). Basic response characteristics of auditory nerve fibers in the grassfrong (Rana temporaria). Hearing Research, 119, 155–163.
Christensen-Dalsgaard, J., Tang, Y., & Carr, C. E. (2011). Binaural processing by the gecko auditory periphery. Journal of Neurophysiology, 105(5), 1992–2004.
Christensen-Dalsgaard, J., Brandt, C., Willis, K. L., Christensen, C. B., Ketten, D., Edds-Walton, P., Fay, R. R., Madsen, P. T., & Carr, C. E. (2012). Specialization for underwater hearing by the tympanic middle ear of the turtle, Trachemys scripta elegans. Proceedings of the Royal Society of London B: Biological Sciences, 279(1739), 2816–2824.
Clack, J. A. (1997). The evolution of tetrapod ears and the fossil record. Brain, Behavior and Evolution, 50, 198–212.
Clack, J. A. (2002). Gaining ground: The origin and evolution of tetrapods. Bloomington: Indiana University Press.
Clack, J. A., & Allin, E. (2004). The evolution of single-and multiple-ossicle ears in fishes and tetrapods. In G. A. Manley, A. N. Popper, & R. R. Ray (Eds.), Evolution of the vertebrate auditory system (pp. 128–163). New York: Springer Science+Business Media.
Coles, R. B., & Guppy, A. (1988). Directional hearing in the barn owl (Tyto alba). Journal of Comparative Physiology A, 163(1), 117–133.
Covey, E., & Carr, C. E. (2004). The auditory midbrain in bats and birds. In J. A. Winer & C. E. Schreiner (Eds.), The inferior colliculus. New York: Springer Science+Business Media.
Cramer, K. S., Fraser, S. E., & Rubel, E. W (2000). Embryonic origins of auditory brain-stem nuclei in the chick hindbrain. Developmental Biology, 224(2), 138–151.
Crawford, A. C., & Fettiplace, R. (1980). The frequency selectivity of auditory nerve fibres and hair cells in the cochlea of the turtle. Journal of Physiology, 306, 79–125.
Crawford, N. G., Faircloth, B. C., McCormack, J. E., Brumfield, R. T., Winker, K., & Glenn, T. C. (2012). More than 1000 ultraconserved elements provide evidence that turtles are the sister group of archosaurs. Biology Letters, 8(1), 1–4.
Díaz, C., Yanes, C., Trujillo-Cenóz, C. M., & Puelles, L. (2000). Cytoarchitectonic subdivisions in the subtectal midbrain of the lizard Gallotia galloti. Journal of Neurocytology, 29(8), 569–593.
Düring, M., Karduck, A., & Richter, H.-G. (1974). The fine structure of the inner ear in Caiman crocodilus. Anatomy and Embryology, 145(1), 41–65.
Eatock, R. A., Manley, G. A., & Pawson, L. (1981). Auditory nerve fibre activity in the tokay gecko. Journal of Comparative Physiology A, 142(2), 203–218.
Edwards, C. J., Alder, T. B., & Rose, G. J. (2002). Auditory midbrain neurons that count. Nature Neuroscience, 5(10), 934–936.
Ehret, G., Moffat, A. J., & Capranica, R. R. (1983). Two-tone suppression in auditory nerve fibers of the green treefrog (Hyla cinerea). Journal of the Acoustical Society of America, 73(6), 2093–2095.
Elliott, T. M., Christensen-Dalsgaard, J., & Kelley, D. B. (2011). Temporally selective processing of communication signals by auditory midbrain neurons. Journal of Neurophysiology, 105(4), 1620–1632.
Endepols, H., Walkowiak, W., & Luksch, H. (2000). Chemoarchitecture of the anuran auditory midbrain. Brain Research Reviews, 33(2), 179–198.
Euston, D. R., & Takahashi, T. T. (2002). From spectrum to space: The contribution of level difference cues to spatial receptive fields in the barn owl inferior colliculus. Journal of Neuroscience, 22(1), 284–293.
Feng, A. S., & Capranica, R. R. (1978). Sound localization in anurans. II. Binaural interaction in superior olivary nucleus of the green tree frog (Hyla cinerea). Journal of Neurophysiology, 41(1), 43–54.
Feng, A. S., & Shofner, W. P. (1981). Peripheral basis of sound localization in anurans: Acoustic properties of the frog’s ear. Hearing Research, 5(2–3), 201–216.
Feng, A. S., Narins, P. M., Xu, C.-H., Lin, W.-Y., Yu, Z.-L., Qiu, Q., Xu, Z.-M., & Shen, J.-X. (2006). Ultrasonic communication in frogs. Nature, 440(7082), 333–336.
Foster, R. E., & Hall, W. C. (1978). The organization of central auditory pathways in a reptile, Iguana iguana. Journal of Comparative Neurology, 178(4), 783–831.
Friedel, P., Young, B., & van Hemmen, J. (2008). Auditory localization of ground-borne vibrations in snakes. Physical Review Letters, 100(4), 048701.
Funabiki, K., Koyano, K., & Ohmori, H. (1998). The role of GABAergic inputs for coincidence detection in the neurones of nucleus laminaris of the chick. Journal of Physiology, 508(3), 851–869.
Gans, E., Willis, K. L., Bierman, H. S., & Carr, C. E. (2012). The interaural canal of the barn owl, Tyto alba. Society for Integrative and Comparative Biology Meeting Planner. Charleston, SC: Society for Integrative and Comparative Biology. http://www.sicb.org/meetings/2012/SICB2012AbstractBook.pdf
Glatt, A. F. (1975a). Vergleichend morphologische Untersuchungen am akustischen System einiger ausgewahlter Reptilien. A. Caiman crocodilus. Revue Suisse De Zoologie: Annales De La Société Zoologique Suisse Et Du Museum D'histoire Naturelle De Geneve, 82(2), 257–281.
Glatt, A. F. (1975b). Vergleichend morphologische Untersuchungen am akustischen System einiger ausgewählter Reptilien. B: Sauria, Testudines. Revue Suisse De Zoologie: Annales De La Société Zoologique Suisse Et Du Museum D'histoire Naturelle De Geneve, 82, 469–494.
Gleich, O., Dooling, R. J., & Manley, G. A. (2005). Audiogram, body mass, and basilar papilla length: Correlations in birds and predictions for extinct archosaurs. Naturwissenschaften, 92(12), 595–598.
Gooler, D. M., Xu, J., & Feng, A. S. (1996). Binaural inhibition is important in shaping the free-field frequency selectivity of single neurons in the inferior colliculus. Journal of Neurophysiology, 76(4), 2580–2594.
Grothe, B. (2003). Sensory systems: New roles for synaptic inhibition in sound localization. Nature Reviews Neuroscience, 4(7), 540–550.
Grothe, B., Carr, C. E., Casseday, J. H., Fritzsch, B., & Köppl, C. (2004). The evolution of central pathways and their neural processing patterns. In G. A. Manley, A. N. Popper, & R. R. Fay (Eds.), Evolution of the vertebrate auditory system. New York: Springer Science+Business Media.
Grothe, B., Pecka, M., & McAlpine, D. (2010). Mechanisms of sound localization in mammals. Physiological Reviews, 90(3), 983–1012.
Hartline, P. (1971a). Physiological basis for detection of sound and vibration in snakes. Journal of Experimental Biology, 54, 349–371.
Hartline, P. (1971b). Mid-brain responses of the auditory and somatic vibration systems in snakes. Journal of Experimental Biology, 54, 373–390.
Hartline, P., & Campbell, H. (1969). Auditory and vibratory responses in the midbrains of snakes. Science, 193(3872), 1221–1223.
Hedges, S. B., & Poling, L. L. (1999). A molecular phylogeny of reptiles. Science, 283(5404), 998–1001.
Hetherington, T. (2008). Comparative anatomy and function of hearing in aquatic amphibians, reptiles, and birds. In J. G. M. Thewissen & S. Nummela (Eds.), Sensory evolution on the threshold: Adaptations in secondarily aquatic vertebrates. Berkeley: University of California Press.
Higgs, D. M., Brittan-Powell, E. F., Soares, D., Souza, M. J., Carr, C. E., Dooling, R. J., & Popper, A. N. (2002). Amphibious auditory responses of the American alligator (Alligator mississipiensis). Journal of Comparative Physiology A, 188(3), 217–223.
Hill, K. G., Lewis, D. B., Hutchings, M. E., & Coles, R. B. (1980). Directional hearing in the Japanese quail (Coturnix coturnix japonica). Journal of Experimental Biology, 86, 135–151.
Hillery, C. M., & Narins, P. M. (1987). Frequency and time domain comparison of low-frequency auditory fiber responses in two anuran amphibians. Hearing Research, 25(2–3), 233–248.
Hoke, K. L., Burmeister, S. S., Fernald, R. D., Rand, A. S., Ryan, M. J., & Wilczynski, W. (2004). Functional mapping of the auditory midbrain during mate call reception. Journal of Neuroscience, 24(50), 11264–11272.
Holmes, G. M. (1903). On the comparative anatomy of the nervus acusticus. Transactions of the Royal Irish Academy, 32, 101–144.
Hunt, R. H., & Watanabe, M. E. (1982). Observations on maternal behavior of the American alligator, Alligator mississippiensis. Journal of Herpetology, 16(3), 235–239.
Hyson, R. L. (2005). The analysis of interaural time differences in the chick brain stem. Physiology & Behavior, 86(3), 297–305.
Jaslow, A. P., Hetherington, T. E., & Lombard, R. E. (1988). Structure and function of the amphibian middle ear. In B. Fritzsch, M. J. Ryan, W. Wilczynski, T. E. Hetherington, & W. Walkeoviak (Eds.), The evolution of the amphibian auditory system (pp. 69–92). New York: John Wiley & Sons.
Jeffress, L. A. (1947). A place theory of sound localization. Journal of Comparative and Physiological Psychology, 41(1), 35–39.
Jørgensen, M. B., & Christensen-Dalsgaard, J. (1997a). Directionality of auditory nerve fiber responses to pure tone stimuli in the grassfrog, Rana temporaria. I. Spike rate responses. Journal of Comparative Physiology A, 180, 493–502.
Jørgensen, M. B., & Christensen-Dalsgaard, J. (1997b). Directionality of auditory nerve fiber responses to pure tone stimuli in the grassfrog, Rana temporaria. II. Spike timing. Journal of Comparative Physiology A, 180, 503–511.
Joris, P. X., Smith, P. H., & Yin, T. C. T. (1998). Coincidence detection in the auditory system: 50 Years after Jeffress. Neuron, 21, 1235–1238.
Kelley, D. B. (2004). Vocal communication in frogs. Current Opinion in Neurobiology, 14(6), 751–757.
Kennedy, M. C. (1974). Auditory multiple-unit activity in the midbrain of the Tokay gecko (Gekko gecko, L.). Brain, Behavior and Evolution, 10(1–3), 257–264.
Kennedy, M. C., & Browner, R. H. (1981). The torus semicircularis in a gekkonid lizard. Journal of Morphology, 169(3), 259–274.
Klump, G. M. (2000). Sound localization in birds. In R. J. Dooling, R. R. Fay, & A. N. Popper (Eds.), Comparative hearing: Birds and reptiles (pp. 249–307). New York: Springer Science+Business Media.
Konishi, M. (1970). Comparative neurophysiological studies of hearing and vocalizations in songbirds. Zeitschrift für vergleichende Physiologie, 66, 257–272.
Köppl, C. (1994). Auditory nerve terminals in the cochlear nucleus magnocellularis: Differences between low and high frequencies. Journal of Comparative Neurology, 339, 438–446.
Köppl, C. (1997). Frequency tuning and spontaneous activity on the auditory nerve and cochlear nucleus magnocelluaris of the barn owl Tyto alba. Journal of Neurophysiology, 77, 364–377.
Köppl, C. (2009). Evolution of sound localisation in land vertebrates. Current Biology, 19(15), R635–R639.
Köppl, C., & Carr, C. E. (1997). Low-frequency pathway in the barn owl’s auditory brainstem. Journal of Comparative Neurology, 378, 265–282.
Köppl, C., & Carr, C. E. (2008). Maps of interaural time difference in the chicken’s brainstem nucleus laminaris. Biological Cybernetics, 98(6), 541–559.
Krützfeldt, N. O. E., Logerot, P., Kubke, M. F., & Wild, J. M. (2010). Connections of the auditory brainstem in a songbird, Taeniopygia guttata.II. Projections of nucleus angularis and nucleus laminaris to the superior olive and lateral lemniscal nuclei. Journal of Comparative Neurology, 518(11), 2135–2148.
Larsen, O. N., Dooling, R. J., & Michelsen, A. (2006). The role of pressure difference reception in the directional hearing of budgerigars (Melopsittacus undulatus). Journal of Comparative Physiology A, 192(10), 1063–1072.
Leake, P. A. (1974). Central projections of the statoacoustic nerve in Caiman crocodilus. Brain, Behavior and Evolution, 10(1–3), 170–196.
Lesica, N. A., Lingner, A., & Grothe, B. (2010). Population coding of interaural time differences in gerbils and barn owls. Journal of Neuroscience, 30(35), 11696–11702.
Lyson, T. R., Bever, G. S., Bhullar, B. A. S., Joyce, W. G., & Gauthier, J. A. (2010). Transitional fossils and the origin of turtles. Biology Letters, 6(6), 830–833.
Manley, G. (1970a). Comparative studies of auditory physiology in reptiles. Zeitschrift für vergleichende Physiologie, 363–381.
Manley, G. A. (1970b). Frequency sensitivity of auditory neurons in the Caiman cochlear nucleus. Zeitschrift für vergleichende Physiologie, 66, 251–256.
Manley, G. A. (1974). Activity patterns of neurons in the peripheral auditory system of some reptiles. Brain, Behavior and Evolution, 10(1–3), 244–256.
Manley, G. A. (1981). A review of the auditory physiology of reptiles. Progress in Sensory Physiology, 2, 49–134.
Manley, G. A. (2000). Cochlear mechanisms from a phylogenetic viewpoint. Proceedings of the National Academy of Sciences of the USA, 97(22), 11736–11743.
Manley, G. A. (2002). Evolution of structure and function of the hearing organ of lizards. Journal of Neurobiology, 53(2), 202–211.
Manley, G. A. (2010). An evolutionary perspective on middle ears. Hearing Research, 263, 3–8.
Manley, G. A., & Köppl, C. (1998). Phylogenetic development of the cochlea and its innervation. Current Opinion in Neurobiology, 8(4), 468–474.
Marbey, D., & Browner, R. H. (1985). The reconnection of auditory posterior root fibers in the red-eared turtle, Chrysemys scripta elegans. Hearing Research, 1–4.
Marin, F., & Puelles, L. (1995). Morphological fate of rhombomeres in quail/chick chimeras: A segmental analysis of hindbrain nuclei. European Journal of Neuroscience, 7, 1714–1738.
McAlpine, D., Jiang, D., & Palmer, A. R. (2001). A neural code for low-frequency sound localization in mammals. Nature Neuroscience, 4(4), 396–401.
McCormick, C. A. (1999). Anatomy of central auditory pathways in fish and amphibians. In A. N. Popper & R. R. Fay (Eds.), Comparative hearing: Fish and amphibians (pp. 155–217). New York: Springer-Verlag.
Melssen, W. J., Epping, W. J., & van Stokkum, I. H. (1990). Sensitivity for interaural time and intensity difference of auditory midbrain neurons in the grassfrog. Hearing Research, 47(3), 235–256.
Miller, M. R. (1980). The cochlear nuclei of snakes. Journal of Comparative Neurology, 192(4), 717–7736.
Miller, M. R., & Kasahara, M. (1979). The cochlear nuclei of some turtles. Journal of Comparative Neurology, 185, 221–236.
Moiseff, A., & Konishi, M. (1981). The owl’s interaural pathway is not involved in sound localization. Journal of Comparative Physiology A, 144, 299–304.
Morona, R., & González, A. (2009). Immunohistochemical localization of calbindin-D28k and calretinin in the brainstem of anuran and urodele amphibians. Journal of Comparative Neurology, 515(5), 503–537.
Moss, C. F., & Carr, C. E. (2012). Comparative audtion. In M. Gallagher & R. J. Nelson (Eds.), Handbook of psychology (pp. 115–156). Hoboken: John Wiley & Sons.
Northcutt, R. G., & Kaas, J. H. (1995). The emergence and evolution of mammalian neocortex. Trends in Neurosciences, 18(9), 373–379.
Overholt, E. M., Rubel, E. W., & Hyson, R. L. (1992). A circuit for coding interaural time differences in the chick brainstem. Journal of Neuroscience, 12(5), 1698–1708.
Passek, K. M., & Gillingham, J. C. (1999). Absence of kin discrimination in hatchling American alligators, Alligator mississippiensis. Copeia, 1999(3), 831–835.
Patterson, W. (1966). Hearing in the turtle. Journal of Auditory Research, 6, 453–464.
Pecka, M., Brand, A., Behrend, O., & Grothe, B. (2008). Interaural time difference processing in the mammalian medial superior olive: The role of glycinergic inhibition. Journal of Neuroscience, 28(27), 6914–6925.
Peña, J. L., Viete, S., Funabiki, K., Saberi, K., & Konishi, M. (2001). Cochlear and neural delays for coincidence detection in owls. Journal of Neuroscience, 21(23), 9455–9459.
Popper, A. N., & Fay, R. R. (2011). Rethinking sound detection by fishes. Hearing Research, 273(1–2), 25–36.
Pritz, M. B. (1974). Ascending connections of a thalamic auditory area in a crocodile, Caiman crocodilus. Journal of Comparative Neurology, 153(2), 199–213.
Ridgway, S. H., Wever, E. G., McCormick, J. G., Palin, J., & Anderson, J. H. (1969). Hearing in the giant sea turtle, Chelonia mydas. Proceedings of the National Academy of Sciences of the USA, 64, 884–890.
Rose, G. J., & Capranica, R. R. (1985). Sensitivity to amplitude modulated sounds in the anuran auditory nervous system. Journal of Neurophysiology, 53(2), 446–465.
Ryugo, D. K., & Parks, T. N. (2003). Primary innervation of the avian and mammalian cochlear nucleus. Brain Research Bulletin, 60(5–6), 435–456.
Schnupp, J. W. H., & Carr, C. E. (2009). On hearing with more than one ear: Lessons from evolution. Nature Neuroscience, 12(6), 692–697.
Shen, X. X., Liang, D., Wen, J. Z., & Zhang, P. (2011). Multiple genome alignments facilitate development of NPCL markers: A case study of tetrapod phylogeny focusing on the position of turtles. Molecular Biology and Evolution, 28(12), 3237–3252.
Smolders, J. W., & Klinke, R. (1986). Synchronized responses of primary auditory fibre-populations in Caiman crocodilus (L.) to single tones and clicks. Hearing Research, 24(2), 89–103.
Sneary, M. (1988). Auditory receptor of the red-eared turtle: II. Afferent and efferent synapses and innervation patterns. Journal of Comparative Neurology, 276, 588–606.
Stecker, G. C., Harrington, I. A., & Middlebrooks, J. C. (2005). Location coding by opponent neural populations in the auditory cortex. Public Library of Science Biology, 3(3), e78.
Szpir, M. R., Sento, S., & Ryugo, D. K. (1990). Central projections of cochlear nerve fibers in the alligator lizard. Journal of Comparative Neurology, 295(4), 530–547.
Szpir, M. R., Wright, D. D., & Ryugo, D. K. (1995). Neuronal organization of the cochlear nuclei in alligator lizards: A light and electron microscope investigation. Journal of Comparative Neurology, 357, 217–241.
Takahashi, T. T. (1989). Construction of an auditory space map. Annals of the New York Academy of Sciences, 563, 101–113.
Takahashi, T. T., & Konishi, M. (1988a). Projections of the cochlear nuclei and nucleus laminaris to the inferior colliculus of the barn owl. Journal of Comparative Neurology, 274(2), 190–211.
Takahashi, T. T., & Konishi, M. (1988b). Projections of nucleus angularis and nucleus laminaris to the lateral lemniscal nuclear complex of the barn owl. Journal of Comparative Neurology, 274, 212–238.
Tang, Y., Christensen-Dalsgaard, J., & Carr, C. E. (2012). Organization of the auditory brainstem in a lizard, Gekko gecko. I. Auditory nerve, cochlear nuclei, and superior olivary nuclei. Journal of Comparative Neurology, 520(8), 1784–1799.
ten Donkelaar, H. J., Bangma, G. C., Barbas-Henry, H. A., de Boer-van Huizen, R., & Wolters, J. G. (1987). The brain stem in a lizard, Varanus exanthematicus. Advances in Anatomy, Embryology, and Cell Biology, 107, 1–168.
Todd, N. P. M. (2007). Estimated source intensity and active space of the American alligator (Alligator Mississippiensis) vocal display. Journal of the Acoustical Society of America, 122(5), 2906.
Tollin, D. J. (2008). Encoding of interaural level differences for sound localization. In A. L. Basbaum, A. Kaneko, G. M. Shepherd, G. Westheimer, P. Dallos, & D. Oertel (Eds.), The senses: A comprehensive reference (Vol. 3, pp. 631–654). San Diego: Academic Press.
Wagner, H. (2005). Microsecond precision of phase delay in the auditory system of the barn owl. Journal of Neurophysiology, 94(2), 1655–1658.
Wagner, H., Mazer, J. A., & von Campenhausen, M. (2002). Response properties of neurons in the core of the central nucleus of the inferior colliculus of the barn owl. European Journal of Neuroscience, 15(8), 1343–1352.
Wagner, H., Asadollahi, A., Bremen, P., Endler, F., Vonderschen, K., & von Campenhausen, M. (2007). Distribution of interaural time difference in the barn owl’s inferior colliculus in the low- and high-frequency ranges. Journal of Neuroscience, 27(15), 4191–4200.
Wagner, H., Brill, S., Kempter, R., & Carr, C. E. (2009). Auditory responses in the barn owl’s nucleus laminaris to clicks: Impulse response and signal analysis of neurophonic potential. Journal of Neurophysiology, 102(2), 1227–1240.
Walkowiak, W. (1992). Acoustic communication in the fire-bellied toad: An integrative neurobiological approach. Ethology Ecology & Evolution, 4(1), 63–74.
Wang, Y., & Karten, H. J. (2010). Three subdivisions of the auditory midbrain in chicks (Gallus gallus) identified by their afferent and commissural projections. Journal of Comparative Neurology, 518, 1199–1219.
Webb, J. F., Montgomery, J. C., & Mogdans, J. (2008). Bioacoustics and the lateral line system of fishes. In J. F. Webb, R. R. Fay, & A. N. Popper (Eds.), Fish bioacoustics(pp. 145–182). New York: Springer Science+Business Media.
Weston, J. K. (1936). The reptilian vestibular and cerebellar gray with fiber connections. Journal of Comparative Neurology, 65, 93–199.
Wever, E. G. (1978). The reptile ear: Its structure and function. Princeton, NJ: Princeton University Press.
Wever, E. G., & Vernon, J. A. (1956a). Sound transmission in the turtle’s ear. Proceedings of the National Academy of Sciences of the USA, 42(5), 292–299.
Wever, E. G., & Vernon, J. A. (1956b). Auditory responses in the common box turtle. Proceedings of the National Academy of Sciences of the USA, 42(12), 962–965.
Wever, E. G., & Vernon, J. A. (1956c). The sensitivity of the turtle’s ear as shown by its electrical potentials. Proceedings of the National Academy of Sciences of the USA, 42(4), 213–220.
Wilczynski, W., & Capranica, R. R. (1984). The auditory system of anuran amphibians. Progress in Neurobiology, 22(1), 1–38.
Wilczynski, W., & Ryan, M. J. (2010). The behavioral neuroscience of anuran social signal processing. Current Opinion in Neurobiology, 20(6), 754–763.
Wilczynski, W., Rand, A. S., & Ryan, M. J. (2001). Evolution of calls and auditory tuning in the Physalaemus pustulosus species group. Brain, Behavior and Evolution, 58(3), 137–151.
Wild, J. M., Krützfeldt, N. O. E., & Kubke, M. F. (2010). Connections of the auditory brainstem in a songbird, Taeniopygia guttata. III. Projections of the superior olive and lateral lemniscal nuclei. Journal of Comparative Neurology, 518(11), 2149–2167.
Willis, K. L., McCormick, C. A., & Carr, C. E. (2011). The organization of the hindbrain auditory nuclei of turtles follows the sauropsid pattern. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience. Online. http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=8e1b3d78-7471-4315-a0c5-fd54ff3be697&cKey=735c9c20-fd67-4f21-afd2-09d9d511f545&mKey=8334be29-8911-4991-8c31-32b32dd5e6c8
Willis, K. L., Christensen-Dalsgaard, J., Ketten, D. R., & Carr, C. E. (2013). Middle ear cavity morphology is consistent with an aquatic origin for testudines. (A. Iwaniuk, Ed.) Public Library of Science ONE, 8(1), e54086.
Winter, P., & Schwartzkopff, J. (1961). Form and cell number of the acoustic nerve center in the medulla oblongata of the owl (Striges). Experientia, 17, 515–516.
Witmer, L. M., & Ridgely, R. C. (2009). New insights into the brain, braincase, and ear region of tyrannosaurs (Dinosauria, Theropoda), with implications for sensory organization and behavior (P. Dodson, Ed.) The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology, 292(9), 1266–1296.
Witmer, L. M., Ridgely, R. C., Dufeau, D. L., & Sermones, M. C. (2008). Using CT to peer into the past: 3D visualization of the brain and ear regions of birds, crocodiles, and non-avian dinosaurs. In H. Endo & R. Frey (Eds.), Anatomical imaging: Towards a new morphology (pp. 67–88). Tokyo: Springer-Verlag.
Yan, K., Tang, Y.-Z., & Carr, C. E. (2010). Calcium-binding protein immunoreactivity characterizes the auditory system of Gekko gecko. Journal of Comparative Neurology, 518(17), 3409–3426.
Young, B. (2003). Snake bioacoustics: Toward a richer understanding of the behavioral ecology of snakes. Quarterly Review of Biology, 78(3), 303–325.
Young, S. R., & Rubel, E. W. (1983). Frequency-specific projections of individual neurons in chick brainstem auditory nuceli. Journal of Neuroscience, 3(7), 1373–1378.
Zakon, H. H., & Wilczynski, W. (1988). The physiology of the anuran eighth nerve. In B. Fritzsch, M. J. Ryan, W. Wilczynski, T. Hetherington, & W. Walkowiak (Eds.), The evolution of the amphibian auditory system (pp. 125–155). New York: John Wiley & Sons.
Zardoya, R., & Meyer, A. (2001). The evolutionary position of turtles revised. Naturwissenschaften, 88(5), 193–200.
Zhang, H., Xu, J., & Feng, A. S. (1999). Effects of GABA-mediated inhibition on direction-dependent frequency turing in the frog inferior colliculus. Journal of Comparative Physiology A, 184, 85–98.
Acknowledgments
This work was supported by awards from the Danish National Science Foundation 09-065990 and Carlsberg Foundation 2009-01-0684 (J. Christensen-Dalsgaard ), the Velux Foundation (Denmark) and NIH DC00436 (C. E. Carr), by NIH P30 DC0466 to the University of Maryland Center for Comparative and Evolutionary Biology of Hearing, and by training grant DC-00046 from the National Institute of Deafness and Communicative Disorders of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Willis, K.L., Christensen-Dalsgaard, J., Carr, C.E. (2013). Auditory Brain Stem Processing in Reptiles and Amphibians: Roles of Coupled Ears. In: Köppl, C., Manley, G., Popper, A., Fay, R. (eds) Insights from Comparative Hearing Research. Springer Handbook of Auditory Research, vol 49. Springer, New York, NY. https://doi.org/10.1007/2506_2013_24
Download citation
DOI: https://doi.org/10.1007/2506_2013_24
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9076-0
Online ISBN: 978-1-4614-9077-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)