Abstract
Lymphomas represent about 5% of all malignant neoplasms of the head and neck area. Frequently, lymphoma is not limited to the head and neck region, but also involves other parts of the body. The workup of a patient with lymphoma requires a multidisciplinary approach. This chapter approaches lymphoma as a systemic disease describing the imaging workup of patients with newly diagnosed head and neck lymphoma. This includes discussion of commonly applied imaging techniques such as chest X-ray, computed tomography (CT), ultrasound, magnetic resonance imaging, and metabolic imaging (PET-CT). Both nodal disease and non-nodal lymphoma manifestations (e.g., in Waldeyer’s ring, larynx, orbit, lacrimal gland, parotid gland, sinonasal cavities, thyroid, and bone) are discussed and illustrated with examples from daily practice. Frequently, imaging findings are nonspecific. However, some imaging patterns can suggest the diagnosis of lymphoma.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
The most frequent group of neoplasms in the neck is the carcinomas, followed by the lymphomas. Only 5% of all neoplasms in the neck are malignant lymphomas. Lymphomas are neoplasms of the lymphoreticular system. They arise from lymphocytes and their derivates. Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL) are the most common malignancies of the hematopoietic system observed in the head and neck. Frequently, lymphoma is not limited to the head and neck region but also involves other parts of the body. This chapter approaches lymphoma as a systemic disease that can manifest itself in many forms in the head and neck. In many instances, the imaging findings are nonspecific and tissue sampling remains the mainstay of making the diagnosis. However, some imaging patterns can strongly suggest the diagnosis of lymphoma.
1.1 Epidemiology
Lymphomas are the fifth most frequently occurring type of cancer in The United States comprising 5–6% of all malignancies. Hodgkin’s lymphoma (HL) accounts for about 10% of all lymphomas and the remaining 90% are referred to as non-Hodgkin’s lymphoma (NHL). Around 65,540 new cases of NHL were diagnosed in the United States in 2007 (Shankland et al. 2012). The annual incidence rate of lymphomas in 1995–1999 was 19 cases per 100,000 persons but seems to rise gradually (Walter et al. 2015). Rates of lymphomas historically have been about 40% higher in urban counties than in rural counties. The incidence rates of lymphomas in the United States tend to exceed those of most other countries. The rapid increase (1–5% annually) in lymphoma incidence in the 1970 and 1980s has been exceeded only by the increase in lung cancer in women and malignant melanomas in both sexes. The increase has been seen for males and females, for whites and blacks, and in all age groups (especially over 65 years of age), except for children. Malignant lymphomas in the head and neck usually presents as a neck lump, caused by lymphadenopathy (Cartwright et al. 1999; Vose et al. 2002).
1.2 Etiology
The majority of lymphomas arise in lymph nodes, but primary extranodal disease now accounts for 20–30% of all cases. The most frequent primary extranodal sites are the stomach, small intestine, skin, and brain. The incidence of extranodal disease has increased more rapidly than nodal disease.
Immunodeficiency, including both congenital and acquired conditions, especially in patients on immunosuppressive drugs after organ transplantation, is strongly associated with an increased lymphoma risk (Pickhardt and Wippold 2nd 1999). Viruses like the Epstein–Barr virus appear to be important cofactors. A history of lymphatic malignancies in close relatives has been repeatedly shown to increase the risk of NHL by two- to threefold. Lymphomas may also cluster within families, not by an inherited genetic susceptibility, but because of shared environmental determinants (Cartwright et al. 1999; Vose et al. 2002).
1.3 Pathology and Classifications
In 1832, Thomas Hodgkin described a disease “lymphogranulomatosis maligna” that nowadays bears his name. From that moment on new lymphoma subtypes were recognized that closely resembled but were not exactly the disease discovered by Thomas Hodgkin, and were therefore epiphrased as NHL. Since the early 1960s in the previous century, several pathologists have attempted to produce classification systems in order to clarify the growing group of NHLs. Each new system was a revision of its predecessor adding new concepts and providing new names to the same entities. In 1982 a consensus system called the “Working Formulation” (WF) was produced combining several previous systems: the British National Lymphoma Investigation’s Classification, the Rappaport Classification, the Lukes and Collins Classification, the Kiel Classification, the Dorfman Classification, and the first World Health Organization (WHO) Classification. The WF lumped all diseases into three groups depending on their clinical behavior; the low-, intermediate-, and high-grade lymphomas abbreviated as LG-NHL, IG-NHL, and HG-NHL.
The combination of several subtypes in just three groups was intended to help the clinician to distinguish lymphomas with more or less the same clinical course and comparable patient management. Soon, this lumping of different diseases in three groups without any biological basis was found to be artificial. In 1994, the WF was replaced by the Revised European-American Classification of Lymphoid Neoplasms, abbreviated as the REAL Classification (Harris et al. 1994). The basis of this system was a better understanding of the putative normal counterparts and their functions within specific sites of a normal lymph node, like the follicles, the germinal centers, and the mantle- and marginal zones. The REAL Classification is based on a subdivision in three groups: B cell neoplasms, T cell/natural killer (NK) cell neoplasms, and Hodgkin’s disease. The subsequent WHO Classifications (e.g., the 2001 and 2016 editions) are a further refinement of the REAL system.
Clinicians still tend to communicate in terminology, which is a mixture derived from the WF, the REAL, and the WHO systems. Table 1 simplifies the four most important lymphoma entities, their nomenclature, and their prevalence among the lymphomas (Harris et al. 2000; WHO 2001; Swerdlow et al. 2016). The indolent lymphomas are the former LG-NHL, the aggressive lymphomas used to be called IG-NHL or HG-NHL.
2 Hodgkin’s Lymphoma
Originally, Thomas Hodgkin described in 1832 a disease “lymphogranulomatosis maligna” that afterward was called Hodgkin’s disease. Since the WHO Classification of 2001, this entity is called HL. Historically, HL is further divided into four subtypes (see also Sect. 3; WHO classification Sect. 3.3, 1(a)–(d)):
-
1.
Nodular sclerosis (NS-HL; 40–60% of all HL)
-
2.
Lymphocyte predominant (LP-HL; ~2% of all HL)
-
3.
Mixed cellularity (MC-HL; 30–40% of all HL)
-
4.
Lymphocyte depleted (LD-HL; ~5% of all HL)
Most HL patients present in early stages predominantly with lymphadenopathy in the neck and upper mediastinum (see also Sect. 7). Usually, these patients complain about a neck lump with or without systemic symptoms such as night sweats, fever, and weight loss. Extranodal spread in HL is very rare, splenic involvement being the most prevalent. Usually, only patients in advanced stage disease show organ involvement by HL. In those cases bone marrow infiltration, intrapulmonary spread (either as deposits or as a direct extension from hilar nodes into the lung parenchyma), liver and other organ involvement can be seen. From an imaging point of view, the following aspects should be emphasized:
-
Neck nodes involved by HL usually have the same appearance as other lymphadenopathies. Sometimes, the imaging presentation is more characteristic and the diagnosis of lymphoma can be suggested by the radiologist (Table 3).
-
NS-HL poses a problem in response assessment after therapy. Histologically, the malignant component of HL is embedded in a large amount of fibrous tissues (hence the name “sclerosis”). Frequently, residual masses are seen on ultrasound, CT, or MRI investigations of the neck and mediastinum after successful treatment of NS-HL. These masses, though diagnosed as “partial response” on imaging do not necessarily contain viable lymphoma.
3 Non-Hodgkin’s Lymphomas (NHL) and Specific Entities
Worldwide, the incidence of NHL is about 5–10 times higher than the incidence of HL, largely dependent on regional differences. Of all cases of NHL, 80% is of B cell origin and 20% is T cell derived (Cartwright et al. 1999; Vose et al. 2002). The WHO Classification (Swerdlow et al. 2016) of lymphoid neoplasms describes the following diseases:
3.1 B Cell Neoplasms
-
1.
Precursor B cell neoplasm
-
(a)
Precursor B lymphoblastic leukemia/lymphoma (precursor B cell acute lymphoblastic leukemia)
-
(a)
-
2.
Mature (peripheral) B cell neoplasms
-
(a)
B cell chronic lymphocytic leukemia/small lymphocytic lymphoma
-
(b)
B cell prolymphocytic leukemia
-
(c)
Lymphoplasmacytic lymphoma
-
(d)
Splenic marginal zone B cell lymphoma (±villous lymphocytes)
-
(e)
Hairy cell leukemia
-
(f)
Plasma cell myeloma/plasmacytoma
-
(g)
Extranodal marginal zone B cell lymphoma of mucosa-associated lymphoid tissues (MALT) type
-
(h)
Nodal marginal zone B cell lymphoma (±monocytoid B cells)
-
(i)
Follicular lymphoma
-
(j)
Mantle-cell lymphoma
-
(k)
Diffuse large B cell lymphoma
-
(l)
Mediastinal large B cell lymphoma
-
(m)
Primary effusion lymphoma
-
(n)
Burkitt’s lymphoma/Burkitt cell leukemia
-
(a)
3.2 T Cell and Natural Killer (NK)-Cell Neoplasms
-
1.
Precursor T cell neoplasm
-
(a)
Precursor T lymphoblastic lymphoma/leukemia (precursor T cell acute lymphoblastic leukemia)
-
(a)
-
2.
Mature (peripheral) T cell neoplasms
-
(a)
T cell prolymphocytic leukemia
-
(b)
T cell granular lymphocytic leukemia
-
(c)
Aggressive NK-cell leukemia
-
(d)
Adult T cell lymphoma/leukemia (HTLV1+)
-
(e)
Extranodal NK/T cell lymphoma, nasal type
-
(f)
Enteropathy-type T cell lymphoma
-
(g)
Hepatosplenic gamma–delta T cell lymphoma
-
(h)
Subcutaneous panniculitis-like T cell lymphoma
-
(i)
Mycosis fungoides/Sezary syndrome
-
(j)
Anaplastic large-cell lymphoma, T/null cell, primary cutaneous type
-
(k)
Peripheral T cell lymphoma, not otherwise characterized
-
(l)
Angioimmunoblastic T cell lymphoma
-
(m)
Anaplastic large-cell lymphoma, T/null cell, primary systemic type
-
(a)
3.3 Hodgkin’s Lymphoma (Hodgkin’s Disease)
-
1.
Nodular lymphocyte-predominant Hodgkin’s lymphoma
-
2.
Classical Hodgkin’s lymphoma
-
(a)
Nodular sclerosis Hodgkin’s lymphoma (grades 1 and 2)
-
(b)
Lymphocyte-rich classical Hodgkin’s lymphoma
-
(c)
Mixed cellularity Hodgkin’s lymphoma
-
(d)
Lymphocyte depletion Hodgkin’s lymphoma
-
(a)
Note: The follicular (Sect. 3.1, 2(i)) and diffuse large B cell lymphomas (Sect. 3.1, 2(k)) make up more than half of all lymphomas diagnosed (see also Table 1).
4 Workup
The workup of a patient with lymphoma requires a multidisciplinary approach involving close cooperation among the medical oncologist or hematologist–oncologist, the head and neck surgeon, the radiation oncologist, the pathologist, and the radiologist.
4.1 Diagnosis
The diagnostic process to fully document new lymphoma patients, whether or not localized in the neck, comprises the following minimum requirements:
-
Full history (including presence of fever, night sweats and weight-loss; the so-called B symptoms) and physical examination with emphasis on all peripheral lymph node regions (neck, axilla and groin), examination of Waldeyer’s ring, liver, spleen, and skin.
-
Full blood count including a differential count of the leukocytes.
-
Representative excision biopsy of an entire enlarged lymph node (in nodal disease).
-
Bone marrow biopsy.
-
Imaging of all lymphatic regions.
4.2 Initial Imaging
Standard radiological workup of a newly diagnosed patient with head and neck lymphoma should include:
-
Posteroanterior and lateral chest X-ray.
-
Contrast-enhanced computed tomography (CECT) of the neck, chest, abdomen, and pelvis.
-
Ultrasound of the neck may be useful as an adjunct study.
-
Magnetic resonance imaging (MRI) may substitute CECT of the neck, especially if Waldeyer’s ring is involved or extranodal head and neck disease is present.
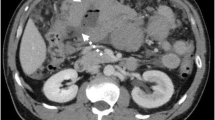
This imaging strategy is usually sufficient to stage a lymphoma patient. CECT and MRI have completely replaced lymphangiography as a diagnostic tool in lymphoma patients. In 2014, fluorodeoxyglucose (FDG) positron emission tomography (PET) computed tomography (CT) was formally accepted into standard staging for (FDG-avid) lymphomas (Cheson et al. 2014). FDG PET-CT is also used for response assessment of lymphomas (Cheson et al. 2007; Meignan et al. 2009). The use of PET-CT is strongly recommended before treatment for patients with FDG-avid lymphomas (e.g., diffuse large B cell lymphomas and Hodgkin’s lymphoma) to better delineate and stage the disease (Fig. 1).
Patient with Hodgkin’s lymphoma (HL). Initial staging with MRI of the neck shows extensive lymphadenopathy. (a) Enlarged left retropharyngeal node. (b) Bilateral neck and left axillary adenopathy (presenting symptom). PET study confirms the MR-findings in the neck (c, d). (e) Periaortic lymph nodes (slim arrows) and lymphadenopathy in the liver hilus (thick arrow) also seen on abdominal CT (not shown), indicating Stage 3 disease HL. Note: Normal activity in the heart, right ureter, and bladder
Recent improvements in MRI technology have resulted in the availability of sufficiently fast and diagnostic sequences to perform “whole-body magnetic resonance imaging” (WB-MRI). This MR technique lacks the use of ionizing radiation which is an obvious advantage when compared to CT and PET-CT. Especially when combined with recently developed functional WB-MRI techniques such as diffusion-weighted imaging (DWI) (Kwee et al. 2008). Earlier data showed that initial staging of malignant lymphoma using WB-MRI (without DWI and with DWI) produced equal results to staging using CT (Fig. 2) in the majority of patients (Kwee et al. 2009). Especially, in young patients with lymphoma it is important to minimize radiation exposure. Recent reports show that WB-MRI could potentially serve as a good radiation-free alternative to FDG-PET/CT (Littooij et al. 2014; Petralia and Padhani 2018).
Pediatric patient with Hodgkin’s lymphoma. Initial staging and response assessment 3 months post chemotherapy with PET-CT. (a) Fused coronal PET-CT image reveals marked FDG uptake in bilateral neck nodes, right axillary nodes (presenting symptom), and bilateral mediastinal nodes (arrows), indicating Stage 2 disease. Note: Normal FDG uptake in the heart and right kidney. (b) Coronal T2 short tau inversion recovery (STIR) image showing good correlation with PET-CT (arrows). Note: Left neck and mediastinal nodes not shown on this section. (c) Post chemotherapy fused coronal PET-CT image shows complete nodal regression and absence of abnormal FDG uptake. (d) Coronal STIR WB-MRI image with good correlation with PET-CT demonstrating complete nodal regression and no areas of abnormal increased signal intensity indicative of complete response
However, worldwide, CT remains the commonly used “workhorse” imaging modality for staging malignant lymphoma because of its widespread availability and relatively low cost (Kwee et al. 2008).
In MR imaging of the head and neck, diffusion-weighted imaging (DWI) has evolved as an important functional technique with a variety of potential applications. DWI can be easily incorporated in diagnostic MR protocols since DWI sequences typically only take a few minutes to obtain. In addition, DWI can be performed on standard 1.5- and 3-T clinical MR systems (Thoeny et al. 2012). In (head and neck) lymphomas an interesting application of DWI is discrimination between squamous cell carcinoma (SCC) and lymphoma based on differences in ADC values. ADCs reported for both nodal and extranodal locations were significantly lower in lymphoma than in SCC (King et al. 2007; Sumi et al. 2007). This difference can be explained by the greater cellularity of lymphomas which leads to increased diffusion restriction and hence a lower ADC (Thoeny et al. 2012). Abdel Razek et al. determined ADC values in patients with (head and neck) non-Hodgkin lymphoma (NHL). The mean ADC value was lower in patients with NHL compared to patients with HL (Abdel Razek et al. 2006).
4.3 Staging
Designed for Hodgkin’s lymphoma the Ann-Arbor system is now used for staging all lymphomas (Table 2). The system was slightly modified after the Cotswold Convention (Lister et al. 1989; Smithers 1971).
5 Treatment
Management of lymphomas is very diverse and depends fully on the specific lymphoma subtype, the stage in which the patient is diagnosed, comorbidity, the performance status, and biological age of the patient. As a concept, lymphoma patients can be treated by chemotherapy, radiotherapy, and immunotherapy, and usually in a combined modality approach.
Some frequently applied regimens are the following:
-
Chemotherapy, single agent; Chlorambucil (oral), Cyclophosphamide (oral), Fludarabin (oral and iv.): in case of indolent lymphomas, predominantly follicular lymphoma.
-
Chemotherapy, multi-agent; CVP (=COP), Chlorambucil + Vincristine (=Oncovin®) + prednisone in case of indolent lymphomas, R-CHOP (Rituxan®; CHOP = COP + Adriamycin), DHAP (Dexamethasone + high dose Ara-C + Cisplatin), VIM (VP-16 (=Etoposide) + Ifosphamide + Mesna) in case of aggressive lymphomas, predominantly DLCL, BEAM (BCNU + Etoposide + Ara-C + Melphalan) as a conditioning regimen before stem cell transplantations for aggressive lymphomas, ABVD (Adriamycin + Bleomycin + Vinblastin + Dacarbazine), MOPP (Mitoxin + Oncovin + Procarbazin + Prednisone), MOPP-ABV for Hodgkin’s lymphoma.
-
IF-RT (=involved field radiotherapy); all lymphomas; irradiation on the affected site only, no prophylactic irradiation to adjacent areas.
-
IN-RT (=involved node radiotherapy); irradiation to the affected individual lymph nodes only, refraining from irradiation of the entire nodal basin.
-
EF-RT (=extended field radiotherapy); all lymphomas; irradiation on the affected site plus prophylactic irradiation to adjacent areas.
-
[S]TNI = [sub]total nodal irradiation; Hodgkin’s lymphoma; this regimen of irradiation is now considered obsolete.
-
Combined modality regimens; 3–4 × CHOP + IF-RT: aggressive lymphomas, predominantly DLCL in stage I or II, 3–4 × ABVD + IF-RT: Hodgkin’s lymphoma, stage I or II.
6 Response Assessment
In principle, all imaging studies performed in the initial staging process will be repeated after completion of treatment. Imaging during treatment may also be indicated to decide on further management.
In hematology, response used to be assessed by the “Cheson-criteria” (Cheson 2008), but these criteria have been refined in the so-called Lugano Classification (Cheson et al. 2014). This applies also to response evaluation in head and neck lymphoma. This is in contrast to response assessment in solid tumors, such as carcinomas and sarcomas, for which the RECIST criteria are used (Therasse et al. 2000).
FDG PET-CT has now become part of the routine diagnostic workup of the majority of oncologic patients, including patients with lymphoma. A major advantage of FDG PET-CT, over conventional imaging techniques such as CT and MRI, is its ability to distinguish between viable tumor and residual mass lesions that contain only necrosis or fibrosis. The widespread use of PET in lymphoma patients warranted a reassessment of the previously established response criteria. In these “Deauville response criteria for lymphomas” recommendations for the use of PET or PET-CT both for pretreatment imaging and for posttreatment response evaluation are included (Meignan et al. 2009) (Fig. 2).
7 Nodal Disease
Per definition, this is lymphatic malignancy occurring in preexisting nodal chains (in the head and neck). In hematology, especially in Hodgkin Lymphoma, lymph nodes are typically involved by contiguous spread. This is in contrast to solid tumors (i.e., squamous cell carcinoma of the head and neck) where specific lymph node groups are at a higher risk for metastatic involvement based on the preferential lymphatic drainage from the primary tumor site.
7.1 The Common Sites
For all imaging modalities depicting nodal disease, including US, CT, and MRI, the cervical lymph nodes are described in terms of levels (Som et al. 2000; UICC 2016). In head and neck nodal disease, levels I–V are most frequently involved (Fig. 3).
In clinically suspected neck adenopathy, US may be helpful because it can be used to guide fine needle aspiration cytology (FNAC). An experienced cytopathologist can reliably diagnose lymphoid lesions from FNAC-smears, especially in the differential diagnosis from carcinomas. CT and MRI are better equipped for local tumor mapping and staging. For further subtyping of the lymphoma, surgical removal of an entire lymph node is subsequently required.
For practical purposes, nodal involvement with HL cannot be exclusively differentiated from nodes with NHL whatever imaging modality is used. In the head and neck, both entities typically present with multiple, uni- or bilateral non-necrotic enlarged lymph nodes involving usual and unusual nodal chains. However, certain imaging features are more characteristic for HL than for NHL.
General Features in Favor of Hodgkin lymphoma Nodes
-
HL presents in lymph nodes in about 98% of cases. When multiple nodes are present, the involved nodal groups are contiguous in about 90% of cases. This suggests a unifocal origin of the disease and subsequent dissemination through lymphatic pathways.
-
Levels III and IV most frequently involved.
-
Combination with mediastinal lymphadenopathy is frequent.
-
Involvement of extranodal sites such as Waldeyer’s ring is uncommon.
General Features in Favor of Non-Hodgkin lymphoma Nodes
-
NHL presents in a generalized fashion (including bone marrow infiltration) much more often than HL.
-
Levels II–IV often involved.
-
NHL often involves extranodal tissue, both Waldeyer’s ring (extranodal lymphatic) and extranodal extralymphatic (orbit, salivary glands, sinonasal, etc.) tissue.
Specific lymph node imaging features for US, CT, and MRI are shown in Table 3.
Note: A minority of patients has enlarged lymph nodes with central necrosis on cross-sectional imaging. This imaging pattern is much more frequently seen in patients with head and neck squamous cell cancer. Central necrosis in lymphoma patients usually indicates a higher malignancy grade.
7.2 The Uncommon Sites
Manifestations of nodal disease may also be seen in more uncommon sites including pre-tracheal nodes (level VI) and retropharyngeal nodes (Fig. 1). Other nodal sites, not included in the UICC nodal classification, include occipital, facial, buccal, periparotid, and intraparotid lymph nodes. All these nodes may be sites of origin of lymphoma (Vega et al. 2005; Aiken and Glastonbury 2008; Watal et al. 2018). There are no differences in imaging appearance between these nodal groups and the level I–V nodes discussed above (Table 3).
8 Extranodal Disease
Per definition, this is lymphatic malignancy occurring outside preexisting nodal chains. Extranodal lymphoma can be separated in extranodal lymphatic and extranodal extralymphatic disease (Hermans 2004). Extranodal areas predisposed to develop lymphoma are sites that are normally rich in lymphoid tissue such as Waldeyer’s ring (extranodal lymphatic disease). Extranodal extralymphatic sites include the orbit, parotid gland, nasal cavity, paranasal sinuses, and the thyroid gland. Although anatomically in close proximity, lymphomas arising in these sites have distinct clinical characteristics. Factors that appear to influence the disease pattern include concurrent conditions, such as Sjögren’s syndrome, and geographic factors particularly concerning sinonasal lymphomas (see also Sect. 8.4).
The clinical presentation of patients with extranodal NHL often mimic those of patients with squamous cell carcinoma (SCC) arising at the same site. Approximately 10% of patients with NHL present with extranodal primary lesions in the head and neck.
The treatment and prognosis of patients with head and neck lymphoma depends on the pathological subtype of disease and extent of involvement at time of presentation. The most common lymphoma is the diffuse large B cell lymphoma (65%) in an early stage at presentation. In the parotid gland, however, the indolent histologies (marginal zone lymphoma and follicular lymphoma) prevail. Predominantly patients between 50 and 60 years of age are affected. The male-to-female ratio is 1.6:1, with the exception of lymphomas of the salivary glands, orbit, and thyroid, which occur equally or more frequently in women.
The imaging findings of extranodal head and neck lymphomas are essentially the same as those found in SCC. In 80% of patients with primary extranodal NHL in the head and neck, additional nodal involvement is present. If a primary lesion is associated with large, homogeneously enhancing lymph nodes without central necrosis, the possibility of lymphoma may be suggested. However, this combination of imaging findings may also be encountered in SCC. Therefore, the diagnosis of lymphoma remains based on histologic tissue examination.
8.1 Waldeyer’s Ring and the Upper Aerodigestive Tract
Waldeyer’s ring comprises the lymphoid tissues located superiorly in the nasopharynx, laterally in both tonsillar fossae and inferiorly in the base of tongue. More than half of extranodal head and neck lymphomas occur in Waldeyer’s ring, the tonsil being the most prevalent subsite (40–50% of cases). All subtypes of NHL can be found in Waldeyer’s ring, but deposits of Hodgkin’s lymphoma are rare (Yuen and Jacobs 1999; Vega et al. 2005).
8.1.1 Nasopharynx
Patients with nasopharyngeal NHL may present with intermittent hearing loss secondary to unilateral or bilateral Eustachian tube obstruction (Fig. 4) or may have persistent epistaxis or nasal obstruction. Imaging of nasopharyngeal NHL shows two morphological types: a circumscribed, bulky, mucosa-based mass that has not invaded the deep structures around the pharynx (Fig. 4), and a mass with a much more infiltrative spread pattern. The latter type of primary nasopharyngeal lymphoma may invade the skull base and spread along nerves in a manner similar to that of SCC (King et al. 2003; Aiken and Glastonbury 2008). Both diseases are commonly associated with cervical adenopathy (Fig. 4). In the diagnostic phase of a patient with a nasopharyngeal mass, diffusion-weighted MR imaging may be used as an adjunct in differentiation between nasopharyngeal carcinoma (NPC) and lymphoma. The ADC values in lymphoma were found to be significantly lower than in NPC (Fong et al. 2010) (Fig. 4).
Nasopharyngeal NHL. Adult patient with bilateral neck swelling and conductive hearing loss. (a) Axial T2-weighted image at the level of the nasopharynx shows a bulky mass (M) without parapharyngeal and/or clival invasion. Note: Bilateral mastoid effusion (arrows) explaining the conductive hearing loss. (b) Coronal contrast-enhanced T1-weighted image with fat saturation of the neck shows bilateral lymphadenopathy (arrows). Note: Non-necrotic enhancing node on the left side and necrotic non-enhancing lymphadenopathy on the right side. (c) b600 axial diffusion-weighted image showing diffuse increased signal intensity of the nasopharyngeal mass indicative of (marked) diffusion restriction
In children and adolescents nasopharyngeal lymphoid tissue is prominent. These so-called “adenoids,” or nasopharyngeal tonsils are located in the roof of the nasopharynx. Such tissue should not be mistaken as an abnormal mass. Normal lymphoid tissues show uniform enhancement after contrast medium injection and, on MRI, display a typical high signal intensity without invasion of adjacent structures.
8.1.2 Tonsillar Fossa
Patients with NHL of the tonsil usually have a unilateral sore throat or obstructive symptoms. Clinically, especially in younger patients, these lesions are difficult to differentiate from reactive hypertrophy. Frequently the diagnosis of NHL is delayed while patients are treated with prolonged courses of antibiotics or even with incision for a suspected tonsillar abscess.
Hermans et al. (1994) described two patterns of tonsillar involvement:
-
(a)
Involvement of only the tonsillar fossa. Imaging in these cases shows a unilateral or bilateral enlarged tonsil (Fig. 5), without infiltration of surrounding tissues.
-
(b)
Tonsillar involvement with extension to the pharyngeal wall. In this subgroup, two types of extension have been described. An infiltrating growth pattern, in which there is extension in the parapharyngeal fat plane and into the extrapharyngeal deep core tissues (masticator space, parotid space, etc.); this pattern mimics the growth pattern of carcinomas. The other subtype in this group consists of an exophytic pattern of growth in which the tumor stayed inside the pharyngeal walls but extended outside of the tonsillar margins expanding into the oral cavity (Fig. 6). Complete circumferential thickening of the pharyngeal wall without deep infiltration has been reported as quite specific for lymphoma (Hermans et al. 1994).
Tonsillar NHL. Axial T2-weighted sequence (TIRM) demonstrates bilateral high signal intensity (SI) soft-tissue masses in the tonsil. Note that there is no invasion of the surrounding deep-tissue planes. There is an associated enlarged level II lymph node on the right with the same high SI appearance
Tonsillar NHL. Contrast-enhanced multislice CT images. (a) Axial contrast-enhanced CT shows a soft-tissue mass situated in the right tonsil (arrows). There is anterior extension (within the pharyngeal wall) along the glossotonsillar sulcus with slight displacement of the right tongue base. A grossly enlarged lymph node is present in level II (arrowhead). (b) Coronal reformatting shows the medial extension of the mass (arrows) with involvement of the soft palate. The level II lymph node contains a large area of central necrosis (arrowhead). (Courtesy R. Hermans, MD, PhD, Leuven, Belgium)
The signal intensity on T2-weighted, T1-weighted, and T1-weighted contrast-enhanced MRI images is as a rule homogeneous and similar to that of normal tonsils. Large tumors can be mildly heterogeneous showing small foci of necrosis. Lymphadenopathy in the ipsilateral upper internal jugular chain can be seen and in those cases, the nodes will be of similar signal intensity to the primary tumor (Fig. 5). In 20% of tonsillar NHL gastrointestinal involvement can be diagnosed simultaneously (Hanna et al. 1997).
8.1.3 Base of Tongue
Patients with lymphoma in the base of tongue may have obstructive symptoms, sore throat and occasionally dysphagia or a change in voice. Clinically, these lesions are often submucosal, bulging and soft on palpation. As mentioned, the high concentration of lymphoid tissue in the base of tongue (tonsilla lingualis) predisposes this site to develop lymphoma. The typical imaging appearance on CT (Fig. 7) or MRI is a bulky, exophytic mass centered in the tongue base. Enhancement pattern and signal intensities are identical to tonsillar NHL.
Base of tongue base NHL. A female patient with dysphagia. Contrast-enhanced multislice CT images. (a) Axial contrast-enhanced CT shows a large homogeneously enhancing mass centered in the left base of tongue. Note that there is no invasion of the surrounding deep-tissue planes. (b) Sagittal reformatting demonstrating the exophytic nature of the mass
8.1.4 Larynx
Laryngeal involvement with NHL is very rare. Laryngeal lymphomas tend to have a large submucosal component most frequently centered in the supraglottis (Aiken and Glastonbury 2008). The imaging characteristics are nonspecific and comparable to SCC. Multiple lesions strongly suggest the diagnosis of NHL (Hermans et al. 1994) (Fig. 8). In addition, a laryngeal tumor with a large supraglottic submucosal component (Fig. 9) should alert the radiologist to the possibility of NHL (King et al. 2004).
8.2 Orbit
There are a variety of tumors and tumor-like lesions in the orbit, both benign and malignant. In an unselected series of 1264 orbital lesions, 810 (64%) were benign and 454 (36%) were malignant. The percentage of malignant lesions was 20% in children, 27% in young adults and middle-aged patients, and 58% in older patents (age range, 60–92 years). Lymphoma was the most common malignancy in older patients, representing 10% of cases (Shields et al. 2004). Lymphoid tumors of the orbit in children are extremely rare.
Besides malignant lymphoma, a spectrum of less malignant lymphoid orbital tumors exists ranging from the benign pseudolymphoma or pseudotumor (also known as idiopathic orbital inflammatory syndrome; IOIS) to the reactive and atypical lymphoid hyperplasia. These two latter entities mimic orbital lymphoma, both from an imaging and from a histological point of view. The incidence of IOIS and lymphoid tumors seems to be equal (Yan et al. 2004). Any orbital tissue or combination of tissues may be involved in malignant lymphoma, as well as the less malignant lymphoid orbital tumors.
Anatomically, true lymphoid tissue is found in the subconjunctival and lacrimal glands, predisposing these sites for development of lymphoma. Orbital lymphomas can be subdivided into three groups: conjunctival, lacrimal, and intra-orbital (true intra-ocular lymphomas are very rare). Orbital lymphomas are mostly indolent, low-grade lesions. Marginal zone lymphoma (MZL), synonym MALT lymphoma, followed by follicular lymphoma as the most prevalent diagnosis (Lee et al. 2005). If MZL is diagnosed, special attention should be focused to the other MZL-prevalent sites like the salivary glands, Waldeyer’s ring, and the stomach.
Treatment of orbital lymphomas is moderate dose irradiation (25 Gy for indolent histologies, such as MZL and 36 Gy for aggressive subtypes such as DLBCL) to the entire orbit with over 90% of local control (Yahalom et al. 2015).
For imaging of the orbit, CT and MRI, or the combination of both, can be used (Priego et al. 2012). When using MRI, diffusion-weighted imaging (DWI) can be used to differentiate orbital lymphomas from other pathologies because these display the lowest ADC among orbital mass lesions (Politi et al. 2010).
8.2.1 Conjunctiva
Patients with lymphoma arising in the conjunctiva complain of local irritation, itching, ptosis, or the sensation of a mass. Imaging shows unilateral or bilateral symmetrical swelling of the conjunctival tissues. These are smooth, sharply marginated ovoid lesion that shows moderate to strong enhancement (Fig. 10). In case of conjunctival involvement, differentiation has to be made from preseptal cellulitis, which is usually unilateral, and of sinonasal origin; preseptal IOIS is rare (Hermans et al. 1994).
8.2.2 Intra-orbital Lymphoma
Painless proptosis, diplopia, and visual disturbance are the common presenting symptoms of patients with intra-orbital lymphoma. On imaging, orbital lymphomas are homogeneous usually sharply marginated intra- or para-conal masses that occur most often in the anterior portion of the orbit, the retrobulbar area (Fig. 11), or in the superior orbital compartment. Mild to moderate enhancement is usually present. Based on the imaging findings alone, there is a differential diagnosis for these types of lesions including malignant lymphoma, pseudotumor (IOIS), reactive hyperplasia, lacrimal gland tumors, optic nerve tumors, and Graves’ orbitopathy. This differential can be narrowed when the imaging features are interpreted in conjunction with the clinical signs and symptoms. A somewhat characteristic feature of orbital lymphoid tumors is the tendency to mold themselves around intra-orbital structures without (bony) destruction (Tailor et al. 2013) (Fig. 11).
Intra-orbital NHL. T1-weighted axial MR images. (a) Non-enhanced image at the level of the optic nerve shows diffuse infiltration of the intraconal space of the left orbit. (b) Post-contrast fat-suppressed image showing moderate homogeneous enhancement of the intraconal mass. Note that the lesion is “molding” around the optic nerve
8.2.3 Lacrimal Gland
Orbital lymphoma has a predilection for the lacrimal gland, which is involved in about 40% of cases (Tailor et al. 2013). Lacrimal gland lymphoma may present with epiphora or as a painless mass noticed for cosmetic reasons. If lymphoma is restricted to the gland itself, the lesion is characteristically located in the supero-lateral orbital compartment. Cross-sectional imaging shows smooth, unilateral or bilateral (Fig. 12), enlargement of the lacrimal gland. If the gland is grossly enlarged, medial and forward displacement of the globe is the clue to the primary location. When using MRI, the addition of diffusion-weighted imaging (DWI) is recommended because lacrimal gland (and other orbital) lymphomas typically display very low ADC values (Politi et al. 2010) (Fig. 13).
Lacrimal gland NHL (marginal zone B cell lymphoma). (a) Axial T1-weighted image shows smooth enlargement of the left lacrimal gland (arrow). (b) Post-contrast fat-suppressed image reveals homogeneous enhancement of the gland (arrow). (c) b1000 diffusion-weighted image showing increased signal intensity of the left lacrimal gland (arrow). (d) ADC map at the same level displays decreased ADC indicative of (marked) diffusion restriction (arrow)
8.3 Salivary Glands
Primary lymphoma of the salivary glands is an uncommon tumor. Primary MZL (marginal zone lymphoma) may occur in Sjögren’s syndrome, as a consequence of continuous antigenic stimulation. The parotid gland is affected most often (80% of reported cases) followed by the submandibular gland (20%). There are only isolated reports of primary lymphoma in minor salivary glands; most commonly involved sites include the palate and gingiva.
8.3.1 Parotid Gland
Lymphoma of the parotid gland may arise within the parotid parenchyma (primary) or within the intraparotid lymph nodes (secondary).
Primary lymphoma of the parotid gland is rare, accounting for less than 5% of parotid tumors. These lymphomas are classified as MALT (mucosa-associated lymphoid tissue) lymphomas (see also Table 1). These low-grade lymphomas may involve any portion of the gastrointestinal tract where lymphoid tissue is part of the mucosal defense system. Lymphoid tissue is normally present in the parotid gland and absent in the submandibular and sublingual gland. When MALT lymphoma occurs in the salivary gland, as in other extranodal sites such as the stomach, it is usually an indolent neoplasm that tends to remain localized for long periods of time (Abbondanzo 2001; Palacios et al. 2004; Tonami et al. 2002, 2003). Patients with primary lymphoma of the parotid gland typically present with a painless parotid-region mass or with parotitis with progressive enlargement of the gland. After systemic workup most patients are found to have early stage localized disease, i.e., Stage IIE (see also Table 2).
Patients with Sjögren’s syndrome have an increased risk of developing primary parotid lymphoma. Sjögren’s syndrome, or sicca syndrome, is an autoimmune disease characterized by keratoconjunctivitis sicca, dryness of mucous membranes, telangiectasias of the face, and bilateral parotid enlargement (Fig. 14). The syndrome is often associated with rheumatoid arthritis and Raynaud’s phenomenon. The risk of lymphoma in these patients has been estimated to be approximately 44 times the incidence expected in a generally healthy population (Yuen and Jacobs 1999). It may affect any area of the head and neck but may have its first manifestations in the parotid gland. Often these patients are scanned to survey for the possibility of lymphoma.
Primary MALT lymphoma of the parotid gland. A female patient with Sjögren’s syndrome with progressive parotid enlargement. T2-weighted (TIRM) MR images. (a) Coronal image confirms bilateral parotid swelling. This is caused by innumerable small cysts indicative of chronic sialadenitis with cystic enlargement of terminal salivary ducts. Note: Multiple non-enlarged level IV nodes bilaterally. (b) Axial plain T1-weighted image shows an additional infiltrative mass located in the superficial lobe of the right parotid gland invading the masseter muscle. Biopsy of this mass revealed MALT lymphoma
Lymphoma may also involve the salivary glands (usually the parotids) secondary to systemic lymphoma.
For imaging of suspected salivary gland tumors, US, CT, as well as MRI can be used. Because of their superficial position, the parotid, the submandibular, and the sublingual glands can be imaged with high-resolution US transducers (Gritzmann et al. 2003). This is especially helpful in the diagnostic phase because US can be used to perform fine needle aspiration cytology.
MRI and CT are better equipped for local tumor mapping and staging purposes. Primary lymphoma is characterized by, partial or total, replacement of the parotid gland by an infiltrating soft tissue mass (Fig. 14). Multiple solid, intraparotid masses are suggestive of secondary lymphoma (Fig. 15). These enlarged intraparotid lymph nodes are usually sharply marginated round to ovoid lesions with homogeneous attenuation. However, nodal necrosis can occur.
On MRI, primary and secondary lymphomas are characterized by homogeneous intermediate signal intensity (SI) on all imaging sequences. There is also a tendency to “fade” into the SI of the parotid gland on T2-weighted and contrast-enhanced, fat-suppressed T1-weighted MR images. Low ADC values of the mass on diffusion-weighted imaging (DWI) may help to suggest the diagnosis of lymphoma (Kato et al. 2012).
In the setting of multiple intraparotid and/or periparotid lesions, the age of the patient is important in establishing a differential diagnosis. In younger patients, acute viral or bacterial infections should be considered. Acute otitis media can present with reactive lymphadenopathy of periparotid lymph nodes. Multiple intraparotid cystic lesions should alert the radiologist to the possibility of HIV infection. These cystic lesions (also called benign lymphoepithelial cysts; BLEC) can serve as the initial clinical manifestation of HIV seropositivity (Sekikawa and Hongo 2017). Most commonly, with HIV infection there is also a diffuse cervical adenopathy.
The differential diagnosis for elderly patients with multiple intraparotid masses should include lymphoma. Other lesions to be considered include Warthin’s tumors and intraparotid metastases from SCC of the skin for unilateral involvement, and Warthin’s tumors and melanoma for bilateral involvement.
8.4 Sinonasal Cavities
The incidence of nasal and paranasal lymphoma in the United States and Europe is low. However, lymphomas of the paranasal sinuses and nasal cavity are relatively prevalent in certain parts of Central and South America and East Asia, including Korea, and seem to be associated with Epstein–Barr virus infections (Koom et al. 2004).
Lymphomas arising in the sinonasal cavities are NHL and frequently are observed in patients who have disseminated lymphoma or AIDS. In the nasal cavity the extranodal natural killer (NK)/T cell lymphoma, nasal type (see also Sect. 3.2; entity 2(e)) is the most prevalent type (Yahalom et al. 2015).
Sinonasal lymphoma most frequently occurs in the nasal cavity and maxillary sinus. Less often, lymphoma is found in the ethmoid sinuses, and only rarely it is found in the sphenoid and frontal sinuses. Patients with maxillary sinus lymphoma present with facial fullness, symptoms of sinusitis, or odontogenic pain. Initial symptoms of nasal cavity lymphoma include nasal obstruction and/or epistaxis. Proptosis and epiphora, i.e., orbital and lacrimal gland extension indicate more widespread disease. Rarely, malignant lymphoma can present as a mass in the canine fossa. If isolated, this most likely represents lymphoma arising in the infraorbital lymph node group, i.e., nodal disease (Tart et al. 1993).
On CT and MRI imaging studies, lymphomas of the sinonasal cavities must be differentiated from the much more common entities of sinusitis, polyposis, as well as granulomatous processes such as Wegener’s granulomatosis, and benign and malignant neoplasms (Aiken and Glastonbury 2008).
The imaging appearance is nonspecific. Lymphomas in the sinonasal cavities tend to be bulky soft tissue masses with moderate enhancement. The growth pattern may be expansile. However, in more advanced cases, aggressive bone erosion may be observed. Based on imaging alone, this more aggressive appearance of lymphoma cannot be differentiated from carcinoma (Fig. 16).
Sinonasal NHL. Contrast-enhanced CT images. (a) Axial image shows a soft-tissue mass with homogenous enhancement centered in the left maxillary sinus. Extensive bone destruction. The mass is extending into the nasal cavity and the soft tissues of the cheek. Subtle bony erosion of the postero-lateral sinus wall with beginning infiltration of the infratemporal fossa. (b) Coronal image (bone window setting) showing the extensive bony destruction. Note: Retention cyst in the right maxillary antrum
8.5 Thyroid
Lymphoma of the thyroid gland is an uncommon disease occurring primarily in older women. It may arise primarily from the thyroid or involve the gland as part of a systemic disease.
There is a strong association between thyroid lymphoma and Hashimoto’s thyroiditis. Hashimoto’s thyroiditis is an autoimmune disease characterized by circulating antithyroglobulin resulting in follicular atrophy, fibrosis, and enlargement and firmness of the gland. Surveillance of these patients by yearly physical examination and US will facilitate discovery of lymphoma at an early stage. The most common histologies are the diffuse large B cell lymphomas (up to 70% of cases) and MALT lymphomas (in 6–27% of cases). Most patients have a short history of an enlarging thyroid or a neck mass causing tracheal compression or swallowing problems. Treatment for MALT lymphomas includes radiation therapy alone. In case of diffuse large B cell lymphoma, patients will be treated by a combination of radiation with chemotherapy (Yahalom et al. 2015).
The imaging approach is based on prior clinical evaluation. Small lesions are ideally assessed with US, which is capable of discriminating between solid and cystic nodules. US-guided fine needle aspiration cytology/biopsy provides tissue for pathologic examination of thyroid nodules. On US, thyroid lymphoma may present as a focal mass or diffuse replacement of the thyroid gland. The mass is typically diffusely hypoechoic. CT and MRI are indicated for larger tumors, especially when extending outside the gland (Weber et al. 2000).
On CT, thyroid lymphoma may be seen as a focal mass, but more commonly as diffuse enlargement of the gland (Kim et al. 2002). On non-contrast CT the tumor demonstrates low attenuation. Following contrast administration, these tumors moderately enhance (Fig. 17). Associated cervical adenopathy may occur. Calcification and necrosis are unusual (Aiken and Glastonbury 2008). These two latter features are much more frequently seen in thyroid goiter.
The MRI appearance of thyroid lymphoma is usually isointense on T1-weighted images with moderate homogeneous enhancement following contrast administration (Fig. 18). On T2-weighted sequences, the signal intensity is homogeneously bright (Takashima et al. 1995). Frequently occurring vascular invasion, associated lymphadenopathy and extension into the retropharyngeal area and mediastinum are depicted adequately by both CT and MRI (Fig. 18).
Thyroid lymphoma. Axial contrast-enhanced MR image shows diffuse enlargement of the thyroid gland with homogenous enhancement. Extensive retropharyngeal, as well as intralaryngeal extension. Note: Enlarged level III lymph node on the right with similar enhancement as the thyroid lymphoma. (Courtesy R. Hermans, MD, PhD, Leuven, Belgium)
8.6 Bone
Bony disease in the setting of head and neck lymphoma is uncommon. Secondary infiltration and/or destruction (mimicking SCC) may occur (Fig. 16). However, there are some primary hematological bone disease entities in the head and neck.
8.6.1 Primary Lymphoma of Bone
The most common lymphoma affecting bone is the diffuse large B cell lymphoma. Primary lymphoma of bone occurs most frequently in the mandible followed by the maxilla. In the mandible, most often there are ill-defined lytic destructive areas of variable size. These imaging features are nonspecific and the differential diagnosis includes other primary neoplasm of bone, such as osteosarcoma and Ewing’s sarcoma.
The jaw is a common site of presentation for the African type of Burkitt’s lymphoma. This B cell lymphoma occurs most frequently in children and young adults with 50% of cases arising in the maxilla or mandible (Fig. 19). This disease is found especially in Africa and other underdeveloped countries, much less frequently in the United States and Europe. Association with Epstein–Barr Virus is postulated (Muwakkit et al. 2004; Rao et al. 2000; Weber et al. 2003).
8.6.2 Multiple Myeloma (Kahlers’ Disease)
Multiple myeloma (MM) is characterized by a malignant proliferation of plasma cells with monoclonal immunoglobulin or immunoglobulin fragments in the patient’s urine. This proliferation of neoplastic cells is associated with bone destruction and involves the bone marrow of the axial skeleton. Although single lesions occasionally occur (solitary plasmacytoma of bone), MM typically shows diffuse involvement of multiple bones. The typical radiographic appearance is that of “punched-out” round to ovoid, regular radiolucencies in the skull and long bones with no circumferential bone sclerosis (Faria et al. 2018). In the mandible, these lesions show a predilection for the angle, ramus, and molar tooth regions. Solitary plasmacytomas of bone usually occur in the vertebra and skull.
8.6.3 Extramedullary Plasmacytoma
Extramedullary plasmacytoma (EMP) is a rare soft-tissue malignancy composed of plasma cells. Eighty percent of EMPs arise in the head and neck, most often occurring in the nasal cavity followed by the paranasal sinuses (Mendenhall et al. 2003). On CT, EMPs of the sinonasal cavities are smooth, homogeneous enhancing polypoid masses that remodel surrounding bone (Fig. 20). On MR imaging, they have intermediate signal intensity on all imaging sequences. EMPs show moderate to marked heterogeneous enhancement (Ching et al. 2002).
8.7 Skin
NHL in the head and neck may also be localized in the skin.
The cutaneous lymphomas can be distinguished in B and T cell types. Specific for the cutaneous T cell lymphomas are the so-called mycosis fungoides and the Sezary syndromes, but they are more likely to be seen on the trunk and extremities than the head and neck (Foss 2004).
Crosti’s syndrome should be mentioned. Originally called the reticulohistiocytoma of the dorsum by Crosti in 1951, this disease is nowadays considered a primary cutaneous B cell lymphoma of follicular center cell origin. This localized skin disease on the back of the head and trunk has a very slowly progressive course, with many patients showing no systemic involvement even after prolonged follow-up (Ziemer et al. 2008).
When palpation is felt to be insufficient for local staging, CT or MRI can be used for mapping pre-therapeutic disease extent and document response post-therapy.
9 Conclusion
Lymphoma should be approached as a systemic disease that can manifest itself in many forms in the head and neck. Frequently, the imaging findings are nonspecific and tissue sampling remains the mainstay of making the diagnosis. Sometimes, involvement of specific subsites or specific imaging patterns can strongly suggest the diagnosis of lymphoma. However, it should be kept in mind that lymphoma is a possible cause in any infiltrative soft tissue mass in the head and neck, irrespective of the location. In the workup of a patient with a head and neck lesion, the radiologist may be the first one to suggest this diagnosis.
References
Abbondanzo SL (2001) Extranodal marginal-zone B-cell lymphoma of the salivary gland. Ann Diagn Pathol 5:246–254
Abdel Razek AAK, Soliman NY, Elkhamary S et al (2006) Role of diffusion-weighted MR imaging in cervical lymphadenopathy. Eur Radiol 16:1468–1477
Aiken AH, Glastonbury C (2008) Imaging Hodgkin and non-Hodgkin lymphoma in the head and neck. Radiol Clin North Am 46:363–378
Cartwright R, Brincker H, Carli PM et al (1999) The rise in incidence of lymphomas in Europe 1985–1992. Eur J Cancer 35:627–633
Cheson BD (2008) New response criteria for lymphomas in clinical trials. Ann Oncol 19:iv35–iv38
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3067
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Ching AS, Khoo JB, Chong VF (2002) CT and MR imaging of solitary extramedullary plasmacytoma of the nasal tract. AJNR Am J Neuroradiol 23:1632–1636
Faria KM, Brandão TB, Silva WG et al (2018) Panoramic and skull imaging may aid in the identification of multiple myeloma lesions. Med Oral Pathol Oral Cir Bucal 23:e38–e43. https://doi.org/10.4317/medoral.22123
Fong D, Bhatia KSS, Yeung D et al (2010) Diagnostic accuracy of diffusion-weighted MR imaging for nasopharyngeal carcinoma, head and neck lymphoma and squamous cell carcinoma at the primary site. Oral Oncol 46:603–606
Foss F (2004) Mycosis fungoides and the Sezary syndrome. Curr Opin Oncol 16:421–428
Gritzmann N, Rettenbacher T, Hollerweger A et al (2003) Sonography of the salivary glands. Eur Radiol 13:964–975
Hanna E, Wanamaker J, Adelstein D et al (1997) Extranodal lymphomas of the head and neck. A 20-year experience. Arch Otolaryngol Head Neck Surg 123:1318–1323
Harnsberger RH (2004) Diagnostic imaging head and neck, 1st edn. Amirsys, Salt Lake City, UT
Harris NL, Jaffe ES, Diebold J et al (2000) World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol 17:3835–3849
Harris NL, Jaffe ES, Stein H et al (1994) A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 84:1361–1392
Hermans R (2004) Extranodal lymphoma—neck. Cancer Imaging 4:1–5
Hermans R, Horvath M, De Schrijver T et al (1994) Extranodal non-Hodgkin lymphoma of the head and neck. J Belg Radiol 77:72–77
Kato H, Kanematsu M, Goto H et al (2012) Mucosa-associated lymphoid tissue lymphoma of the salivary glands: MR imaging findings including diffusion-weighted imaging. Eur J Radiol 81:e612–e617. https://doi.org/10.1016/j.ejrad.2011.12.035
Kim HC, Han MH, Kim KH et al (2002) Primary thyroid lymphoma: CT findings. Eur J Radiol 46:233–239
King AD, Ahuja AT, Yeung DKW et al (2007) Malignant cervical lymphadenopathy: diagnostic accuracy of diffusion-weighted MR imaging. Radiology 245:806–813
King AD, Lei KI, Richards PS et al (2003) Non-Hodgkin’s lymphoma of the nasopharynx: CT and MR imaging. Clin Radiol 58:621–625
King AD, Yuen EH, Lei KI et al (2004) Non-Hodgkin lymphoma of the larynx: CT and MR imaging findings. Am J Neuroradiol 25:12–15
Koom WS, Chung EJ, Yang WI et al (2004) Angiocentric T-cell and NK/T-cell lymphomas: radiotherapeutic viewpoints. Int J Radiat Oncol Biol Phys 59:1127–1137
Kwee TC, Quarles van Ufford HME, Beek FJ et al (2009) Whole-body MRI, including diffusion-weighted imaging, for the initial staging of malignant lymphoma. Investig Radiol 44:683–690
Kwee TC, Takahara T, Ochiai R et al (2008) Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol 18:1937–1952
Lee JL, Kim MK, Lee KH et al (2005) Extranodal marginal zone B-cell lymphomas of mucosa-associated lymphoid tissue-type of the orbit and ocular adnexa. Ann Hematol 84:13–18
Lister TA, Crowther D, Sutcliffe SB et al (1989) Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol 7:1630–1636
Littooij AS, Kwee TC, Barber I et al (2014) Whole-body MRI for initial staging of paediatric lymphoma: prospective comparison to an FDG-PET/CT-based reference standard. Eur J Radiol 24:1153–1165
Meignan M, Gallamini A, Haioun C (2009) Report on the first international workshop on interim-PET scan in lymphoma. Leuk Lymphoma 50:1257–1260
Mendenhall WM, Mendenhall CM, Mendenhall NP (2003) Solitary plasmacytoma of bone and soft tissues. Am J Otolaryngol 24:395–399
Muwakkit SA, Razzouk BI, Shabb NS et al (2004) Clinical presentation and treatment outcome of children with Burkitt lymphoma in Lebanon: a single institution’s experience. J Pediatr Hematol Oncol 26:749–753
Palacios E, Larusso G, Rojas R et al (2004) Lymphoma of the parotid gland in Sjőgren’s syndrome. Ear Nose Throat J 83:156
Petralia G, Padhani AR (2018) Whole-body magnetic resonance imaging in oncology: indications. Magn Reson Imaging Clin N Am 26:495–507
Pickhardt PJ, Wippold FJ 2nd (1999) Neuroimaging in posttransplantation lymphoproliferative disorder. AJR Am J Roentgenol 172:1117–1121
Politi LS, Forghani R, Godi C et al (2010) Ocular adnexal lymphoma: diffusion-weighted MR imaging for differential diagnosis and therapeutic monitoring. Radiology 256:565–574
Priego G, Majos C, Climent F et al (2012) Orbital lymphoma: imaging features and differential diagnosis. Insight Imaging 3:337–344
Rao CR, Gutierrez MI, Bhatia K et al (2000) Association of Burkitt’s lymphoma with the Epstein-Barr virus in two developing countries. Leuk Lymphoma 39:329–337
Sekikawa Y, Hongo I (2017) HIV-associated benign lymphoepithelial cysts of the parotid glands confirmed by HIV-1 p24 antigen immunostaining. BMJ Case Rep. https://doi.org/10.1136/bcr-2017-221869
Shankland KR, Armitage JO, Hancock BW (2012) Non-Hodgkin lymphoma. Lancet 380(9844):848–857. https://doi.org/10.1016/S0140-6736(12)60605-9
Shields JA, Shields CL, Scartozzi R (2004) Survey of 1264 patients with orbital tumors and simulating lesions. Ophthalmology 111:997–1008
Smithers DW (1971) Summary of papers delivered at the Conference on Staging in Hodgkin’s Disease (Ann Arbor). Cancer Res 31:869–870
Som P, Curtin H, Mancuso A (2000) Imaging-based classification for evaluation of neck metastatic adenopathy. Am J Roentgenol 174:837–844
Sumi M, Ichikawa Y, Nakamura T (2007) Diagnostic ability of apparent diffusion coefficients for lymphomas and carcinomas in the pharynx. Eur Radiol 17:2631–2637
Swerdlow SH, Campo E, Pileri SA et al (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127:2375–2390
Tailor TD, Gupta D, Dalley RW et al (2013) Orbital neoplasms in adults: clinical, radiologic and pathologic review. Radiographics 33:1739–1758
Takashima S, Nomura N, Noguchi Y et al (1995) Primary thyroid lymphoma: evaluation with US, CT and MRI. J Comput Assist Tomogr 19:282–288
Tart RP, Mukherji SK, Avino AJ et al (1993) Facial lymph nodes: normal and abnormal CT appearance. Radiology 188:695–700
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Thoeny HC, De Keyzer F, King AD (2012) Diffusion-weighted MR imaging in the head and neck. Radiology 263:19–32
Tonami H, Matoba M, Kuginuki Y et al (2003) Clinical and imaging findings of lymphoma in patients with Sjőgren’s syndrome. J Comput Assist Tomogr 27:517–524
Tonami H, Matoba M, Yokota H et al (2002) Mucosa-associated lymphoid tissue lymphoma in Sjőgren’s syndrome: initial and follow-up imaging features. Am J Roentgenol 179:485–489
UICC (2016) TNM classification of malignant tumours, 8th edn. Wiley Blackwell, Hoboken
Vega F, Lin P, Medeiros J (2005) Extranodal lymphomas of the head and neck. Ann Diagn Pathol 9:340–350
Vose JM, Chiu BC, Cheson BD et al (2002) Update on epidemiology and therapeutics for non-Hodgkin’s lymphoma. Hematology 1:241–262
Walter C, Ziebart T, Sagheb K et al (2015) Malignant lymphomas in the head and neck region—a retrospective, single-center study over 41 years. Int J Med Sci 12:141–145. https://doi.org/10.7150/ijms.10483. eCollection 2015
Watal P, Bathla G, Thaker S et al (2018) Multimodality imaging spectrum of the extranodal lymphomas in the head and neck—a pictorial review. Curr Probl Diagn Radiol 47:340–352. https://doi.org/10.1067/j.cpradiol.2017.07.007
Weber AL, Rahemtullah A, Ferry JA (2003) Hodgkin and non-Hodgkin lymphoma of the head and neck: clinical, pathologic, and imaging evaluation. Neuroimaging Clin N Am 13:371–392
Weber AL, Randolph G, Aksoy FG (2000) The thyroid and parathyroid glands. CT and MR imaging and correlation with pathology and clinical findings. Radiol Clin North Am 38:1105–1129
World Health Organization Classification of Tumors (2001) In: Jaffe ES, Harris HL, Stein H et al (eds) Tumors of haematopoietic and lymphoid tissues. Oxford University Press, Oxford, pp 162–167
Yahalom J, Illidge T, Specht L et al (2015) Modern radiation therapy for extranodal lymphomas: field and dose guidelines from the international lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys 92:11–31
Yan J, Wu Z, Li Y (2004) The differentiation of idiopathic inflammatory pseudotumor from lymphoid tumors of orbit: analysis of 319 cases. Orbit 23:245–254
Yuen A, Jacobs C (1999) Lymphomas of the head and neck. Semin Oncol 26:338–345
Ziemer M, Bauer HI, Fluhr J et al (2008) Primary cutaneous follicle center lymphoma – ‘Crosti lymphoma’. Am J Clin Dermatol 9:133–136
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pameijer, F.A., Haas, R.L.M. (2020). Neck Lymphoma. In: Hermans, R. (eds) Head and Neck Cancer Imaging. Medical Radiology(). Springer, Cham. https://doi.org/10.1007/174_2020_232
Download citation
DOI: https://doi.org/10.1007/174_2020_232
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-64734-6
Online ISBN: 978-3-030-64735-3
eBook Packages: MedicineMedicine (R0)