Abstract
Current computed tomography (CT) technology enables the noninvasive imaging of the coronary arteries in daily clinical practice. Patients with a low to intermediate likelihood of coronary artery disease (CAD) and having equivocal findings at electrocardiogram (ECG) or stress tests are considered suitable candidates to undergo noninvasive coronary CT angiography. The excellent negative predictive value close to 100 % enables the exclusion of obstructive CAD with certainty. In order to obtain images without artifacts, CT scanner systems need to provide a high temporal and spatial resolution. In addition, several techniques must be applied and combined for minimizing the exposure of patients to ionizing radiation. CT data acquisition needs to be synchronized to the simultaneously recorded ECG for either retrospective, prospective, or high-pitch scanning. Depending on the heart rate of the patients, ECG pulsing windows must be flexibly adjusted. Owing to the steadily increasing temporal resolution of modern CT systems, coronary CT angiography today is possible even at high and/or irregular heart rates.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
According to the Centers for Disease Control and Prevention (CDC), coronary artery disease (CAD) is considered to be the leading cause of mortality in industrialized countries (Mozaffarian et al. 2015). Prior to cardiac CT imaging, conventional catheter coronary angiography (CCA) was the only means to directly visualize the coronary arteries. However, CCA is relatively expensive, causes inconvenience to patients, and is associated with a small, albeit non-negligible, periprocedural morbidity. Currently, about only one-third of CCA procedures are subsequently followed by a percutaneous coronary intervention (Maier et al. 2006). This indicates that CCA mostly is used as a mere diagnostic tool. The associated economic burden of this invasive approach prompted a search for alternative, cost-effective, and noninvasive means for coronary imaging (Achenbach and Daniel 2001).
Continuous technical innovations, especially due to the introduction of multi-detector and dual-source CT scanners, have enabled isotropic submillimeter spatial resolution and sub-second gantry rotation times. Various CT protocol developments and software modifications such as automatic attenuation-based tube current modulation, tube potential adaptation, electrocardiography (ECG) pulsing, and iterative image reconstruction enabled the decrease of ionizing radiation dose in one order of magnitude over the last two decades (den Harder et al. 2016). Currently, the most common and most acknowledged indication for coronary CT angiography is in patients with stable angina having a low to intermediate pretest probability of CAD (Taylor et al. 2010).
2 Technical Issues
2.1 Radiation Dose
Tireless efforts to continuously lower radiation dosages have sparked major technological advances in CT imaging. Apart from restrictions of the total number of cardiac CT examinations by close adherence to guidelines and recommendations, major radiation dose reduction strategies include technological issues such as automatic tube current modulation (or exposure control), tube voltage optimization, scan length adjustment, evolution of ECG gating techniques, and iterative image reconstruction post-processing (den Harder et al. 2016). Optimal selection and combination of each of the aforementioned techniques help in keeping the radiation exposure to each individual patient “as low as reasonably achievable” (the so-called ALARA principle).
2.1.1 ECG Gating
Combining the electrocardiogram (ECG) of the patient with CT data acquisition allows for imaging at defined time points of the cardiac cycle. At normal heart rates, least cardiac motion occurs during diastole, when the ventricles are passively filling. Three approaches for so-called ECG gating are currently in use: (1) retrospective ECG gating with spiral data acquisition, (2) prospective ECG gating with a sequential (or step-and-shoot) data acquisition, and (3) prospective ECG gating with spiral data acquisition and high pitch.
In retrospective ECG gating techniques, partially overlapping multi-detector CT projections are continuously acquired in the spiral mode and the ECG signal is simultaneously recorded. Software algorithms are then used to sort the data from different phases of the cardiac cycle by progressively shifting the temporal window of acquired helical projection data relative to the R wave. Every position of the heart is covered by a detector row at every point of the cardiac cycle. Therefore, the scanner table continuously moves but advances no more than the total width of the active detectors for each heartbeat (Desjardins and Kazerooni 2004). For gapless volume coverage of the heart in each cardiac phase, a low table feed (pitch <1) is required, which has to be adapted to the heart rate of the patient. The advantage of this scan mode is the availability of image data at each time point of the cardiac cycle, which increases the confidence of reading. In addition, this scan mode can be applied also at higher heart rates. The downside of this scan mode, however, is the comparably high associated effective radiation dose ranging between 8 and 10 mSv when ECG pulsing (see below) is used. However, combining the technique with tube current and tube voltage optimization and iterative reconstruction allows for lowering of the associated effective radiation dose to 4–6 mSv.
Prospective ECG gating uses the ECG signal to limit scanning to time points defined prior to data acquisition, usually during diastole. Projection data are acquired during slightly more than half a gantry rotation. The total number of slices produced per heartbeat during this half rotation of the gantry is proportional to the number of rows of active detectors. As axial (rather than helical) scanning is used, the table is not moving during, but only in-between data acquisition. The table has to move by the total collimation width after each acquisition (sequential or step-and-shoot mode). A meta-analysis showed that prospective ECG gating is associated with an average radiation dose of only 2.7 mSv (von Ballmoos et al. 2011). Currently, prospective ECG gating is the most widely used technique for data acquisition in cardiac CT (den Harder et al. 2016; Menke et al. 2013).
Since the commercial launch of dual-source CT technology in 2005, further reduction of ionizing radiation was achieved by the use of prospective ECG gating with high pitch. In this mode, data is acquired in a spiral fashion while the table runs with a high pitch (e.g., 3.2 with third-generation dual-source CT, equivalent to a table feed of 737 cm/s). Using this technique yields a “snapshot” scan of the entire heart within one cardiac cycle, usually during diastole. While pitch in a single-source CT is limited to values of approximately 1.5 to ensure gapless volume coverage along the z-axis, a dual-source CT system contains a second x-ray tube at 90° offset, which acquires scan data at the same angular position at a quarter rotation later. This facilitates a maximum pitch of 3.2–3.4, depending on the scanner generation, within a limited scan field of view (FOV) covered by both detectors. A quarter rotation of data per measurement system is used for image reconstruction, and each individual axial image has a temporal resolution of a quarter of the gantry rotation time. The ECG is used to trigger prospectively the start of table motion and the start of the high-pitch spiral. A requirement for usage of this technique is generally a stable sinus rhythm with heart rates ≤60 bpm with second-generation and ≤70 bpm with third-generation dual-source CT scanners. Associated radiation doses of the high-pitch mode can be as low as 0.2–0.4 mSv.
2.1.2 ECG Pulsing
The use of ECG pulsing allows for modulation of the tube current in the retrospective ECG-gated mode with spiral data acquisition. The peak tube output is generated during a selected pulsing window. During the remaining parts of the cardiac cycle, the tube output is reduced to approximately 25 %. Dual-source CT allows for further reduction of the tube current to 5 % in the remaining periods. Most scanners allow to adjust the ECG pulsing window width according to the heart rate, in order to be as narrow as possible for maximal radiation exposure reduction, while being as wide as reasonable to obtain diagnostic image quality at the same time (Leschka et al. 2007). Modern ECG pulsing algorithms are able to automatically detect arrhythmia and switch off ECG pulsing during ectopic heartbeats.
2.1.3 Tube Current Modulation and Tube Voltage Adaptation
Tube current modulation is defined as automatic adjustment of the tube current to account for differences in body shape and attenuation depending on the body region scanned. Lowering and optimizing the tube voltage represent another important approach for dose reduction, as radiation dose varies with the square of the tube voltage. Recently, automatic tube potential selection algorithms became available which automatically lower the tube potential according to patient size, diagnostic task, and scanner tube current limits. This measurement is reported to yield a dose reduction between 20 and 50 % in nonobese patient populations.
2.2 Image Reconstruction
2.2.1 Data Reconstruction
The minimum data that is required to reconstruct a CT image is 180° of one gantry rotation with a single-source CT and 90° with a dual-source CT. The data may be reconstructed either utilizing mono-segment or multiple-segment reconstruction techniques. With mono-segment reconstructions, the time needed to complete a half rotation in single-source CT or a quarter rotation in dual-source CT determines the temporal resolution of the scanner. The gantry rotation time is defined as the time that is required to complete one full rotation of the x-ray tube and detector elements around the subject. The temporal resolution is little greater than half the gantry rotation time in mono-segment reconstruction. The temporal resolution is therefore dependent on the rotational speed of the gantry, which is limited by considerable centrifugal forces of up to 28 g.
Dual-source CT scanners overcome the limitation of gantry rotation speed by introducing a second x-ray tube and corresponding detector that is mounted with an offset of 90° to the rotating gantry (Flohr et al. 2006). With this configuration, only about a quarter rotation is required (90° plus fan beam angle) for reconstructing cardiac CT images. Current third-generation dual-source scanners possess a temporal resolution of up to 66 ms at a gantry rotation time of 0.25 s.
A software-based approach to increase temporal resolution in single-source CT scanners represents the multiple-segment reconstruction algorithms. The basic principle behind these algorithms is that the scan projection data required to reconstruct an axial slice are selected from segmental scans obtained during sequential heart cycles at the same z-position, in contrast to mono-segment reconstructions, where data from only a single heartbeat is reconstructed. Usually, up to four segments can be sampled, which results in an increased temporal resolution by the factor of four (Herzog et al. 2007). The disadvantage of multi-segment reconstruction techniques is that any misregistration results in a degradation of the image quality by introducing blurring artifacts. Thus, its use is limited in patients with variable heart rates and with inter-heartbeat variability of the coronary artery position.
2.3 Reconstruction Phases
Coronary artery motion shows a biphasic pattern of rapid movement. The maximum of motion is observed during the ventricular contraction at early to mid-systole and during rapid filling in early diastole. During isovolumetric relaxation at mid-diastole and during mid- to late systole, coronary motion is relatively quiescent. With increasing heart rates, the minimum mid-diastolic velocity increases, while the width of the mid-diastolic velocity successively decreases and eventually disappears. At heart rates greater than approximately 80 beats per minute (bpm), the lowest velocities occur in systole rather than in diastole. The motion of the left anterior and left circumflex artery follows the motion of the left ventricle. Therefore, their best reconstruction interval is in mid-diastole, at 50–80 % of the R-R interval, when the left ventricle is relatively quiescent. As the contraction of the right atrium is causative for the motion of the right coronary artery, reconstructions of the right coronary artery are best performed at late systole and early diastole, at 30–60 % of the R-R interval, representing a time interval with the right atrium being relatively free of motion. Since the diastole shortens more than the systole with increasing heart rates, imaging of the right coronary artery in late systole and early diastole is less prone to motion artifacts when shortening of the diastole occurs.
These heart rate-dependent variations in reconstruction phases must be taken into account when implementing the technique of ECG pulsing for radiation dose reduction when the retrospective ECG-gated mode with spiral acquisition is applied. Then, flexible adjustments of the pulsing width at different heart rates are recommended (Leschka et al. 2007).
2.3.1 Filtered Back Projection
Until recently, CT images were almost exclusively reconstructed with filtered back projection (FBP), largely due to the fact that FBP generates diagnostic images at a low level of computational complexity. In FBP the x-ray beam assumes a pencil shape, and the x-ray source is aligned in a parallel fashion to a linear x-ray detector array. For image generation, the x-ray source is rotated over a certain angle, allowing for intensities to be measured at the detector. These intensities are described as an integral function for a specific angle and the shift in the position of the x-ray tube. After this, the reconstruction process involves solving an integral equation by inversion or so-called back projection. Despite its ability to rapidly reconstruct images, FBP has various limitations: in particular, increased image noise, which is most prominent in low tube current imaging, poor contrast resolution, and streak artifacts. This is primarily due to the inherent failure of the FBP algorithm to account for image noise that results from Poisson statistical variations in the number of photons across the imaging plane, leading to an inverse relationship between radiation dose and image noise.
2.3.2 Iterative Reconstruction
Iterative reconstruction (IR) is a technique that incorporates statistical modeling in image reconstruction with the main aim to reduce image noise. In principle, IR techniques attempt to localize and selectively remove image noise by frequent comparison with a canvas. This is achieved through a process of modeling the imaging acquisition process, including fluctuations in photon statistics, the optics system, and various aspects of x-ray interactions to generate an expected dataset, which is then iteratively compared to the actually acquired dataset. The differences between these two are used to identify and remove noise, and the process is repeated multiple times until the updated data converges with or approximates the expected data to maximize image optimization. This technique can be also used for reducing the radiation dose (usually through reduced tube current) of up to 50 %, while maintaining the image quality of the examination (Alkadhi and Leschka 2011; Gordic et al. 2016). Similar to the options tube current modulation and tube voltage adaptation, IR can be applied for all scan protocols described above.
2.3.3 Post-processing
While 2D techniques such as planar or curved multiplanar reformations (MPR) as well as maximum intensity projection (MIP) images are widely used in coronary imaging, 3D imaging post-processing techniques such as volume rendering (VR) are mainly used to convey complex anatomy (Dappa et al. 2016), as it might be useful for understanding the anatomy of coronary artery bypass grafts.
The VR technique consists of two steps: classification of each voxel and image projection. Classification determines how each point on the artificial rays that pass through the data contributes to the pixel value on the picture. For separation of different tissues (e.g., bone and soft tissue), a trapezoid is used for each tissue type. The tissue represented in each voxel of the volume dataset is determined by using predefined attenuation threshold levels and is assigned to a specific color and opacity. The weighted sum of the percentage of each tissue type represented in the voxel is calculated to determine the overall color and transparency of each voxel. This step is performed for each voxel and for the whole volume dataset. The 3D volume is displayed by using a projection technique. This is done by simulating rays of light, which are projected through the 3D volume that contains the classified voxels (Luccichenti et al. 2005; Lell et al. 2006). While helpful in visualizing 3D spatial relationships, these rendered images are not of diagnostic quality as they may mask anatomical information and, hence, pathology.
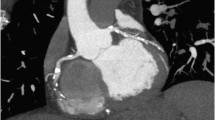
More recently a new technique for 3D visualization of cross-sectional image data from CT has been introduced: cinematic rendering (CR). CR generates photo-realistic anatomic depictions (Fig. 1). It works with random sampling computational algorithms and uses different light maps to generate a realistic depiction of medical data (Dappa et al. 2016). While first studies have started using the CR technique for visualizing anatomy from CT data, regulatory approval for clinical use is still pending.
3 Accuracy
The diagnostic accuracy of coronary CT angiography is challenged by numerous factors including the average heart rate, heart rate variability, and vessel wall calcifications. Briefly, the higher the spatial and temporal resolution of a CT scanner, the less impact these issues have on the accuracy of the technique (Gordic et al. 2014). Severe vessel wall calcifications remain an issue leading to a reduced image quality and, hence, to a reduced accuracy. Blooming from calcification can obscure parts of the vessel lumen, causing an overestimation of the stenosis assessments. As a result, specificity of the technique is diminished due to false-positive findings (Raff et al. 2005; Ong et al. 2006).
Multiple single- and multi-center studies have investigated the accuracy of coronary CT angiography for detecting anatomically significant stenosis with ≥50 % luminal narrowing in comparison with CCA as a reference. Across all published meta-analyses on the accuracy of coronary CT angiography using at least 64-slice CT systems with prospective triggering, the pooled sensitivity was 98.7 % (Fig. 2) (Menke et al. 2013; Meinel et al. 2015). The high sensitivity translates into an excellent negative predictive value of 95–100 %. A fully diagnostic coronary CT angiography that demonstrates no plaques or stenoses can exclude obstructive CAD with certainty (Fig. 3) (Meinel et al. 2015). In contrast to the high sensitivity and excellent negative predictive value, the reported specificity (89 %) and positive predictive value (60–80 %) remains lower (Menke et al. 2013; Arbab-Zadeh et al. 2012). The main reason for that is the abovementioned overestimation of coronary stenosis and resulting false-positive ratings in heavily calcified plaques.
A 61-year-old male patient with atypical chest pain. Curved multiplanar reformations of the LAD (a), LCX (b), and RCA (c) and 3D cinematic rendering (d) show no significant stenosis. Images were acquired with the high-pitch mode on a third-generation dual-source CT scanner; the estimated effective radiation dose was 0.4 mSv
To ensure best performance of the involved medical professionals, dedicated training programs and consensus guidelines were introduced. In light of increasing demand for standardized reporting in medical imaging, the Society of Cardiovascular Computed Tomography (SCCT) has recently launched a coronary CT angiography reporting system called CAD-RADs™ (Cury et al. 2016). It is intended to decrease variability among medical practitioners and may provide further benefit by linking the final imaging diagnosis with suggestions for further patient management. The recommendations of CAD-RADs™ for coronary CT angiography results in patients with stable chest pain in the outpatient setting are provided in Table 1.
4 Prognosis
In a meta-analysis of mostly symptomatic patients (n = 7,335) with suspected CAD followed for a median of 20 months, the presence of any stenosis greater than 50 % at coronary CT angiography was associated with a tenfold higher risk of cardiovascular events. The finding of any CAD inferred a 4.5-fold increased risk and each coronary segment involved increased the risk for adverse outcomes by 23 % (Bamberg et al. 2011). In a more recent analysis from the CONFIRM registry (n = 17,793), including mainly patients with chronic chest pain, it was shown that the number of proximal segments with mixed or calcified plaques and the number of proximal segments with ≥50 % were those coronary CT angiography parameters with the strongest predictive value for all-cause mortality at a median follow-up of 2.3 years (Hadamitzky et al. 2013a). The long-term prognostic value of coronary CT angiography was seen in 1,584 patients after a median of 5.6 years. The annual rates of major adverse cardiac events (MACE) were 0.2 % for patients with no CAD and 1.1 % of patients with obstructive CAD according to CT (Hadamitzky et al. 2013b). Similarly, in another study reporting the median 6.9-year follow-up in 218 patients, annual MACE rates were 0.3, 2.7, and 6.0 % in patients with normal coronary CT angiography, nonobstructive CAD, and obstructive CAD, respectively (Dougoud et al. 2014).
The available prognostic data suggests that the warranty period with an excellent prognosis after a coronary CT angiography study negative for any CAD extends beyond 5 years, and the presence of any CAD, obstructive CAD, and the burden of atherosclerotic changes at coronary CT angiography is strongly predictive for cardiac outcomes in patients with chronic chest pain (Dougoud et al. 2014; Meinel et al. 2015).
5 Indications
In 2010 the American College of Cardiology reported appropriate use criteria for cardiac CT (Taylor et al. 2010). The four main indications were (1) the evaluation of chest pain in patients without known CAD at low (if uninterpretable ECG or unable to exercise) and intermediate risk of CAD, (2) the detection of CAD in patients without known CAD and reduced left ventricular ejection fraction, (3) the coronary evaluation before noncoronary cardiac surgery in patients at intermediate risk of CAD, and (4) the assessment of coronary anomalies (Taylor et al. 2010).
5.1 Coronary Artery Disease
Several prospective trials have evaluated the clinical use of coronary CT angiography and the relevance of CT findings in the context of suspected stable coronary artery disease. They include the PROMISE (Douglas et al. 2015) and SCOT-HEART (Investigators S-H 2015) trials, which demonstrated that coronary CT angiography is clinically useful as an alternative to (PROMISE) or in addition to functional testing (SCOT-HEART). Four large randomized trials (CT-STAT, ACRIN-PA, ROMICAT II, and CT-COMPARE) compared coronary CT angiography to the current standard of care in patients with acute chest pain (Goldstein et al. 2011; Litt et al. 2012; Hoffmann et al. 2012; Hamilton-Craig et al. 2014). Their clinical implementations illustrated the reliability of a negative coronary CT angiography in identifying patients for discharge from the emergency department (Cury et al. 2016). Here, coronary CT angiography allows for fast and safe triage of patients. In clinical practice, individuals demonstrating severe coronary stenosis are often directly referred to invasive catheter angiography, and patients with intermediate stenosis, high overall plaque burden, or equivocal findings typically undergo functional testing (Taylor et al. 2010; Meinel et al. 2015).
Coronary CT angiography can also be performed after heart transplantation, as indicated by a recent meta-analysis reporting a high sensitivity and negative predictive value for the exclusion of allograft vasculopathy with CT (Wever-Pinzon et al. 2014).
Cardiac arrhythmias still pose a challenge even to modern CT scanners. As radiation saving ECG gating techniques cannot be applied, higher radiation doses occur and the image quality might still be non-diagnostic. Still, coronary CT angiography may be appropriate as an alternative to CCA and/or functional testing in patients with ventricular tachycardia, ventricular fibrillation, or frequent premature ventricular contractions (Wolk et al. 2014). Further applications are seen in healthy patients with a new onset of atrial fibrillation, as investigation of an underlying ischemic cause is recommended. A meta-analysis illustrated a high diagnostic accuracy at the cost of increased radiation dosages for coronary CT angiography in patients with atrial fibrillation (Meinel et al. 2015; Vorre and Abdulla 2013).
5.2 Coronary Artery Anomalies
Coronary artery anomalies (CAAs) are a diverse group of congenital disorders, whose manifestations and pathophysiological mechanisms are highly variable (Angelini 2007). Anomalies of the coronary arteries may be found incidentally in 0.3–1 % of healthy individuals (Angelini et al. 2002). In a study by Eckart et al. of 126 non-traumatic sudden deaths in young adults, cardiac abnormalities were found in 64 cases (51 %), with coronary artery abnormalities being the most common cardiac abnormality found in 39 of 64 patients (61 %) (Eckart et al. 2004).
Detecting coronary anomalies by CCA is challenging in the diagnostic work-up of symptomatic patients, as coronary artery anomalies are difficult to visualize and their course may be delineated inaccurately. The latter problem is aggravated by aberrant coronary arteries that run retro-aortal, inter-arterial (between ascending aorta and pulmonary artery), pre-pulmonary, or septal. Furthermore, catheterization of the origin of anomalous coronary arteries is technically challenging, and the exact three-dimensional course of the anomalous arteries is difficult to obtain in angiography (Cheng et al. 2010). Coronary CT angiography is superior to CCA in defining the ostial origin and proximal path of anomalous coronary branches (Shi et al. 2004). 3D visualization of spatial relationships to surrounding structures using rendering techniques provides valuable information to clinicians, especially prior to surgery (Fig. 4) (Alkadhi 2009).
A 46-year-old female patient with coronary anomaly. Transverse thick maximum intensity projection image (a) and 3D cinematic rendering (b) show a single left coronary artery with the origin of the RCA from the left main artery (arrow) with inter-arterial course of the proximal RCA between the ascending aorta (asterisk) and pulmonary trunk (plus)
5.3 Coronary Stents and Bypass Grafts
The evaluation of patients with coronary stents remains a limitation for coronary CT angiography. Due to blooming artifacts from the metal strut, the assessment of the in-stent lumen is typically limited to larger stent sizes (diameter >3 mm). For bypass grafts, coronary CT angiography has high accuracy in assessing the patency of grafts (see Fig. 1) (Higashigaito et al. 2016), but the assessment of the downstream native vessels is frequently challenging because of small vessel diameters and extensive calcifications in case of known severe CAD. However, coronary CT angiography provides valuable information concerning anatomic relationships between sternum and bypass grafts before redo-sternotomies (Meinel et al. 2015; Gasparovic et al. 2005).
6 Summary
Fast-paced technological advances have established coronary CT angiography as a reliable and highly accurate diagnostic tool with low radiation exposure. Its approved indications include, but are not limited to, evaluation of stable chest pain in patients without known CAD at low to intermediate risk, detection of CAD in patients without known CAD and reduced left ventricular ejection fraction, coronary evaluation before noncoronary cardiac surgery at intermediate risk of CAD, and assessment of coronary anomalies. In patients with acute chest pain, coronary CT angiography is indicated for optimizing triage in the emergency department. A fully diagnostic coronary CT angiography that demonstrates no plaques or stenoses excludes CAD with certainty. In order to obtain a high level of diagnostic accuracy, CT scanner systems need to provide high temporal and spatial resolutions, and patient selection needs to be steered by adherence to evidence-based indication guidelines.
References
Achenbach S, Daniel WG (2001) Noninvasive coronary angiography – an acceptable alternative? N Engl J Med 345(26):1909–1910
Alkadhi H (2009) Praxisbuch Herz-CT. Springer, Heidelberg, XVI, 177 S. p.
Alkadhi H, Leschka S (2011) Radiation dose of cardiac computed tomography – what has been achieved and what needs to be done. Eur Radiol 21(3):505–509
Angelini P (2007) Coronary artery anomalies: an entity in search of an identity. Circulation 115(10):1296–1305
Angelini P, Velasco JA, Flamm S (2002) Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation 105(20):2449–2454
Arbab-Zadeh A, Miller JM, Rochitte CE, Dewey M, Niinuma H, Gottlieb I et al (2012) Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. J Am Coll Cardiol 59(4):379–387
Bamberg F, Sommer WH, Hoffmann V, Achenbach S, Nikolaou K, Conen D et al (2011) Meta-analysis and systematic review of the long-term predictive value of assessment of coronary atherosclerosis by contrast-enhanced coronary computed tomography angiography. J Am Coll Cardiol 57(24):2426–2436
Cheng Z, Wang X, Duan Y, Wu L, Wu D, Liang C et al (2010) Detection of coronary artery anomalies by dual-source CT coronary angiography. Clin Radiol 65(10):815–822
Cury RC, Abbara S, Achenbach S, Agatston A, Berman DS, Budoff MJ et al (2016) Coronary Artery Disease – Reporting and Data System (CAD-RADS): an Expert Consensus Document of SCCT, ACR and NASCI: endorsed by the ACC. JACC Cardiovasc Imaging 9(9):1099–1113
Dappa E, Higashigaito K, Fornaro J, Leschka S, Wildermuth S, Alkadhi H (2016) Cinematic rendering – an alternative to volume rendering for 3D computed tomography imaging. Insights Imaging 1–8. doi:10.1007/s13244-016-0518-1
den Harder AM, Willemink MJ, de Jong PA, Schilham AM, Rajiah P, Takx RA et al (2016) New horizons in cardiac CT. Clin Radiol 71(8):758–767
Desjardins B, Kazerooni EA (2004) ECG-gated cardiac CT. AJR Am J Roentgenol 182(4):993–1010
Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B et al (2015) Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 372(14):1291–1300
Dougoud S, Fuchs TA, Stehli J, Clerc OF, Buechel RR, Herzog BA et al (2014) Prognostic value of coronary CT angiography on long-term follow-up of 6.9 years. Int J Card Imaging 30(5):969–976. doi:10.1007/s10554-014-0420-1
Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN et al (2004) Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med 141(11):829–834
Flohr TG, McCollough CH, Bruder H, Petersilka M, Gruber K, Suss C et al (2006) First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol 16(2):256–268
Gasparovic H, Rybicki FJ, Millstine J, Unic D, Byrne JG, Yucel K et al (2005) Three dimensional computed tomographic imaging in planning the surgical approach for redo cardiac surgery after coronary revascularization. Eur J Cardiothorac Surg 28(2):244–249
Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW et al (2011) The CT-STAT (coronary computed tomographic angiography for systematic triage of acute chest pain patients to treatment) trial. J Am Coll Cardiol 58(14):1414–1422
Gordic S, Husarik DB, Desbiolles L, Leschka S, Frauenfelder T, Alkadhi H (2014) High-pitch coronary CT angiography with third generation dual-source CT: limits of heart rate. Int J Card Imaging 30(6):1173–1179
Gordic S, Desbiolles L, Sedlmair M, Manka R, Plass A, Schmidt B et al (2016) Optimizing radiation dose by using advanced modelled iterative reconstruction in high-pitch coronary CT angiography. Eur Radiol 26(2):459–468
Hadamitzky M, Achenbach S, Al-Mallah M, Berman D, Budoff M, Cademartiri F et al (2013a) Optimized prognostic score for coronary computed tomographic angiography: results from the CONFIRM registry (COronary CT Angiography EvaluatioN For Clinical Outcomes: an InteRnational Multicenter Registry). J Am Coll Cardiol 62(5):468–476
Hadamitzky M, Taubert S, Deseive S, Byrne RA, Martinoff S, Schomig A et al (2013b) Prognostic value of coronary computed tomography angiography during 5 years of follow-up in patients with suspected coronary artery disease. Eur Heart J 34(42):3277–3285
Hamilton-Craig C, Fifoot A, Hansen M, Pincus M, Chan J, Walters DL et al (2014) Diagnostic performance and cost of CT angiography versus stress ECG – a randomized prospective study of suspected acute coronary syndrome chest pain in the emergency department (CT-COMPARE). Int J Cardiol 177(3):867–873
Herzog C, Nguyen SA, Savino G, Zwerner PL, Doll J, Nielsen CD et al (2007) Does two-segment image reconstruction at 64-section CT coronary angiography improve image quality and diagnostic accuracy? Radiology 244(1):121–129
Higashigaito K, Husarik DB, Barthelmes J, Plass AR, Manka R, Maisano F et al (2016) Computed tomography angiography of coronary artery bypass grafts: low contrast media volume protocols adapted to tube voltage. Investig Radiol 51(4):241–248
Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT et al (2012) Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 367(4):299–308
Lell MM, Anders K, Uder M, Klotz E, Ditt H, Vega-Higuera F et al (2006) New techniques in CT angiography. Radiographics 26(Suppl 1):S45–S62
Leschka S, Scheffel H, Desbiolles L, Plass A, Gaemperli O, Valenta I et al (2007) Image quality and reconstruction intervals of dual-source CT coronary angiography: recommendations for ECG-pulsing windowing. Investig Radiol 42(8):543–549
Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW et al (2012) CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med 366(15):1393–1403
Luccichenti G, Cademartiri F, Pezzella FR, Runza G, Belgrano M, Midiri M et al (2005) 3D reconstruction techniques made easy: know-how and pictures. Eur Radiol 15(10):2146–2156
Maier W, Abay M, Cook S, Togni M, Zeiher A, Meier B et al (2006) The 2002 European registry of cardiac catheter interventions. Int J Cardiol 113(3):299–304
Meinel FG, Bayer RR 2nd, Zwerner PL, De Cecco CN, Schoepf UJ, Bamberg F (2015) Coronary computed tomographic angiography in clinical practice: state of the art. Radiol Clin N Am 53(2):287–296
Menke J, Unterberg-Buchwald C, Staab W, Sohns JM, Seif Amir Hosseini A, Schwarz A (2013) Head-to-head comparison of prospectively triggered vs retrospectively gated coronary computed tomography angiography: meta-analysis of diagnostic accuracy, image quality, and radiation dose. Am Heart J 165(2):154–63.e3
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M et al (2015) Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation 131(4):e29–322
Ong TK, Chin SP, Liew CK, Chan WL, Seyfarth MT, Liew HB et al (2006) Accuracy of 64-row multidetector computed tomography in detecting coronary artery disease in 134 symptomatic patients: influence of calcification. Am Heart J 151(6):1323.e1–1323.e6
Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA (2005) Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol 46(3):552–557
SCOT-HEART Investigators (2015) CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 385(9985):2383–2391
Shi H, Aschoff AJ, Brambs HJ, Hoffmann MH (2004) Multislice CT imaging of anomalous coronary arteries. Eur Radiol 14(12):2172–2181
Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O’Gara P et al (2010) ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol 56(22):1864–1894
von Ballmoos MW, Haring B, Juillerat P, Alkadhi H (2011) Meta-analysis: diagnostic performance of low-radiation-dose coronary computed tomography angiography. Ann Intern Med 154(6):413–420
Vorre MM, Abdulla J (2013) Diagnostic accuracy and radiation dose of CT coronary angiography in atrial fibrillation: systematic review and meta-analysis. Radiology 267(2):376–386
Wever-Pinzon O, Romero J, Kelesidis I, Wever-Pinzon J, Manrique C, Budge D et al (2014) Coronary computed tomography angiography for the detection of cardiac allograft vasculopathy: a meta-analysis of prospective trials. J Am Coll Cardiol 63(19):1992–2004
Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM et al (2014) ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol 63(4):380–406
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Mannil, M., Alkadhi, H. (2017). Noninvasive Coronary Artery Imaging. In: Nikolaou, K., Bamberg, F., Laghi, A., Rubin, G.D. (eds) Multislice CT. Medical Radiology(). Springer, Cham. https://doi.org/10.1007/174_2016_89
Download citation
DOI: https://doi.org/10.1007/174_2016_89
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42585-6
Online ISBN: 978-3-319-42586-3
eBook Packages: MedicineMedicine (R0)