Abstract
A revolution in radiography has occurred in the last three decades; digital radiography has replaced screen-film radiography. To understand digital radiography, one must begin with the fundamental principles, which have not changed since Roentgen’s time. The conversion of X-rays into a visible image, however, has changed from screen-film to digital radiography. A discussion on the characteristics of digital radiography and its most common forms, computed radiography (CR) and digital flat-panel radiography follows. The fundamentals of digital image processing are discussed, including preprocessing, latitude reduction, and contrast modification. Advanced technologies are also described, including structured phosphors, slot scanners, dual-sided CR, irradiation side sampling flat panels, and gaseous avalanche detectors. The potential application of dual energy subtraction radiography and tomosynthesis to pediatric thoracic radiography is also considered. The chapter concludes with a discussion on radiation dose optimization in pediatric chest radiography including the newest standards for exposure indicators, dose area product, dose reporting, and informatics initiatives to support dose reporting.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Compute Radiography
- Digital Radiography
- Image Receptor
- Dose Area Product
- International Electrotechnical Commission
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The fundamental principles in creating a radiographic projection image remain unchanged from the time of Roentgen. That is, a polyenergetic beam of X-rays is produced by high voltage acceleration of electrons into a high-Z target such as tungsten where their kinetic energy is transformed into radiant energy. This X-ray beam, shaped by collimation, is directed toward a patient. The beam is differentially attenuated by portions of the patient’s anatomy that differ in density, thickness, or composition, creating a shadow when projected on a flat surface. Because the X-rays are too high in energy to be detected by the human eye, a process of conversion into a visible image must be employed in order for the shadow to be appreciated by a human observer. It is the process of conversion that has undergone a revolution during the last three decades, replacing screen-film radiography with digital radiography.
The design and terminology of the digital radiography is rooted in screen-film radiography. Many radiologists and technologists were trained in screen-film radiography. Much of the technical literature reflects the perspective of screen-film radiography. Many of our misconceptions about the new technology are caused by our attempt to apply familiar concepts from screen-film radiography. There are still hospitals using screen-film radiography today. To fully understand this new technology, a review of conventional screen-film radiography is worthwhile.
2 Conventional Screen-Film Radiography
Conventional screen-film radiography relies on conversion of X-rays into light through photoelectric interactions with an intensification screen. The fluorescent light from the screen creates a latent image by activating silver halide grains in the film so they are able to be reduced to elemental silver by a chemical developer. This developed image is then fixed by removing all remaining silver halide using acetic acid. The negative image is then viewed by transillumination. Dark areas, such as the lungs, show low attenuation and light areas, such as bones, show high attenuation of the original X-ray beam. The contrast that is visible in the conventional radiograph is governed by a number of well-known factors such as the X-ray beam quality, i.e., the effective energy, the patient anatomy in the beam, the ratio of scattered radiation to primary X-rays at the image receptor, the inherent properties of the screen-film combination manifested in the characteristic Hurter and Driffield curve, the specifics of the chemical development, and the manner of transillumination. All these variables were fine-tuned over more than a century of research and development and clinical practice. The result was a single rendering of the X-ray shadow that could be appreciated by a human observer and served as a permanent record of the radiographic examination.
The screen-film combination produced a diagnostic quality image under relatively limited conditions of exposure, so that exposure techniques needed to be tightly controlled. Radiographic technique guides were designed to accommodate different anatomical views and patient sizes while still delivering the necessary X-ray exposure to the image receptor. The wide variation in patient sizes from neonate to adult made it necessary to design specialized technique guides for pediatric radiographic imaging. Automatic exposure control (AEC), the primary mechanism for controlling exposure factor technique for routine adult thoracic radiography, was unsuitable for most pediatric chest examinations because the physical dimensions of the AEC ion chambers did not correspond to appropriate pediatric anatomical regions, and even with smaller “pediatric chambers” the task of assuring proper registration of the anatomy of a small, non-compliant patient with a small AEC chamber was impractical. Mis-registration can result in either over- or under-exposure, and either can produce non-diagnostic screen-film radiographs. For this reason, manual or fixed technique is preferred in most pediatric radiographic imaging at most centers, although there is no universal agreement.
The optical density in the developed film depended on exposure in a unique manner specific to the screen-film combination, so a variety of screen-film cassettes were designed for different radiographic examinations. Selections included general purpose, extremity, and chest cassettes. Screen-film combinations for chest imaging were designed to produce a long enough latitude to capture the low attenuation regions of the lungs, as well as the higher attenuation regions of the mediastinum and bony structures of the thorax. The pinnacle of screen-film technology for the chest was the asymmetric screen-film combination where the front and rear intensification screens were not only of different thicknesses, but also composed of different materials.
The speed of a screen-film combination was the inverse of the amount of x-ray exposure necessary to produce one optical density unit (above base-plus-fog) in the film when developed according to the manufacturer’s specifications. Speed classes were created to categorize screen-film combinations for comparison to the speed of traditional calcium tungstate screens, so-called par speed screens, which were assigned a speed class of 100. These screens were termed “slow,” 200 speed class was also known as “medium,” and 400 speed class was called “fast.” Screen-film combinations of 800 and 1600 speed class were also manufactured and often used in bedside radiography.
It is possible to use the same material to produce screen-film combinations of different speed classes. The thicker the layer of screen material, the more light is produced by the same amount of X-ray exposure, and hence a faster screen-film system. The drawback of this approach is that the thicker the intensification screen, the more light is produced at different depths in the screen and consequently the more unsharpness or blur by the time the light reaches the film. The speed class is inversely related to the spatial resolution in the resulting radiograph. Slow speed screen-film systems, used in extremity cassettes, are also called “detail” cassettes because of their higher resolution.
In order to produce even more light, screen-film cassettes typically had two screens with the film sandwiched between. The photographic emulsion was usually coated on both sides of the polyester base resulting in a double-emulsion film. However, a single emulsion film could be used in conjunction with one or two screens in order to improve the resulting detail. Single emulsion film was typically used with small parts imaging such as mid to distal extremities.
The slower (lower) the speed class, the more the X-ray exposure needed to produce a diagnostic image and the higher the X-ray exposure to the patient. In order to reduce radiation exposure to pediatric patients, many hospitals adopted fast and ultra fast screen-film systems (600 speed or greater), sacrificing the spatial resolution in the radiographic image. Other hospitals that appreciated the visualization of small clinical features in the pediatric thorax deliberately chose medium speed class systems for pediatric radiography. It should be understood that patient radiation doses are small in projection radiography, and even with repeated examinations, the total dose does not approach the magnitude of a single fluoroscopic examination or that from a computed tomographic examination (Willis 2002).
Projection radiography of the pediatric chest is complicated by the variation in size and compliance among children. The same child may be imaged in a posterior–anterior (PA) orientation at an upright exposure station using a long source-to-image distance (SID), an anterior–posterior (AP) orientation at the same upright exposure station, in AP orientation atop a table using a short SID, and in AP orientation either bedside or in an ICU setting (Fig. 1). The variation in acquisition geometry causes variation in the distortion of clinical features projected on the same flat image receptor. This in turn complicates the determination of interval change by the radiologist.
The lack of compliance in children has led to the development of specialized accommodations for immobilization during the examination. Immobilization devices are not completely radiolucent and cast shadows on the projected image (Fig. 2). Because some of the X-rays exiting the patient are attenuated by the device before reaching the image receptor, more X-rays must be used resulting in a small penalty in radiation dose to the patient.
Because there was only one copy of the image, competition arose between physicians who wanted to use the information contained in the radiograph. For example, radiologists needed the image for primary interpretation while intensivists needed the image to monitor the condition of the patient and to provide immediate feedback on therapeutic procedures such as central venous line placement. This competition was resolved by two methods: copy film and double-loading. Upon developing the radiograph, the darkroom technician could immediately produce a copy film which was supplied to the intensivist. In this case, the radiologist would interpret the original radiograph, and the intensivist would have an image of compromised quality. An alternative method was double-loading of cassettes, that is, placing two sheets of film between the two fluorescent intensification screens to produce two radiographs from a single exposure. This practice led to two radiographs of compromised image quality, but satisfied the needs of two geographically dispersed observers. Neither method was optimal for both physicians. Both methods required additional materials and technologist time.
3 Digital Radiography
With the advent of computers and improvement in image distribution using networks, screen-film radiography has been largely supplanted by digital radiography. Digital images are available at multiple locations within a hospital network at the same time. Both the radiologist and the patient care provider can view the same study simultaneously at disparate locations. An extensive review of the technology and its application to chest radiography was published by Schaefer-Prokop et al. (2008).
Historically, digital radiography can be divided into two broad subtypes, computed radiography which requires a separate laser readout step and digital flat panel radiography which integrates the readout step into the imaging detector. These distinctions are blurred with the newer technologies. An alternative categorization divides digital radiography into “cassette-based” and “cassetteless” depending on the form-factor of the image receptor, irrespective of the image acquisition technology (Seibert 2007; Romlein 2007; Willis 2008).
3.1 Computed Radiography
Computed radiography (CR), also known as photo-stimulable phosphor (PSP) radiography, is based on the principle of photo-stimulable luminescence (PSL). A number of crystalline materials having some impurities that cause crystal defects, such as europium-doped barium fluorohalide, are able to store energy for an indeterminate period, and subsequently release that energy when exposed to light. The phenomenon has been known for centuries, but was only recently applied to imaging (Luckey 1975). An excellent review of CR has been published by Rowlands (2002). Coincident with the introduction of CR into clinical practice in the United States, the first reports of its use for radiographic imaging of the pediatric thorax are found (Kogutt et al. 1988; Cohen et al. 1989, 1991; Tarver et al. 1990; Merlo et al. 1991).
Three major film manufacturers competed to field CR products for clinical radiography. Oddly enough the original motivation was simply the manufacture of film. Suppose that a radiographic image could be captured on a single media, and the output to a single type of film. Since the image was digitized as an intermediate step in the process, it could be modified so that the resulting film image mimicked the image that would have been produced by any one of a dozen screen-film combinations. Multiple identical copies of the image could be printed, resolving the competition between the radiologist and patient caregiver for a single unique radiograph. It is important to understand that digital imaging was not originally intended to replace film, rather to make the manufacture of film more efficient and was likely to produce and sell more film!
The physical mechanism of PSL is not completely understood. The interaction of X-rays with the PSP material excites electrons, which can de-excite by prompt emission of light (fluorescence). Some of the excited electrons do not immediately give up their energy; instead they are trapped at a higher than normal energy state in local potential energy “wells” associated with defects in the crystal lattice (also known as “color centers”). The trapped electrons constitute the latent image. Over time the trapped electrons can escape on their own, but the fading of the stored signal is very gradual. When the PSP is exposed to light of a particular wavelength using a laser reader, the trapped electrons absorb enough energy to escape their traps and de-excite with the emission of visible light. The amount of emitted light is proportional to the original amount of X-ray exposure, so that it faithfully represents the projected X-ray shadow. The light can be collected and amplified by a photomultiplier tube and converted by an analog-to-digital converter into a digital value that is representative of the original X-ray exposure to the PSP. The original design of the laser readers was 90 s intended to compete with automatic film processor cycle time. The current generation of laser readers requires about 30–40 s to process the latent image.
3.2 Digital Flat-Panel Radiography
Digital radiography without the intermediate latent image and physical processing required by PSP-based radiography was first reported in the early 1980s. Scanned projection radiography was accomplished by means of a flying spot scanner that involved mechanical motion of a specially collimated X-ray generator and detector. The detector was a sodium iodide (NaI) scintillation crystal attached to a photomultiplier tube. Like CR, early application to pediatric chest radiography was reported almost simultaneously (Heller et al. 1982; Kushner 1983). Early reports also considered scanned linear arrays, such as the scout view of a Computed Tomography system as a means to generate a DR image of the chest.
Early DR systems relied on video cameras, and later charge-coupled device (CCD) arrays. Video cameras had very limited spatial resolution. CCD arrays have very small dimensions, therefore, a large field of view (FOV) must be minified somehow to conform to the small CCD array. This can be accomplished either by optical lenses or by tapered fiber optic bundles. Both of these methods have large losses in efficiency.
The amorphous silicon (a-Si) thin film transistor (TFT) array is the technology that made DR practical for medical imaging. The TFT array is bonded to the X-ray detector, eliminating the need for a separate reader step. Typical processing time is 10 s or less, much faster than CR. DR systems require some sort of X-ray conversion material. These flat-panel detectors are divided into indirect DR systems and direct DR systems based on the method of conversion. Indirect DR systems use an X-ray conversion layer, or scintillator, that converts X-rays into visible light by means of fluorescence. This is functionally similar to the intensification screen of the screen-film system, or to the input phosphor of an imaging intensifier for a fluoroscopy system. Gadolinium oxysulfide (Gd2O2S) and cesium iodide (CsI) are used in commercial indirect DR systems. Both of these materials convert X-rays into visible light. Visible light is easier to convert into an electronic signal (charge).
Direct DR systems use a thick amorphous selenium (a-Se) layer which converts X-rays directly into charge without the intermediate fluorescence step. High voltage across the amorphous selenium causes the charges (electron/hole pairs) that are created to migrate directly to the TFT elements where they are collected without lateral diffusion. This gives direct DR exceptional sharpness (spatial resolution). Direct DR systems are most often found in mammography applications.
4 Advanced Detector Technologies
4.1 Structured Phosphor
X-ray conversion layers differ in a fundamental way. Gd2O2S crystals are contained in a binder medium without any particular structure. On the other hand, CsI crystals are needles arranged parallel to each other (Fig. 3). This structure channels the fluorescence within each crystal toward the TFT array and discourages lateral diffusion of the light. For this reason, indirect DR systems that use CsI are expected to have better sharpness than those that use Gd2O2S. Alternatively, CsI conversion layers can be designed to provide better efficiency than Gd2O2S with the same sharpness, simply by making them thicker.
Photomicrographs of unstructured and structured phosphors. a unstructured or powder phosphor. Gadolinium oxysulfide (GOS; Gd2O2S) is used in DR and barium fluorobromide (BaFBr) is used in CR. b structured or needle phosphor. Cesium iodide (CsI) is used in DR and cesium bromide (CsBr) is used in CR. Courtesy of AGFA Healthcare
The same principle can be applied to CR. Photo-stimulable phosphor materials are typically crystals contained in a binder without any organized structure. However, cesium bromide (CsBr) crystals can be made into an organized structure like CsI, and can also be doped to produce photo-stimulated luminescence (Leblans et al. 2000). The CsBr structured phosphor has the same advantages as CsI for restricted lateral diffusion of light over unstructured phosphors.
4.2 Slot Scanner
Scattered radiation degrades contrast in projection radiography. The anti-scatter grid is a well-known countermeasure, but has a penalty in requiring more X-ray exposure to get the same signal at the image receptor, with a corresponding increase in patient radiation dose. The amount of scattered radiation depends on the volume of tissue in the X-ray field—even for small patients this volume can be substantial when the entire image receptor is exposed at once. The mechanical slot-scanner moves a fan-shaped beam of X-rays across the FOV. In this way, a smaller volume of tissue is irradiated at one time, and the amount of scattered radiation is much less. This reduction in scatter is achieved without any dose penalty to the patient.
Slot scanners have shown significantly reduced scatter fraction compared with anti-scatter grids (Samei et al. 2004; Liu et al. 2008) and have shown some improved detection of simulated nodules and interstitial lung pathology in phantom testing (Kroft et al. 2004, 2005) but there is no information on use in clinical pediatric chest imaging.
4.3 Dual-Sided CR
The light from PSL is emitted in all directions. Conventional CR scanner designs collect light from the same side of the imaging plate that is stimulated by the laser, collecting at best only one-half of the PSL that is emitted. If the phosphor material is coated onto a translucent base, then it is possible to collect PSL from the back side of the imaging plate as well. Combining the signal collected from the back with the signal from the front improves the efficiency of detection with a small penalty in sharpness. Uffmann et al. (2005) reported improvement in the detection of simulated nodules using dual-sided CR compared to single-sided CR.
4.4 Irradiation Side Sampling Digital Flat Panel
This flat panel detector is designed so that the X-ray beam exiting the patient passes through the TFT array before reaching the CsI X-ray conversion layer. The TFT array is relatively radio-transparent, so there is little loss of signal. The majority of X-ray interactions generate fluorescence closer to the TFT array, so that there is less unsharpness from spreading of the light. In addition, the CsI crystals are grown on a substrate and then reversed and optically coupled to the TFT array. In this way, the fluorescence exits the top of the crystals, instead of the base of the crystals so that there is less diffusion of light from needle-to-needle. This detector is intended to produce better spatial resolution than conventional indirect DR designs.
4.5 Gaseous/Avalanche Detectors
A novel xenon (Xe) high-pressure (6 atm) gas-filled detector has been incorporated into a commercial imaging product primarily intended for orthopedic imaging. The detector has a high voltage electrode and an antenna array that amplifies photoelectrons produced from interactions with the X-ray beam into an avalanche that is collected by printed microstrips. The imaging system incorporates two of these detectors each paired with an X-ray tube collimated into a fan beam. The two fan beam/detector pairs are oriented orthogonally to each other. The subject stands upright in the imaging system and the fan beam/detector pairs descend from their highest extent to scan the patient simultaneously in the PA/AP and lateral orientation. The images are created line-by-line.
Commercial gas/avalanche slot scanners have been used for scoliosis examinations with a reduction in skin dose by a factor of 6–9 over CR systems with improved subjective image quality ratings (Deschenes et al. 2010; Despres et al. 2005). The time required to scan the thorax is 4–5 s, which may limit its utility for pediatric chest radiography, considering the potential for body motion, breathing, and multiple cardiac cycles during acquisition.
5 Digital Image Processing
The principal advantage of the digital image compared to the conventional radiograph is the ability for multiple caregivers simultaneously to view the study in disparate locations. In addition to the advantage of availability, the digital image can be modified from its original state creating an infinite variety of possible presentations. In fact, modification of the original digital image is not just a cosmetic enhancement; this is absolutely required in order to render it usable for clinical diagnosis. By changing processing, one can enhance the image to identify abnormalities such as pneumothorax or highlight catheter placement.
A plethora of schemes and brand names exist for digital image processing. These are often cited by sales personnel as basis for differentiation among products. Irrespective of the specific method of acquisition, functional categories of image processing can be identified.
5.1 Preprocessing
Preprocessing (sometimes called “pre-acquisition processing”) involves corrections that are applied to the raw digital data. These include, in the case of CR and linear scan systems, corrections for nonuniform light collection efficiency in one dimension across the image receptor, and in the case of DR and other two-dimensional fixed array systems, corrections for gain, and offset nonuniformity among individual detector elements and amplifiers as well as correction of nonfunctional (“dead”) detector elements (Fig. 4).
Preprocessing may also include rescaling of the numerical values of the digital data so that they bear a particular mathematical relationship to the X-ray exposure that produced them. For example, the raw data may have a linear relationship to the X-ray exposure and preprocessing may transform them so that they are linear with respect to the logarithm of exposure. Preprocessing is applied automatically according to data gathered during calibration protocols. Preprocessing is generally transparent to the radiologist, unless there is an error in the calibration. The digital image that results from preprocessing is called the “original data” in IEC terminology, “for-processing data” in DICOM terminology, and may be called “raw, ranged data” for some systems that incorporate auto-ranging in their preprocessing (Fig. 5).
PA chest image of a 12-year-old female. a “for processing” image and grayscale histogram. Note the lack of contrast between soft tissue and bone in the “for processing” image. The wide histogram ranges from 234 through 8784 but the relevant image is centered at 1785. In the “for processing” image, grayscale values increase linearly with exposure. This DR system rescales the grayscale values during processing so that their numerical values increase with the negative logarithm of exposure. b “for presentation” image and grayscale histogram. The “values of interest” (VOI) have been determined, the window-width and window-level adjusted so that contrast is appropriate for the final, displayed image
5.2 Latitude Reduction
DR systems have an extremely wide latitude compared to conventional screen-film systems, that is, they are able to capture X-ray exposures over a range of ten-thousand versus a range of one-hundred for screen-film radiography. DR systems are much more tolerant of underexposure and overexposure, reducing the need to retake images for these reasons. The range of exposures present in the X-ray shadow of any particular anatomic projection is only about a range of one-hundred. This means that the original DR image has extremely low contrast compared to a screen-film image. The primary purpose of digital image processing is therefore to determine the digital “values of interest” (VOI) that correspond to clinical features in the image and to remap those values to increase contrast, while sacrificing contrast elsewhere. Contrast is increased for clinical features by selectively reducing the overall latitude.
5.2.1 Exposure Recognition. Detection of Collimator Boundaries or Anatomy
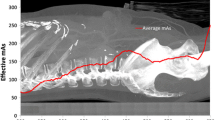
The first task in determining the VOI is to locate the area within the field of view (FOV) that has received substantial X-ray exposure. The common method for accomplishing this is to locate the boundaries of collimation, which is fairly easy considering that the exposure outside the collimators is much less than inside the collimators, even if patient anatomy is in the beam. A more sophisticated method used by one manufacturer is to locate the edges of the projected anatomy within the FOV. Both these methods are subject to interferences from shielding and high density materials such as orthopedic implants that overlie edges of the X-ray field. Collimation is notoriously variable in pediatric chest radiography, causing one manufacturer to develop special pediatric examinations that rely on a neural network to identify the VOI.
5.2.2 Window-Width and Window-Level Adjustment According to Grayscale Histogram
Most DR systems perform an analysis of a grayscale histogram which is a bar-graph of the number of picture elements (pixels) within the VOI versus their signal value (Fig. 5). The histogram analysis may involve some expectations about the shape of the histogram for the specific anatomic view, and results in a window-width (WW) and window-level (WL) for the default presentation of the digital image. The histogram analysis may also report a value indicative of the X-ray exposure that the image receptor received.
There are three important consequences of adjustment of the WW and WL by this automated method. First, over and underexposure are compensated by shifting the WL. Second, the latitude of images from patients of different sizes or acquired using different kVps are matched by changing the WW. Third, contrast of features within the VOI is maximized by displaying the digital image with the WW and WL.
5.2.3 Contrast Modification
The digital image can be subjected to any number of exotic processes to modify the contrast of specific features for specific purposes. All of these processes involve sacrificing contrast of some details in order to increase contrast of others. The simplest process is remapping of the digital values according to a nonlinear look-up-table (LUT) to achieve a screen-film-like appearance. A more complex process often used is called an unsharp-mask, where a blurred version of the images is subtracted from the original image to create a mask, which is added back to the original image to enhance the contrast of high frequency features, i.e., edge enhancement (Fig. 6). Even more complex methods decompose the digital image into frequency bands and apply filters or amplification (boost) in order to improve sharpness, reduce noise, or generate different contrast according to the size of objects in the image.
These automated processes are controlled by the specification of numerous adjustable parameters, some of which are under operator control and some of which may only be known to manufacturer personnel. The appropriate amount of image processing depends on the anatomic view and thickness. For example, the processing for a bedside neonatal chest image will differ greatly from the processing for an upright adult chest image. There are no standards for digital image processing, so the nomenclature and parameter settings are unique from manufacturer to manufacturer. Obtaining the same look from two different manufacturers is possible, but complicated. Customization of image processing parameters for every possible radiographic view and patient size is extremely labor intensive, and is not adequately facilitated for the user in current DR systems.
6 Dose Reporting
6.1 Exposure Indicators
As mentioned in Sect. 5.2.2 above, histogram analysis of the digital image provides an indication of the radiation exposure to the image receptor. Until recently, there was no standardization of the mathematical form, calibration conditions, or units of exposure for exposure indicators. Each manufacturer had its own proprietary exposure indicator. The diversity in the relationship of these indicators to the receptor exposure, e.g., linear versus logarithmic, direct or inverse, confused technologists and radiologists. A parallel effort by the American Association of Physicists in Medicine (AAPM; Shepard et al. 2009) and the International Electrotechnical Commission (IEC; IEC 62494-1:2008) has created a standard for exposure indicators that is linearly and directly related to the plate exposure (Seibert and Morin 2011) and is being implemented in many new digital radiography products. From a radiologist’s perspective, the standards are very similar and functionally equivalent. The Medical Imaging & Technology Alliance (MITA), representing many digital radiographic equipment manufacturers, has agreed to use the IEC standard (Vastagh 2011).
There are three terms that a radiologist should learn in order to understand the new standard (Don et al. 2012). The Exposure Index (EI) is a measure of the entrance air KERMA at the image receptor, K cal. Its value is compared to a target air KERMA, Exposure Index Target (EI T ), is the “optimized” reference exposure index that is specific for the particular anatomic view and image receptor. A Deviation Index (DI) is then reported to provide feedback on how far the actual exposure was from the target value. According to the IEC standard,
Table 1 illustrates the relationship between the deviation index value and the fraction of the intended exposure to the image receptor.
A deviation index of ±1 corresponds to approximately one mAs station on a well-calibrated X-ray system. These mAs stations follow a geometric sequence known as a “Renard series” (also known as ISO R’10) where each step corresponds to a change of approximately 25 %. A deviation index of ±3 corresponds to a doubling or halving of exposure from the target value.
Traditional exposure indicators and those that follow the new standard are both subject to interferences and require proper calibration to yield meaningful data. In the best case, they represent the exposure to the image receptor. The exposure indicators are not measures of patient dose. With some assumptions about patient thickness, X-ray field size, and technique factors such as kVp, source-to-image distance (SID), and presence of a grid, patient dose can be estimated from the value of the exposure indicator.
6.2 Dose Area Product
Dose Area Product (DAP), or more appropriately KERMA Area Product (KAP), is a quantity that represents the air KERMA at any point along the central axis of the X-ray beam times the field dimensions at that same point. The “dose” in DAP is actually the dose to air, so that DAP and KAP are fundamentally equivalent.Footnote 1 The value of DAP (in units of air KERMA times distance squared) is constant at any distance along the central axis, because the reduction in air KERMA from the inverse square law is counteracted by the divergence of the beam, as illustrated in Fig. 7. If the FOV is known at the entrance surface of the patient, the dose to the patient can be calculated from the entrance air KERMA.
DAP may be measured using an ion chamber attached to the collimator assembly, or it may be calculated from knowledge of the X-ray output and FOV. In the case of DAP, integrated DR systems have a distinct advantage over cassette-based systems and incompletely integrated “add-ons”; integrated systems have knowledge of the radiographic technique and collimation and can therefore estimate DAP. The radiographic technique and FOV can also be reported in the header of the digital image for retrospective analysis. It is important to note that the International Electrotechnical Commission (IEC) requires only ±35 % accuracy in dosimetric indications such as DAP, so radiologists should not regard reported values as absolute.
6.3 Informatics Initiatives for Dose Reporting
In the development of connectivity to support electronic imaging, dose reporting was an afterthought. Early attempts to monitor dose relied on interpreting irradiation events reported via the DICOM Modality Performed Procedure Step (MPPS) or on inspection of DICOM header elements from individual images. These methods are still in use. Recent efforts have begun to define and standardize dose reporting within the existing framework of the Digital Imaging and Communications in Medicine (DICOM) standard and the Integrating the Healthcare Enterprise (IHE®) initiative.
The DICOM Radiation Dose Structured Report (SR) is the information object now intended to contain specific data about the dose delivered during an irradiation event, including projection radiography. The IHE Radiation Exposure Monitoring (REM) profile depends on the DICOM SR templates to retrieve and archive dose information. The emphasis of development of these standards was dose monitoring for Computed Tomography, so their current implementations are imperfect for projection radiography; however, there are active efforts to improve their functionality. When these features are fully standardized, they should facilitate the collection of dose information within a large healthcare enterprise, as well as multi-institutional data collection, such as the American College of Radiology’s Dose Index Registry. These collections will enable the establishment of dose reference levels for comparing local practice patterns with national standards of practice.
7 Advanced Imaging Technologies with Potential Application to Pediatric Thoracic Radiography
7.1 Dual Energy Subtraction
The bony structures in the thorax can interfere with the inspection of soft tissue features in a radiographic projection. The attenuation of bone relative to soft tissue decreases as kVp increases. In fact, high kVp chest imaging is routinely performed to reduce the contrast of bone relative to soft tissue. Because of the differences in attenuation, a low kVp image and a high kVp image can be combined using digital image processing to produce a bone-only and a soft tissue only image. Instead of making two exposures, it is possible to expose two image receptors simultaneously, one behind the other with a copper filter sandwiched in between, to produce a low energy image (front) and a hardened, high energy image (back). Dual energy subtraction is used extensively at some hospitals for adult chest radiography especially for pulmonary nodule detection, but there is only anecdotal incidental use for pediatric chest examinations.
There are other digital image processing methods for removing the bony structures from a single standard digital chest radiograph without a second exposure or second receptor. One method uses a neural network that is trained to detect bones and remove them. Another method detects the edges of bones and then reduces the contrast of the edges so that the bones are less visible to an observer.
7.2 Tomosynthesis
A limitation of projection radiography is that overlying anatomic structures sometimes interfere with the visibility of important clinical features. The lateral view is a practical method for visualizing underlying structures, as are other views such as obliques. Conventional tomography was a method of making an image of a single slice in the patient by moving the X-ray tube and image receptor to blur out details in other planes. With digital radiography, it is possible to acquire many projections from different aspects by tilting the X-ray tube. These images can be combined to reconstruct slices in multiple planes. The reconstruction can be repeated from the original digital images without making any additional exposures. The overall dose to the patient from digital tomosynthesis is much less than Computed Tomography (CT), and the spatial resolution within each slice is superior to CT.
Digital tomosynthesis is not yet widely used in chest radiography and has only been recently introduced into mammography. Digital tomosynthesis has been used in the evaluation of cystic fibrosis in children (Vult von Styern et al. 2012). Reductions in patient dose from CT, such as iterative reconstruction, and breathholding requirements for digital tomosynthesis may limit the application of digital tomosynthesis in pediatrics.
8 Radiation Dose Optimization in Pediatric Chest Radiography
Currently there is considerable enthusiasm for reducing the amount of ionizing radiation used in medical imaging of children. The Image Gently® campaign is an international effort to optimize dose, including their recent “Back-to-Basics” campaign and a review article suggesting ten steps to manage radiation dose (Don et al. 2013). There are many ways to reduce patient dose (Willis 2009), however, optimization cannot be achieved without simultaneous consideration of image quality and the clinical purpose of the examination. The balance of these competing factors is discussed by Uffmann and Schaefer-Prokop (2009). It makes little sense to reduce the radiation dose if the resulting image is so noisy that important clinical features are no longer discernible. Different image receptor technologies also have different efficiencies reflected in the dose that is necessary to detect disease (Kroft et al. 2005). Better diagnostic quality at the same patient dose may be preferable to a lower dose that yields the same image quality.
Consider that the radiation dose to the patient in pediatric chest radiography is already very low, that the potential consequence of exposure to ionizing radiation, e.g., cancer, is displaced far in time from the exposure, and that our best estimates of risk of radiogenic cancer at these exposure levels are speculative and miniscule.
“The American Association of Physicists in Medicine (AAPM) acknowledges that medical imaging procedures should be appropriate and conducted at the lowest radiation dose consistent with acquisition of the desired information. Discussion of risks related to radiation dose from medical imaging procedures should be accompanied by acknowledgement of the benefits of the procedures. Risks of medical imaging at effective doses below 50 mSv for single procedures or 100 mSv for multiple procedures over short time periods are too low to be detectable and may be nonexistent.” (AAPM Position Statement 2011).
For thoracic radiography, as long as there is a valid reason for obtaining the radiograph, the benefits greatly outweigh any long-term, theoretical risk. The consequence of misinterpreting the patient’s condition is real and may be immediate. Heroic efforts to reduce radiation dose in pediatric chest radiography are therefore unwarranted in comparison to the urgency of the examination.
An argument can be made that small improvements in radiation dose have more impact for patients that undergo serial imaging, such as those in the NICU or PICU, rather than those who have routine examinations. While this is true, the urgency of the examination and the potential consequences of misinterpretation are also greater and more immediate, these patients generally have more important medical concerns than the possible risk of cancer later in life.
Nevertheless, we have extensive evidence of deleterious effects of ionizing radiation, and should make judicious use of radiation in our practice of pediatric radiology. Reduction of radiation dose in pediatric chest radiology should be a team effort among the pediatric radiologist, technologist, and the diagnostic medical physicist. It should consider the image quality demanded by the diagnostic task, as well as modifications of digital image processing to accommodate the lower dose to the image receptor. Advances in dose reporting should facilitate oversight of technologist practice by the pediatric radiologist.
Notes
- 1.
Some older systems report “EAP”, or “Exposure Area Product” in traditional units of Roentgen times distance squared.
References
AAPM position statement on radiation risks from medical imaging. PP-25. 12/13/2011 http://www.aapm.org/org/policies/details.asp?id=318&type=PP¤t=true Accessed 29 April 2013
Cohen MD, Long B, Cory DA, Broderick NJ, Smith JA (1989) Digital imaging of the newborn chest. Clin Radiol 40:365–368
Cohen MD, Katz BP, Kalasinski LA, White SJ, Smith JA, Long B (1991) Digital imaging with a photostimulable phosphor in the chest of newborns. Radiology 181(3):829–832
Deschênes S, Charron G, Beaudoin G, Labelle H, Dubois J, Miron MC, Parent S (2010) Diagnostic imaging of spinal deformities: reducing patients radiation dose with a new slot-scanning X-ray imager. Spine 35:989–994
Després P, Beaudoin G, Gravel P, de Guise JA (2005) Physical characteristics of a low-dose gas microstrip detector for orthopedic x-ray imaging. Med Phys 32(4):1193–1204
Don S, Whiting BR, Rutz LJ, Apgar BK (2012) New exposure indicators for digital radiography simplified for radiologists and technologists. Am J Roentgenol 199(6):1337–1341. doi:10.2214/AJR.12.8678
Don S, Macdougall R, Strauss K, Moore QT, Goske MJ, Cohen M, Herrmann T, John SD, Noble L, Morrison G, Lehman L, Whiting BR (2013) Image gently campaign back to basics initiative: ten steps to help manage radiation dose in pediatric digital radiography. Am J Roentgenol 200(5):W431–W436. doi:10.2214/AJR.12.9895
Heller RM, Erickson JJ, Price RR (1982) Pediatric non-angiographic applications of digital radiography. In: Price RR, Rollo FD, Mo-nahan WG, James AE Jr (eds) Digital radiography: a focus on clinical utility. Grune & Stratton, New York, pp 267–277
Kogutt MS, Jones JP, Perkins DD (1988) Low-dose digital computed radiography in pediatric chest imaging. Am J Roentgenol 151:775–779
Kroft LJM, Geleijns J, Mertens BJA, Veldkamp WJH, Zonderland HM (2004) Digital slot-scan charge-coupled device radiography versus AMBER and bucky screen-film radiography for detection of simulated nodules and interstitial disease in a chest phantom. Radiol 231(1):156–163
Kroft LJM, Veldkamp WJH, Mertens BJA, Boot MV, Geleijns J (2005) Comparison of eight different digital chest radiography systems: variation in detection of simulated chest disease. Am J Roentgenol 185:339–346
Kushner DC (1983) Scanning beam low dose digital radiography: initial clinical trials relevant to pediatric radiology [abstr]. Am J Roentgenol 141:847
Leblans P, Struye L, Willems P (2000) A new needle-crystalline computed radiography detector. J Digit Imaging 13(2)Suppl 0031:117–120
Liu X, Shaw CC, Lai CJ, Altunbasa MC, Chen L, Han T, Wang T (2008) Scatter rejection and low-contrast performance of a slot-scan digital chest radiography system with electronic aft-collimation: a chest phantom study. Med Phys 35(6):2391–2402
Luckey GW (1975) Apparatus and method for producing images corresponding to patterns of high-energy radiation. US Patent 3,859,527, 01 Jan 1975
Medical electrical equipment—Exposure index of digital X-ray imaging systems—Part 1: Definitions and requirements for general radiography, International Electrotechnical Commission (IEC), international standard 62494-1-08 Geneva, Switzerland, 2008
Merlo L, Bighi S, Cervi PM, Lupi L (1991) Computed radiography in neonatal intensive care. Pediatr Radiol 21:94–96
Rowlands JA (2002) The physics of computed radiography. Phys Med Biol 47:R123–R166
Romlein J (2007) CR versus DR? Blurred lines of distinction. Applied Radiol December Suppl:8–10
Samei E, Saunders RS, Lo JY, Dobbins JT III, Jesneck JL, Floyd CE, Ravin CE (2004) Fundamental imaging characteristics of a slot-scan digital chest radiographic system. Med Phys 31(9):2687–2698
Schaefer-Prokop C, Neitzel U, Venema HW, Uffmann M, Prokop M (2008) Digital chest radiography: an update on modern technology, dose containment and control of image quality. Eur Radiol 18:1818–1830
Seibert JA (2007) Digital radiography: CR versus DR? Time to reconsider the options, the definitions, and the current capabilities. Applied Radiol December Suppl:4–7
Seibert JA, Morin RL (2011) The standardized exposure index for digital radiography: an opportunity for optimization of radiation dose to the pediatric population. Pediatr Radiol 41:573–581
Shepard SJ, Wang J, Flynn M, Gingold E, Goldman L, Krugh K, Leong DL, Mah E, Ogden K, Peck D, Samei E, Wang J, Willis CE (2009) An exposure indicator for digital radiography: AAPM Task Group 116 (Executive Summary). Med Phys 36(7):2898–2914
Tarver RD, Cohen M, Broderick NJ, Conces DJ Jr (1990) Pediatric digital chest imaging. J Thorac Imaging 5(1):31–35
Uffmann M, Prokop M, Eisenhuber E, Fuchsjager M, Weber M, Schaefer-Prokop C (2005) Computed radiography and direct radiography: influence of acquisition dose on the detection of simulated lesions. Invest Radiol 40(5):249–256
Uffmann M, Schaefer-Prokop C (2009) Digital radiography: the balance between image quality and required radiation dose. Eur J Radiol 72(2):202–208
Vastagh S (2011) Statement by MITA on behalf of the MITA CR-DR group of the X-ray section. Pediatr Radiol 41:566. doi:10.1007/s00247-010-1961-7
Vult von Steyern K, Björkman-Burtscher IM, Bozovic G, Wiklund M, Geijer M (2012) Description and validation of a scoring system for tomosynthesis in pulmonary cystic fibrosis. Eur Radiol 22:2718–2728
Willis CE (2002) Computed radiography: a higher dose? Pediatr Radiol 32:745–750
Willis CE (2008) Digital radiography: CR versus DR? Sometimes recognizing the distinction in technologies makes a difference. Applied Radiol January:25–28
Willis CE (2009) Optimizing digital radiography of children. Eur J Radiol 72(2):266–273
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Willis, C.E., Don, S. (2013). Advances in Chest Radiography Techniques: CR, DR, Tomosynthesis, and Radiation Dose Optimization. In: Garcia-Peña, P., Guillerman, R. (eds) Pediatric Chest Imaging. Medical Radiology(). Springer, Berlin, Heidelberg. https://doi.org/10.1007/174_2013_938
Download citation
DOI: https://doi.org/10.1007/174_2013_938
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37336-7
Online ISBN: 978-3-642-37337-4
eBook Packages: MedicineMedicine (R0)