Abstract
Endolysosomal ion channels are a group of ion channel proteins that are functionally expressed on the membrane of endolysosomal vesicles. The electrophysiological properties of these ion channels in the intracellular organelle membrane cannot be observed using conventional electrophysiological techniques. This section compiles the different electrophysiological techniques utilized in recent years to study endolysosomal ion channels and describes their methodological characteristics, emphasizing the most widely used technique for whole endolysosome recordings to date. This includes the use of different pharmacological tools and genetic tools for the application of patch-clamping techniques for specific stages of endolysosomes, allowing the recording of ion channel activity in different organelles, such as recycling endosomes, early endosomes, late endosomes, and lysosomes. These electrophysiological techniques are not only cutting-edge technologies that help to investigate the biophysical properties of known and unknown intracellular ion channels but also help us to investigate the physiopathological role of these ion channels in the distribution of dynamic vesicles and to identify new therapeutic targets for precision medicine and drug screening.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
1.1 Endolysosome System
In the 1950s, scientists firstly observed subcellular compartments, called organelles, which contain hydrolytic enzymes for digestion. Since then, several scientists were awarded the Nobel Prize in Physiology or Medicine for their work on intracellular compartments and endolysosome-related research. Christian de Duve (awarded in 1974) discovered and described the cellular compartment as a sac-like structure surrounded by a lipid membrane containing acid phosphatase. de Duve named them “lysosome” by combining the lyso, which is derived from λύσις (lysis, Greek for breakdown) and –some, derived from σῶμα (soma, Greek for body) (de Duve et al. 1955). In the 1980s, Randy Schekman (awarded in 2013) and other scientists identified sec genes, encode secretory proteins that mediate intracellular organelle transport in the cell (Novick et al. 1979; Kaiser et al. 1990). In the 1990s, James Rothman (awarded in 2013) discovered SNARE complex that allows intracellular vesicles to dock and fuse with their target membrane controlling the fusion process (Söllner et al. 1993). Thomas Südhof (awarded in 2013) and other scientists identified calcium ions and calcium-sensitive proteins, synaptotagmins, contributing to the binding process of vesicles to the plasma membrane of nerve cells for neurotransmitter release in synaptic transmission (Hata et al. 1993). Christian de Duve, the scientist behind the discovery of the lysosome, discovered an intracellular vesicle trafficking pathway by which cellular cargo is transported to the lysosome for degradation. de Duve named this process “autophagy” meaning “self-degradation,” derived from αυτο (auto; Greek for self) and φᾰγεῖν (phageîn, Greek for eating). In early 1990s, Yoshinori Ohsumi (awarded in 2016) used yeast to identify genes essential for autophagy (Takeshige et al. 1992). Today, we know that endolysosomes contain more than 60 different types of hydrolytic enzymes and more than 50 membrane proteins (Xu and Ren 2015). Endolysosomes mediate the degradation of substances from extracellular environment via endocytosis and intracellular components via autophagy. Besides degradation, these vesicles are also essential for intracellular cargo and membrane trafficking, exporting, ionic homeostasis, and nutrient sensing (Huotari and Helenius 2011; Luzio et al. 2007; Ruivo et al. 2009; Saftig and Klumperman 2009).
The endolysosomal system consists of multifunctional membrane organelles that are specialized for essential functions for the cell, including recycling endosome (RE), early endosome (EE), late endosome (LE), lysosome (LY), and hybrid organelles sharing properties of endolysosomes and other compartments, such as phagophore and phagosome (Repnik et al. 2013; Klionsky et al. 2014). EE, also known as the sorting endosome (SE), is one of the initial destinations for material internalized from the plasma membrane (PM) (Naslavsky and Caplan 2018). EE is a crucial compartment for the sorting of cargoes to various endocytic pathways, e.g., LE/LY maturation pathway for degradation, fast-recycling pathway back to the PM, and a slow-recycling pathway involving passage through the recycling compartment or peripheral RE (Klumperman and Raposo 2014). Multivesicular bodies (MVBs) are formed from endosomes, are spherical compartments surrounded by a limiting membrane which can be filled with intraluminal vesicles (ILVs) (Piper and Luzio 2008).
Endolysosomes can be classified by their specific membrane protein markers, such as Ras-related proteins (Rab; ras related in brain). For example, Rab11 is one of the most common membrane proteins of slow RE. Rab4 is a marker for fast RE. EEA1 (Early Endosome Antigen 1) and Rab5 proteins usually localize to EEs. Rab7 and Rab9 proteins are mainly localized on LE/LY. These small GTPases also play key roles in the formation, functioning, trafficking, and fusion of endolysosomes (Sönnichsen et al. 2000). Rab4 is suggested to control the fast-recycling pathway of the transferrin receptor (TfR) and the G protein-coupled receptor (GPCR) signaling pathway regulating cell surface expression of the β2 adrenergic receptor (β2-AR) (Yudowski et al. 2009). Rab11 is commonly enriched in peripheral RE and regulates the slow-recycling pathway. Other Rab proteins also localize to REs, e.g., Rab8, Rab10, Rab12, Rab14, Rab17, Rab22 (Klumperman and Raposo 2014). Rab5 is involved in biogenesis and fusion of EEs. Rab7 is involved in EE-LE and LY transport and biogenesis (Wandinger-Ness and Zerial 2014). These Rabs share various common effectors and partially mark overlapping membrane compartments. They are also expressed at different levels in different types of cells to regulate distinct recycling pathways (Stenmark 2009). Likewise, different membrane lipid compositions have also been assessed to classify endolysosomes, e.g. phosphoinositides, such as phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2], which is thought to be mostly localized to LE/LY, and phosphatidylinositol 3-phosphate (PI3P) and phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] that are mainly observed on EE and the PM (Li et al. 2013). In the endolysosomal maturation pathway, the luminal pH decreases gradually from neutral to acidic. Acidification of the vesicular lumen (pH ~4.6) supports degradation of endocytic cargoes by hydrolases in lysosomes and autolysosomes.
1.2 Endolysosomal Ion Channels
Physiological functions of endolysosomes, such as transport and degradation, are determined by the ionic homeostasis and membrane potential. The luminal Na+ and K+ concentrations are estimated as 20–140 mM for Na+ and 2–50 mM for K+ (Xu and Ren 2015). The enormous variation in these values is presumably due to the large amount of various ion channels, transporters, and exchangers in the small-size organelles (100–500 nm in diameter for vesicular endolysosomes, ~50 nm in diameter for tubular endolysosomes) (Morgan et al. 2011; Xu and Ren 2015; Freeman and Grinstein 2018). Endolysosomal ion channels mediate ion flux across membranes and thereby control ion distribution and thus ion homeostasis, membrane potential, and Ca2+ release (Xu and Ren 2015; Morgan et al. 2011). Electrophysiological methods are the most predominant techniques to functionally study ion channels. The endolysosomal patch-clamp technique, which will be described in this chapter, has recently been applied in several studies to characterize a number of endolysosomal ion channels (TPCs, TRPMLs, BK, P2X4, TWIK2, TMEM175, LRRC8A, PAC) (Wang et al. 2012; Dong et al. 2010; Chen et al. 2017a; Plesch et al. 2018; Cao et al. 2015; Huang et al. 2014; Bobak et al. 2017; Cang et al. 2015; Li et al. 2020; Osei-Owusu et al. 2021) (Fig. 1). Using a combination of knockout and knockdown animal models, cell biological methods, imaging technologies, pharmacological tools, and the endolysosomal patch-clamp technique, the functions of these channels have been explored in more detail (Patel et al. 2022; Hu et al. 2022; Riederer et al. 2022; Chen et al. 2022).
Localization of endolysosomal ion channels. Distribution of various endolysosomal cation and anion channels based on specific whole-organelle, e.g., recycling endosome (RE), early endosome (EE), late endosome and lysosome (LE/LY), and phagosome patch-clamp recordings. TfR, transferrin receptor; TRPML, transient receptor potential mucolipins; TMEM175, transmembrane protein 175; PAC, proton-activated chloride channel; TPC, two-pore channels; LAMP1, lysosomal-associated membrane protein 1; BK, calcium-activated potassium channel; P2X4, P2X receptor ATP-activated cation channel; LRRC8, leucine-rich repeat-containing protein 8; LC3, microtubule-associated proteins 1A/1B light chain 3B
2 Material and Method of Endolysosomal Patch-Clamp Technique
Detailed electrophysiological characterization of endolysosomal ion channels allows a better understanding of how organellar ion homeostasis is regulated and allows us to evaluate them as potential novel drug targets for human diseases. Erwin Neher and Bert Sakmann developed the patch-clamp technique in the 1970s; this method is still the gold standard approach to investigate plasma membrane ion channels (Sakmann and Neher 1984). In patch-clamping, a glass microelectrode filled with a solution referred to as pipette solution (= cytosolic solution) is used to form a gigaseal with the cell’s surface membrane in the bath solution (= extracellular solution). The electrical circuit is formed between the recording and reference electrode with the cell membrane in between. Then membrane currents or membrane potential is measured by applying voltage pulses or injecting currents through the pipette under the control of an amplifier. While this conventional patch-clamp technique has been very efficacious for investigating ion channels on the plasma membrane of the cell of interest, for channels expressed in endolysosomal organelles, three major challenges limit the application of this conventional method: (1) The size of endolysosomes is usually small (<1 μm in diameter). Thus, endolysosomes are challenging to be resolved under the microscope and are smaller than the opening end of the typical glass micropipette. (2) Special skills are required for the isolation of endolysosomes directly out of the target cell while maintaining the organelles’ integrity. (3) The seal formation and then rupturing the endolysosomal membrane inside the patch pipette to form the whole-endolysosome configuration are difficult, because intracellular organelles do not have a cytoskeleton and therefore do not entirely maintain their structure after breaking in.
Several electrophysiological methods have been employed to overcome the above-mentioned problems:
-
(a)
Lipid bilayer recordings: In the bilayer recordings, purified ion channel proteins or membrane fractions containing the organelles are reconstituted into synthetic phospholipid bilayers (Pitt et al. 2010; Brailoiu et al. 2010).
-
(b)
Conventional patch-clamping for endolysosomal ion channels redirected to the plasma membrane: In this approach, the lysosomal targeting sequences of endolysosomal ion channels are mutated, resulting in endolysosomal proteins being expressed predominantly on the plasma membrane instead of endolysosomal membranes (Bonifacino and Traub 2003).
-
(c)
Solid-supported membrane-based electrophysiology (SSM or SSME) technique: The SSME also refers to as an endolysosomal planar patch-clamp method, was applied to characterize TPC2 and TRPML1 on intact endolysosomes, isolated, e.g., from fibroblasts that endogenously express the channel or HEK293 cell lines that stably express the channel (Schieder et al. 2010; Chen et al. 2014; Grimm et al. 2014; Ruas et al. 2015). Solid-matrix planar glass chips were used in the Port-a-Patch system (Nanion, Munich) for capturing the isolated native or enlarged vesicle on a small aperture (<1 μm in diameter) in a microstructured planar borosilicate chip. This glass chip with a small aperture allows small and even native endolysosomes to be analyzed based on pressure suction control system.
-
(d)
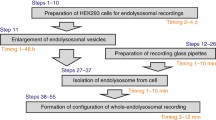
Modified conventional patch-clamp technique: This technique requires basic classical glass-electrode-based patch-clamp instrumentation such as an inverted microscope, an anti-vibration table, a micromanipulator, and electronics. The most important step in endolysosomal patch-clamp is to utilize a glass dissection micropipette to open up the plasma membrane and to push the endolysosome of interest out of the cell (Fig. 2). Here, we will describe the formal processes, alternative plans, and trouble-shootings for the endolysosomal patch-clamp approach and its derivative techniques based on the standard protocol and the methodology of the endolysosomal patch-clamp technique (Zhong and Dong 2015; Chen et al. 2017a, b).
Lateral view of the dissection and isolation process of individual enlarged endolysosomes from a cell attached to a glass coverslip. The isolation-pipette is pressed against the edge of a cell close to the enlarged target vesicle, pressed downward until touching the coverslip (I), and quickly pulled off to dissect a piece of the plasma membrane (II). A rapid pull helps to tear the plasma membrane while reducing the possibility of the cells detaching from the coverslip. Then, the target vesicle is slowly pushed out of the cell (III). The isolated vesicle is isolated next to the host cell in the end (IV). After isolation, new recording pipette will be used for patching
The standard endolysosomal patch-clamp approach consists of subsequent steps including: cell culture, enlargement of vesicles, pipette preparation, organelle isolation, and recording (Chen et al. 2017a). All essential equipment is the same as for traditional electrophysiology: patch-clamp setup (inverted microscope, amplifier, micromanipulator, anti-vibration table, faraday cage, perfusion system) and pipette preparation setup (micropipette puller, microforge). The difference between endolysosomal patch-clamp approach and the conventional patch-clamp technique lies in three key points:
-
1.
The enlargement of the organelle.
-
2.
The separation of the organelle.
-
3.
The pipette preparation.
2.1 Enlargement of the Organelle
For endolysosomal patch-clamp approaches, enlarging the target organelle to a diameter greater than 2 μm is the necessary prerequisite. Except for the occasional observation of large vesicles, most endolysosomes still require pharmacological pre-treatments or genetic tools to enlarge. Different tools can be used to selectively enlarge individual endolysosomal compartments such as RE, EE, LE, LY and other compartments like phagosome, autophagosome, and autolysosomes, making these vesicle populations accessible to endolysosomal patch-clamp. The pharmacological and genetic tools described in Sect. 3 enlarge endolysosomal vesicles by increasing homologous fusion (or, by reducing fission). However, it is still possible for the cells in culture to develop extremely large vesicles spontaneously, such as gigantic autophagosomes in aged cells (Escobar et al. 2015; Chen et al. 2018). To reduce selection bias, the thresholds for size and quality of vesicles have to be set and described in each experiment, e.g., capacitance 1–3 pF, diameter 3–5 μm, and excluding large compartment with solid cytoplasmic content. In addition to enlarging specific endolysosomes, fluorescent-protein-tagging endolysosomal membrane protein/channel variants, such as mCherry-Rab proteins or TPCs/TRPMLs-YFP, are often used to identify specific target vesicles using epi-fluorescence microscopy.
2.2 Isolation of the Organelle
As with the conventional patch-clamp technique, all cell types attached to glass slides can be used for the endolysosomal patch-clamp approach. However, the success rate of endolysosomal patch-clamp is largely determined by specific characteristics of the cells, such as cell adhesion, cytoplasmic size, the toughness of lipid membrane, extracellular matrix, and intracellular matrix. Various conditions may facilitate the isolation and establishment of gigaseals (1–20 GΩ): (1) Coating the coverslips with poly-L-lysine to promote the tight attachment of cells to the coverslip, e.g., exposed to 0.1 mg/ml of poly-l-lysine in 80 mM boric acid and 10 mM borate for at least 24 h (Chen et al. 2017b). (2) Maintaining a high-quality cell culture environment. (3) Inhibiting the synthesis of extracellular matrix, e.g., ß-D-xyloside (Izu and Sachs 1991). (4) Stable micromanipulator to minimize vibration and drift of the manipulator. (5) Phase-contrast microscopy may facilitate to discriminate membrane structures, which helps to identify the edge of the plasma membrane and isolated vesicle. The steps of vesicle isolation are shown in Fig. 2. Besides the major steps shown in Fig. 2, several suggestions may help with the isolation of organelle:
-
Cell confluence: If possible, 30–50% cell density provides suitable clean space on nearby the target cell for dissection of plasma membrane and observation of isolated vesicle. However, theoretically you only need at least one cell to be well adhered to the glass slide. For example, it is difficult to obtain very small numbers of primary cells such as brain microglia (Li et al. 2021). If the cells do not adhere well, the tip of the isolation-pipette can be used to compress the cytoplasm outside the target vesicles, like stapling the cells to a glass slide. Or choose a colony of three to five cells, which sometimes adhere better than a single cell.
-
Target vesicle: The best position of the target vesicle in the cell is on the side close to the micromanipulator arm. If it is on the opposite side, the glass electrode must cross the entire upper edge of the cell, which is likely to cause unwanted interference.
-
Size: The size of the recorded target vesicles should be kept within a certain range (e.g., 3–5 μm in diameter) and the capacitance value (e.g., 1–3 pF) or even the image of each vesicle should be recorded to reduce the experimenter’s selective bias. Sometimes large lysosomes may be observed with residues that have not yet been digested, and these substances usually reduce the chance of gigaseal formation.
-
Pressure: Using a syringe to apply some positive pressure (~2–10 mmHg) to the inside of the glass electrode before the tip of the glass pipette touches the liquid surface may help reduce the amount of material in the solution sticking to the pipette tip and increase the chance of forming a gigaseal. Another benefit of applying positive pressure is that as the tip approaches the vesicle about 1–2 μm, you can observe the balloon-like vesicle being blown up, or slightly deformed, or even rolling in place.
-
Time: As in conventional electrophysiology, cells gradually become apoptotic and rounded when removed from the cell culture incubator. Therefore, in endolysosomal electrophysiology experiments, it is also recommended to complete the isolation preparation as soon as possible within 1 h.
If a second arm of micromanipulator is available, the target cells can be holding with the second glass micropipette and opening of the cells can be performed using the isolation glass micropipette (Zhong and Dong 2015).
2.3 Pipette Preparation and Recording Solutions
For successful seal formation, the pipette tip must be smooth and symmetrical. It is highly recommended to use a multi-step puller and fire-polishing to produce suitable pipette tips (Chen et al. 2017a). The tip opening should be 0.5–0.9 μm in diameter or 5–10 MΩ. For endolysosomal recordings (Fig. 3), unless otherwise stated, the cytoplasmic solution (bath solution) comprised 140 mM K-MSA, 5 mM KOH, 4 mM NaCl, 0.39 mM CaCl2, 1 mM EGTA, and 10 mM HEPES (pH adjusted with KOH to 7.2). Luminal solution (pipette solution) comprises 140 mM Na-MSA, 5 mM K-MSA, 2 mM Ca-MSA, 1 mM CaCl2, 10 mM HEPES, and 10 mM MES (pH adjusted to 4.6 with MSA) (Cang et al. 2013; Sakurai et al. 2015; Chao et al. 2017; Bobak et al. 2017; Li et al. 2020; Gerndt et al. 2020). Solutions are modified from Tyrode’s solution to mimic physiological conditions. MSA (methanesulfonate) is used instead of high concentration of chloride to reduce endogenous Cl− currents (Schieder et al. 2010). MES (2-(N-Morpholino)-ethane sulfonic acid) was applied for buffering acidic pH solution. Bath and pipette solutions must be free of debris and are freshly filtered with a detergent-free 0.2 μm filter before experiment.
Whole-endolysosomal recordings of TPC2. (a) Whole-endolysosomal currents were recorded from human TPC2-expressing human embryonic kidney 293 (HEK293) cells by using endolysosomal patch-clamp. Currents were activated by application of 10 μM PI(3,5)P2. Current-voltage relations were recorded using voltage step protocols from −125 mV to +125 mV, step of 25 mV, holding voltage at +60 mV. (b) Current-voltage relationships determined from similar current families as in A. Currents were determined at the end of the voltage pulses as indicated by the arrow in A and plotted against voltage of the respective voltage step. Data are represented as mean ± SEM. N = 4
3 Different Protocols to Enlarge Specific Types of Endolysosome for Patch-Clamp Analysis
3.1 Whole-Endolysosome Recording
For standard whole-endolysosomal patch-clamp recordings we use vacuolin-enlarged endolysosomes (Dong et al. 2010; Cang et al. 2013; Sakurai et al. 2015; Chao et al. 2017; Bobak et al. 2017; Li et al. 2020; Gerndt et al. 2020). Vacuolin is known to enlarge different types of endosomes and lysosomes (Chen et al. 2017b; Cerny et al. 2004). For endolysosomal vesicle enlargement (>2 μm in diameter), transfected and untransfected cells are pretreated with 1 μM vacuolin-1. The timing of application is determined by the growth rate of each cell type. For native macrophages it is 0.5–1 h; for COS cells 1–2 h; for HEK293 cells 1–12 h; for fibroblasts, cardiac myocytes, and neurons, an overnight treatment with 2 μM vacuolin-1 is suggested (Fig. 4). Notably, the lipid and protein compositions of the vacuolin-enlarged vacuoles may vary between different subtypes of endolysosomes; different endolysosomes (LE/LY, EE, RE…) have various components resulting in altered channel properties, such as PIPs. Additionally, the incubation of cells with sucrose (50 mM) for hours has been applied to enlarge endolysosomal vacuoles for whole-endolysosome patch-clamping (Wang et al. 2017).
Pharmacological tools for endolysosomal patch-clamp approaches. (a) Cartoon illustrating the different pharmacological tools used for the enlargement of endolysosomal compartments. The combination of Wort. (Wortmannin) and Lat.B (Latrunculin B), and the Vicenistatin are selectively enlarging Rab5 and EEA1-positive early endosomes (EE). YM201636 and Apilimod are specific for Rab7 and LAMP1-positive late endosomes (LE) and lysosomes (LY). Vacuolin enlarges RE, EE, LE, LY, and autolysosomes. The distribution of the major endolysosomal calcium permeable ion channels is also shown in the figure. (b) A table shows the different cell types and endolysosomes to enlarge and the compounds, concentration, and time required for swelling vesicles. Notably, it has been reported that no significant difference in channel properties was seen for endolysosomes obtained with pharmacological enlargement or endogenous vesicles (Dong et al. 2010; Grimm et al. 2014)
3.2 Late Endosome and Lysosome Recording
To selectively record ion channel activities on LE/LY, these organelles need to be selectively enlarged. For this purpose we use inhibitors of PIKfyve, the kinase that converts PI3P to PI(3,5)P2. Two compounds are currently available, e.g., YM201636 (Chen et al. 2017a) and apilimod (Cai et al. 2014; Wang et al. 2017). The pretreatment of YM201636 and apilimod specifically enlarges LAMP1-positive LE/LY, but not Rab5-positive EE or Rab11-positive RE. Apilimod can be used at 1 μM or 0.5 μM for 8 h to enlarge LE/LY in COS-1 cells (Cai et al. 2014; Wang et al. 2017). Besides pretreatment of cells with pharmacological tools, transient transfection with TMEM106B has been applied to enlarge LE/LY for whole-LE/LY patch-clamping (Li et al. 2020). TPC2, TRPMLs, BK, and LRRC8 channel activities have been measured using the LE/LY patch-clamp approach (Wang et al. 2017; Chen et al. 2017a, 2020; Gerndt et al. 2020; Li et al. 2020; Spix et al. 2022).
3.3 Early Endosome Recording
Inhibition of Rab5 activity stimulates endosomal membrane homotypic fusion, e.g., transfecting cells with Rab5-Q79L, a dominant-negative Rab5 mutant (Bertl et al. 1992). Enlarged EEs are observed in Rab5-Q79L transfected cells, allowing ion channels on the EE to be examined by patch-clamping (Cang et al. 2015; Osei-Owusu et al. 2021). Vicenistatin, a natural compound that enhances homotypic fusion between EEs, has been reported as a small-molecule tool for EE (Rab5-positive) enlargement, and the vacuoles produced by a pretreatment with 300 nM Vicenistatin for 2 h are feasible for EE patch-clamping (Wang et al. 2017). A combined pretreatment of wortmannin and latrunculin B selectively enlarges Rab5-positive EE for EE patch-clamping (Chen et al. 2017a; Li et al. 2020). Wortmannin is a fungal metabolite that is a non-specific inhibitor of phosphoinositide 3-kinases (PI3K), which induces the enlargement of RE (Carpentier et al. 2013). Latrunculin B was found to disrupt endosomal sorting and impacted actin dynamics (Greene and Gao 2009) which may also facilitate the enlargement of RE. TRPMLs, TMEM175, and PAC have been investigated using EE patch-clamp approach (Wang et al. 2017; Chen et al. 2017a; Gerndt et al. 2020; Plesch et al. 2018; Spix et al. 2022).
3.4 Recycling Endosome Recording
There is no known method to exclusively enlarge the recycling endosome for RE patch-clamping, probably because the half-life of recycling endosomes and sorting endosomes is short (RE, SE t1/2 of 2–30 min) (Maxfield and McGraw 2004). However, by non-specific enlargement of all endolysosomes by vacuolin, while using fluorescent protein markers or RE-specific dye, enlarged RE was identified in HEK293 cells and macrophages. For example, transiently transfection with fluorescent tagging RE markers, e.g., Rab4, Rab11, or Alexa Fluor 555-conjugated Tfn dye (Tfn555), and pretreatment of vacuolin was used to enlarge fast-RE (Rab4-mCherry positive vesicles), slow-RE (Rab11-mCherry positive vesicles), or Tfn555+ RE (Tfn555 stained vesicles), respectively (Plesch et al. 2018; Chen et al. 2020). TRPML2 channel activity has been reported using RE patch-clamp approaches (Plesch et al. 2018; Chen et al. 2020).
3.5 Other Endolysosomal Compartment Recording
Whole-phagosome patch-clamp has been applied to observe TRPML1 currents in erythrocyte-containing phagosomes from macrophages (Samie et al. 2013). Whole-VAC (vacuolar apical compartment) patch-clamp was performed to investigate TRPML1 channel activity on apical vacuoles isolated from gastric parietal cells (Sahoo et al. 2017). For whole-VAC recording, pretreatment of histamine and the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) in parietal cells was applied to swell VAC (Sahoo et al. 2017), which is highly enlarged filling with HCl and water because of the action of ATPase pump (Karvar et al. 2002). For phagosome patch-clamping, endocytic erythrocytes from phagocytosis were considered to be phagosomes (Samie et al. 2013). Inwardly rectifying TRPML1-like currents were observed in the whole-phagosome and whole-VAC configurations (Sahoo et al. 2017).
3.6 Limitations and Challenges
The electrophysiological techniques mentioned in this chapter still have some limitations. In the methods (a) and (b), the main drawback is that the membrane environment is an artificial bilayer or plasma membrane, which is fundamentally different from the endolysosomal membranes. In particular, the specific membrane composition and proteins are different. The absence of possible interaction partners and cofactors results in non-physiological channel gating phenomena or even altering the channel conformation, e.g., Rabs and PIPs (Zhang et al. 2012; Lin-Moshier et al. 2014). The limitations of method (c) are (1) vision control is missing and (2) restriction of solution compositions (e.g., high concentrations of Ca2+ and F− are required for forming gigaseal). Nevertheless, the application of the solid-supported membrane electrophysiology is beneficial when cells are growing only in suspension principally. Although method (d) is currently the most commonly used electrophysiological technique to study endolysosomal ion channels, it generally requires the step of enlarging vesicles and manually separating the selected vesicles. However, this is also a limitation of the capillary glass-electrode-based electrophysiological technology. The lack of cytoskeleton to maintain organelle stability and interference from intracellular fibers remain the biggest reasons for the low success rate of these experiments. In addition to the accumulated experience of electrophysiologists, breakthroughs in electrophysiological techniques require the cooperation of other fields such as advanced high-resolution optical microscopy. This chapter has introduced the cutting-edge techniques for measuring electrophysiological signals in endolysosomes. In the future, with the help of advanced optical systems and high-precision fluorescent small-molecule tools, it will be possible to measure ion channel activity in a single organelle within a cell.
References
Bertl A, Blumwald E, Coronado R, Eisenberg R, Findlay G, Gradmann D, Hille B, Köhler K, Kolb HA, MacRobbie E et al (1992) Electrical measurements on endomembranes. Science 258(5084):873–874
Bobak N, Feliciangeli S, Chen CC, Ben Soussia I, Bittner S, Pagnotta S, Ruck T, Biel M, Wahl-Schott C, Grimm C, Meuth SG, Lesage F (2017) Recombinant tandem of pore-domains in a weakly inward rectifying K+ channel 2 (TWIK2) forms active lysosomal channels. Sci Rep 7(1):649
Bonifacino JS, Traub LM (2003) Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72:395–447
Brailoiu E, Hooper R, Cai X, Brailoiu GC, Keebler MV, Dun NJ, Marchant JS, Patel S (2010) An ancestral deuterostome family of two-pore channels mediates nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J Biol Chem 285(5):2897–2901
Cai X, Xu Y, Kim YM, Loureiro J, Huang Q (2014) PIKfyve, a class III lipid kinase, is required for TLR-induced type I IFN production via modulation of ATF3. J Immunol 192(7):3383–3389
Cang C, Zhou Y, Navarro B, Seo YJ, Aranda K, Shi L, Battaglia-Hsu S, Nissim I, Clapham DE, Ren D (2013) mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell 152(4):778–790
Cang C, Aranda K, Seo YJ, Gasnier B, Ren D (2015) TMEM175 is an organelle K(+) channel regulating lysosomal function. Cell 162(5):1101–1112
Cao Q, Zhong XZ, Zou Y, Zhang Z, Toro L, Dong XP (2015) BK channels alleviate lysosomal storage diseases by providing positive feedback regulation of lysosomal Ca2+ release. Dev Cell 33(4):427–441
Carpentier S, N'Kuli F, Grieco G, Van Der Smissen P, Janssens V, Emonard H, Bilanges B, Vanhaesebroeck B, Gaide Chevronnay HP, Pierreux CE, Tyteca D, Courtoy PJ (2013) Class III phosphoinositide 3-kinase/VPS34 and dynamin are critical for apical endocytic recycling. Traffic 14(8):933–948
Cerny J, Feng Y, Yu A, Miyake K, Borgonovo B, Klumperman J, Meldolesi J, McNeil PL, Kirchhausen T (2004) The small chemical vacuolin-1 inhibits Ca(2+)-dependent lysosomal exocytosis but not cell resealing. EMBO Rep 5(9):883–888
Chao YK, Schludi V, Chen CC, Butz E, Nguyen ONP, Müller M, Krüger J, Kammerbauer C, Ben-Johny M, Vollmar AM, Berking C, Biel M, Wahl-Schott CA, Grimm C (2017) TPC2 polymorphisms associated with a hair pigmentation phenotype in humans result in gain of channel function by independent mechanisms. Proc Natl Acad Sci U S A 114(41):E8595–E8602
Chen CC, Keller M, Hess M, Schiffmann R, Urban N, Wolfgardt A, Schaefer M, Bracher F, Biel M, Wahl-Schott C, Grimm C (2014) A small molecule restores function to TRPML1 mutant isoforms responsible for mucolipidosis type IV. Nat Commun 5:4681
Chen CC, Cang C, Fenske S, Butz E, Chao YK, Biel M, Ren D, Wahl-Schott C, Grimm C (2017a) Patch-clamp technique to characterize ion channels in enlarged individual endolysosomes. Nat Protoc 12(8):1639–1658
Chen CC, Butz ES, Chao YK, Grishchuk Y, Becker L, Heller S, Slaugenhaupt SA, Biel M, Wahl-Schott C, Grimm C (2017b) Small molecules for early endosome-specific patch clamping. Cell Chem Biol 24(7):907–916.e4
Chen Q, Kang J, Fu C (2018) The independence of and associations among apoptosis, autophagy, and necrosis. Signal Transduct Target Ther 3:18
Chen CC, Krogsaeter E, Butz ES, Li Y, Puertollano R, Wahl-Schott C, Biel M, Grimm C (2020) TRPML2 is an osmo/mechanosensitive cation channel in endolysosomal organelles. Sci Adv 6(46):eabb5064
Chen CC, Krogsaeter E, Kuo CY, Huang MC, Chang SY, Biel M (2022) Endolysosomal cation channels point the way towards precision medicine of cancer and infectious diseases. Biomed Pharmacother 148:112751
de Duve C, Pressman BC, Gianetto R, Wattiaux R, Appelmans F (1955) Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J 60:604–617
Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H (2010) PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun 1(4):38
Escobar L, Echeverria OM, Vazquez-Nin GH (2015) Necrosis as programmed cell death. https://www.intechopen.com/books/cell-death-autophagy-apoptosis-and-necrosis/necrosis-as-programmed-cell-death
Freeman SA, Grinstein S (2018) Resolution of macropinosomes, phagosomes and autolysosomes: osmotically driven shrinkage enables tubulation and vesiculation. Traffic 19(12):965–974
Gerndt S, Chen CC, Chao YK, Yuan Y, Burgstaller S, Scotto Rosato A, Krogsaeter E, Urban N, Jacob K, Nguyen ONP, Miller MT, Keller M, Vollmar AM, Gudermann T, Zierler S, Schredelseker J, Schaefer M, Biel M, Malli R, Wahl-Schott C, Bracher F, Patel S, Grimm C (2020) Agonist-mediated switching of ion selectivity in TPC2 differentially promotes lysosomal function. Elife 9:e54712
Greene W, Gao SJ (2009) Actin dynamics regulate multiple endosomal steps during Kaposi’s sarcoma-associated herpesvirus entry and trafficking in endothelial cells. PLoS Pathog 5(7):e1000512
Grimm C, Holdt LM, Chen CC, Hassan S, Müller C, Jörs S, Cuny H, Kissing S, Schröder B, Butz E, Northoff B, Castonguay J, Luber CA, Moser M, Spahn S, Lüllmann-Rauch R, Fendel C, Klugbauer N, Griesbeck O, Haas A, Mann M, Bracher F, Teupser D, Saftig P, Biel M, Wahl-Schott C (2014) High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nat Commun 5:4699
Hata Y, Slaughter CA, Südhof TC (1993) Synaptic vesicle fusion complex containsunc-18 homologue bound to syntaxin. Nature 366(6453):347–351
Hu M, Zhou N, Cai W, Xu H (2022) Lysosomal solute and water transport. J Cell Biol 221(11):e202109133
Huang P, Zou Y, Zhong XZ, Cao Q, Zhao K, Zhu MX, Murrell-Lagnado R, Dong XP (2014) P2X4 forms functional ATP-activated cation channels on lysosomal membranes regulated by luminal pH. J Biol Chem 289(25):17658–17667
Huotari J, Helenius A (2011) Endosome maturation. EMBO J 30(17):3481–3500
Izu YC, Sachs F (1991) Inhibiting synthesis of extracellular matrix improves patch clamp seal formation. Pflugers Arch 419(2):218–220
Kaiser CA, Schekman R (1990) Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell 61(4):723–733
Karvar S, Yao X, Crothers JM, Liu Y, Forte JG (2002) Localization and function of soluble N-ethylmaleimide-sensitive factor attachment protein-25 and vesicle-associated membrane protein-2 in functioning gastric parietal cells. J Biol Chem 277(51):50030–50035
Klionsky DJ, Eskelinen EL, Deretic V (2014) Autophagosomes, phagosomes, autolysosomes, phagolysosomes, autophagolysosomes... wait, I’m confused. Autophagy 10(4):549–551
Klumperman J, Raposo G (2014) The complex ultrastructure of the endolysosomal system. Cold Spring Harb Perspect Biol 6(10):a016857
Li X, Wang X, Zhang X, Zhao M, Tsang WL, Zhang Y, Yau RG, Weisman LS, Xu H (2013) Genetically encoded fluorescent probe to visualize intracellular phosphatidylinositol 3,5-bisphosphate localization and dynamics. Proc Natl Acad Sci U S A 110(52):21165–21170
Li P, Hu M, Wang C, Feng X, Zhao Z, Yang Y, Sahoo N, Gu M, Yang Y, Xiao S, Sah R, Cover TL, Chou J, Geha R, Benavides F, Hume RI, Xu H (2020) LRRC8 family proteins within lysosomes regulate cellular osmoregulation and enhance cell survival to multiple physiological stresses. Proc Natl Acad Sci U S A 117(46):29155–29165
Li Y, Schön C, Chen CC, Yang Z, Liegl R, Murenu E, Schworm B, Klugbauer N, Grimm C, Wahl-Schott C, Michalakis S, Biel M (2021) TPC2 promotes choroidal angiogenesis and inflammation in a mouse model of neovascular age-related macular degeneration. Life Sci Alliance 4(8):e202101047
Lin-Moshier Y, Keebler MV, Hooper R, Boulware MJ, Liu X, Churamani D, Abood ME, Walseth TF, Brailoiu E, Patel S, Marchant JS (2014) The two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc Natl Acad Sci U S A 111(36):13087–13092
Luzio JP, Pryor PR, Bright NA (2007 Aug) Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8(8):622–632
Maxfield FR, McGraw TE (2004) Endocytic recycling. Nat Rev Mol Cell Biol 5(2):121–132
Morgan AJ, Platt FM, Lloyd-Evans E, Galione A (2011) Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem J 439(3):349–374
Naslavsky N, Caplan S (2018) The enigmatic endosome – sorting the ins and outs of endocytic trafficking. J Cell Sci 131(13):jcs216499
Novick P, Schekman R (1979) Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 76(4):1858–1862
Osei-Owusu J, Yang J, Leung KH, Ruan Z, Lü W, Krishnan Y, Qiu Z (2021) Proton-activated chloride channel PAC regulates endosomal acidification and transferrin receptor-mediated endocytosis. Cell Rep 34(4):108683
Patel S, Yuan Y, Chen CC, Jaślan D, Gunaratne G, Grimm C, Rahman T, Marchant JS (2022) Electrophysiology of Endolysosomal two-pore channels: a current account. Cell 11(15):2368
Piper RC, Luzio P (2008) Late endosomes: sorting and partitioning in multivesicular bodies. Traffic 2(9):612–621
Pitt SJ, Funnell TM, Sitsapesan M, Venturi E, Rietdorf K, Ruas M, Ganesan A, Gosain R, Churchill GC, Zhu MX, Parrington J, Galione A, Sitsapesan R (2010) TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+. J Biol Chem 285(45):35039–35046
Plesch E, Chen CC, Butz E, Scotto Rosato A, Krogsaeter EK, Yinan H, Bartel K, Keller M, Robaa D, Teupser D, Holdt LM, Vollmar AM, Sippl W, Puertollano R, Medina D, Biel M, Wahl-Schott C, Bracher F, Grimm C (2018) Selective agonist of TRPML2 reveals direct role in chemokine release from innate immune cells. Elife 7:e39720
Repnik U, Česen MH, Turk B (2013) The endolysosomal system in cell death and survival. Cold Spring Harb Perspect Biol 5(1):a008755
Riederer E, Cang C, Ren D (2022) Lysosomal ion channels: what are they good for and are they druggable targets? Annu Rev Pharmacol Toxicol
Ruas M, Davis LC, Chen CC, Morgan AJ, Chuang KT, Walseth TF, Grimm C, Garnham C, Powell T, Platt N, Platt FM, Biel M, Wahl-Schott C, Parrington J, Galione A (2015) Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J 34(13):1743–1758
Ruivo R, Anne C, Sagné C, Gasnier B (2009) Molecular and cellular basis of lysosomal transmembrane protein dysfunction. Biochim Biophys Acta 1793(4):636–649
Saftig P, Klumperman J (2009) Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol 10(9):623–635
Sahoo N, Gu M, Zhang X, Raval N, Yang J, Bekier M, Calvo R, Patnaik S, Wang W, King G, Samie M, Gao Q, Sahoo S, Sundaresan S, Keeley TM, Wang Y, Marugan J, Ferrer M, Samuelson LC, Merchant JL, Xu H (2017) Gastric acid secretion from parietal cells is mediated by a Ca2+ efflux channel in the tubulovesicle. Dev Cell 41(3):262–273.e6
Sakmann B, Neher E (1984) Patch clamp techniques for studying ionic channels in excitable membranes. Annu Rev Physiol 46:455–472
Sakurai Y, Kolokoltsov AA, Chen CC, Tidwell MW, Bauta WE, Klugbauer N, Grimm C, Wahl-Schott C, Biel M, Davey RA (2015) Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 347(6225):995–998
Samie M, Wang X, Zhang X, Goschka A, Li X, Cheng X, Gregg E, Azar M, Zhuo Y, Garrity AG, Gao Q, Slaugenhaupt S, Pickel J, Zolov SN, Weisman LS, Lenk GM, Titus S, Bryant-Genevier M, Southall N, Juan M, Ferrer M, Xu H (2013) A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev Cell 26(5):511–524
Schieder M, Rötzer K, Brüggemann A, Biel M, Wahl-Schott C (2010) Planar patch clamp approach to characterize ionic currents from intact lysosomes. Sci Signal 3(151):pl3
Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE (1993) SNAP receptors implicated in vesicle targeting and fusion. Nature 362(6418):318–324
Sönnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M (2000) Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol 149(4):901–914
Spix B, Butz ES, Chen CC, Rosato AS, Tang R, Jeridi A, Kudrina V, Plesch E, Wartenberg P, Arlt E, Briukhovetska D, Ansari M, Günsel GG, Conlon TM, Wyatt A, Wetzel S, Teupser D, Holdt LM, Ectors F, Boekhoff I, Boehm U, García-Añoveros J, Saftig P, Giera M, Kobold S, Schiller HB, Zierler S, Gudermann T, Wahl-Schott C, Bracher F, Yildirim AÖ, Biel M, Grimm C (2022) Lung emphysema and impaired macrophage elastase clearance in mucolipin 3 deficient mice. Nat Commun 13(1):318
Stenmark H (2009) Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10(8):513–525
Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y (1992) Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol 119(2):301–311
Wandinger-Ness A, Zerial M (2014) Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol 6(11):a022616
Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, Zhu MX, Clapham DE, Ren D, Xu H (2012) TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell 151(2):372–383
Wang W, Zhang X, Gao Q, Lawas M, Yu L, Cheng X, Gu M, Sahoo N, Li X, Li P, Ireland S, Meredith A, Xu H (2017) A voltage-dependent K+ channel in the lysosome is required for refilling lysosomal Ca2+ stores. J Cell Biol 216(6):1715–1730
Xu H, Ren D (2015) Lysosomal physiology. Annu Rev Physiol 77:57–80
Yudowski GA, Puthenveedu MA, Henry AG, von Zastrow M (2009) Cargo-mediated regulation of a rapid Rab4-dependent recycling pathway. Mol Biol Cell 20(11):2774–2784
Zhang X, Li X, Xu H (2012) Phosphoinositide isoforms determine compartment-specific ion channel activity. Proc Natl Acad Sci U S A 109(28):11384–11389
Zhong XZ, Dong XP (2015) Lysosome electrophysiology. Methods Cell Biol 126:197–215
Acknowledgments
This work was supported by funding from the National Science and Technology Council (MOST110-2320-B002-022-MY3, National Health Research Institutes (NHRI-EX112-11119SC), and National Taiwan University (112L7818) to C.-C. C.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chen, CC. (2023). Electrophysiological Techniques on the Study of Endolysosomal Ion Channels. In: Wahl-Schott, C., Biel, M. (eds) Endolysosomal Voltage-Dependent Cation Channels. Handbook of Experimental Pharmacology, vol 278. Springer, Cham. https://doi.org/10.1007/164_2023_638
Download citation
DOI: https://doi.org/10.1007/164_2023_638
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-31522-0
Online ISBN: 978-3-031-31523-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)