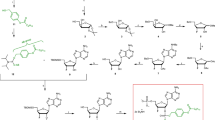
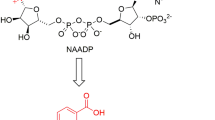
Abstract
Nicotinic acid adenine dinucleotide phosphate (NAADP) is the most potent Ca2+ mobilizing second messenger known to date. Major steps elucidating metabolism and Ca2+ mobilizing activity of NAADP are reviewed, with emphasis on a novel redox cycle between the inactive reduced form, NAADPH, and the active oxidized form, NAADP. Oxidation from NAADPH to NAADP is catalyzed in cell free system by (dual) NADPH oxidases NOX5, DUOX1, and DUOX2, whereas reduction from NAADP to NAADPH is catalyzed by glucose 6-phosphate dehydrogenase. Using different knockout models for NOX and DUOX isozymes, DUOX2 was identified as NAADP forming enzyme in early T-cell activation.
Recently, receptors or binding proteins for NAADP were identified: hematological and neurological expressed 1-like protein (HN1L)/Jupiter microtubule associated homolog 2 (JPT2) and Lsm12 are small cytosolic proteins that bind NAADP. In addition, they interact with NAADP-sensitive Ca2+ channels, such as ryanodine receptor type 1 (RYR1) or two-pore channels (TPC).
Due to its role as Ca2+ mobilizing second messenger in T cells, NAADP’s involvement in inflammation is also reviewed. In the central nervous system (CNS), NAADP regulates autoimmunity because NAADP antagonism affects a couple of T-cell migration and re-activation events, e.g. secretion of the pro-inflammatory cytokine interleukin-17. Further, the role of NAADP in transdifferentiation of IL-17-producing Th17 cells into T regulatory type 1 cells in vitro and in vivo is discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 The Ca2+ Mobilizing Second Messenger NAADP: Things to Know at a Glance
The discovery of Ca2+ mobilizing second messenger nicotinic acid adenine dinucleotide phosphate (NAADP; Fig. 1) started when an (obviously impure) commercial NADP preparation was shown to release Ca2+ from sea urchin egg homogenates (Clapper et al. 1987). Yet it took another 8 years until the structure of the impurity was identified as NAADP (Lee and Aarhus 1995). While the initial characterization of NAADP’s potential to release Ca2+ was carried out in sea urchin egg homogenates, rapidly thereafter NAADP’s activity was reported also in other cell systems, e.g. ascidian oocytes (Albrieux et al. 1998), starfish oocytes (Santella et al. 2000), pancreatic acinar cells (Cancela et al. 1999), or human T-lymphocytes (Berg et al. 2000).
When comparing NAADP’s activity in sea urchin eggs and higher eukaryotic cells, characteristic differences were observed, e.g. regarding NAADP concentrations necessary for desensitization. The underlying mechanism has not yet been fully resolved, but it appears that the NAADP receptors or binding proteins from sea urchin egg homogenates and higher eukaryotic cells are different, as shown by photoaffinity labeling (Lin-Moshier et al. 2012; Walseth et al. 2012b; vs Walseth et al. 2012a). At least the NAADP receptors/binding proteins of higher eukaryotic cells have meanwhile been identified as hematological and neurological expressed 1-like protein (HN1L)/Jupiter microtubule associated homolog 2 (JPT2) (Roggenkamp et al. 2021; Gunaratne et al. 2021). Briefly after the discovery of HN1L/JPT2, a second NAADP receptor/binding protein, Lsm12, was described (Zhang et al. 2021). NAADP receptors/binding proteins of higher eukaryotic cells are not Ca2+ channels on their own, but upon NAADP binding activate Ca2+ channels (Fig. 2). This somewhat unexpected mechanism was first proposed for pancreatic acinar cells (Gerasimenko et al. 2003) and later adopted as basis for a “unifying hypothesis” to explain the fact that different Ca2+ channels were reported to be sensitive to NAADP (Guse 2012). The unifying hypothesis was modified in 2018 proposing more than one NAADP receptor/binding protein and at least two Ca2+ channels that can be activated by NAADP (Guse and Diercks 2018). NAADP-sensitive Ca2+ channels so far identified are type 1 ryanodine receptor (RYR1) (Hohenegger et al. 2002; Dammermann et al. 2009; Wolf et al. 2015) and two-pore channels (TPC) (Zong et al. 2009; Brailoiu et al. 2009; Calcraft et al. 2009) (Fig. 2).
NAADP metabolism and signaling. Receptor evoked formation of NAADP proceeds via a redox cycle between NAADPH and NAADP in T cells. Oxidation of NAADPH is catalyzed by dual NADPH oxidase DUOX2 and reduction of NAADP by glucose 6-phosphate dehydrogenase (G6P-DH). Another proposed way for NAADP synthesis is the base-exchange reaction from NADP that can be catalyzed by CD38 or SARM1. CD38-catalyzed NAADP formation in the lysosomal lumen requires so far not identified transport systems for NADP and nicotinic acid (substrates) into the lysosome and an export system for NAADP. SARM1 activation is regulated by the ratio of [NAD+]/[nicotinamide mononucleotide (NMN)]; excess of NMN binds to the autoinhibitory ARM domain thereby activating SARM1 enzyme function. Once formed, NAADP binds to an NAADP receptor/binding protein, as proposed in the unifying hypothesis (Guse 2012); currently, two such high affinity NAADP receptors/binding proteins were identified, HN1L/JPT2 (Roggenkamp et al. 2021; Gunaratne et al. 2021). While HN1L/JPT2 appears to activate both RYR1 (Roggenkamp et al. 2021) and TPC1 (Gunaratne et al. 2021), Lsm12 was found in a screen toward proteins interacting with TPC2 (Zhang et al. 2021)
Another relevant topic in NAADP’s history is its biosynthesis. The multifunctional NAD-glycohydrolase/ADP-ribosyl cyclase CD38 was early reported to synthesize NAADP from NADP via the “base-exchange reaction” (Aarhus et al. 1995) (Fig. 2). This happens under unphysiological conditions as far as the cytosol is concerned, since the reaction requires excess of nicotinic acid (mM range) and pH 4–5. Taking these “acidic” conditions into account, CD38-catalyzed synthesis of NAADP in the acidic lumen of lysosomes was proposed (Fang et al. 2018; Nam et al. 2020) (Fig. 2). However, intra-lysosomal synthesis of NAADP requires transport of NADP and nicotinic acid into the lysosomal lumen and of NAADP from the lumen back into the cytosol; currently, import of NADP by connexin-43 has been proposed (Nam et al. 2020) while the export protein for NAADP toward the cytosol is unknown. However, alternative enzymes for NAADP synthesis have been described recently, sterile alpha toll/interleukin receptor motif containing-1 (SARM1; reviewed in DiAntonio et al. 2021) and DUOX2 (Gu et al. 2021). According to current knowledge, regulation of SARM1 proceeds via the nicotinamide adenine dinucleotide (NAD)/nicotinamide mononucleotide (NMN) ratio through the autoinhibitory ARM domain of SARM1: a high NAD concentration keeps SARM1 inactive due to autoinhibitory activity of the ARM domain, whereas decreasing endogenous NAD combined with increasing NMN induces a conformational change leading to activation of multifunctional enzymatic activities already known from CD38. These are conversion of NAD to adenosine diphosphoribose (ADPR) or cyclic adenosine diphosphoribose (cADPR), or conversion of NADP through the “base-exchange reaction” to NAADP (Zhao et al. 2019). Thus, so far SARM1 enzymatic activity appears to rather depend on NAD metabolism than on a signaling process evoked by a receptor.
Since the current Handbook of Experimental Pharmacology recently published two comprehensive reviews on NAADP signaling and endo-lysosomal Ca2+ channels (Galione et al. 2022; Rautenberg et al. 2022), in the next chapters I will concentrate on the DUOX2–HN1L–RYR 1 axis, as described for T-lymphocytes (or T cells). Further, NAADP’s relevance for inflammation will be discussed.
2 A Novel Redox Cycle as Hub for NAADP Metabolism
Many cell types express CD38 and despite the non-physiological conditions required for the “base-exchange” reaction in the cytosol (see above), CD38 was considered a reasonable “prime” candidate for NAADP formation. However, when endogenous NAADP was determined in lymphoid tissues spleen and thymus, no significant difference between wild-type tissue and Cd38−/− tissue was observed (Schmid et al. 2011). Similar results were obtained in human myometrial cells (Soares et al. 2007). Further, primary T cells from Cd38−/− mice were neither different from wild-type T cells regarding initial, local Ca2+ signals, defined as Ca2+ microdomains (Wolf et al. 2015), nor regarding global Ca2+ signaling, indicating no acute role for CD38 in NAADP formation. It should be noted here that NAADP formation proceeds very rapidly in T cells; an about sevenfold transient increase over basal concentration upon T-cell receptor (TCR)/CD3 stimulation was observed within 10s post stimulation (Gasser et al. 2006). In 2015, I reviewed metabolism of NAADP mentioning potential novel pathways for NAADP formation, “…in theory there are some more possibilities for NAADP formation: (1) conversion of NAADPH to NAADP by NADPH oxidase, (2) conversion of NAAD to NAADP by a kinase, and (3) conversion of NADP to NAADP by a deamidase” (Guse 2015). While experimental evidence for options (2) or (3) have not been obtained in our hands, and to the best of my knowledge also not by other laboratories, we discovered that (dual) NADPH oxidase family (NOX/DUOX) members produce NAADP from its reduced form, NAADPH, and are involved in generation of NAADP upon TCR/CD3 stimulation (Gu et al. 2021).
The finding that NOX/DUOX enzymes might be involved in NAADP formation is somewhat surprising. The general view of the role of NOX/DUOX is production of reactive oxygen species (ROS), e.g. O2− or H2O2, as main products, whereas NADP, that is generated from NADPH, is considered a less important by-product (Fig. 3a) (comprehensive review of NOX/DUOX by Buvelot et al. 2019). However, the NOX/DUOX catalyzed reactions may also be seen from a different angle, focusing on the product NAADP, as oxidized form of NAADPH (Fig. 3b). In fact, Gu et al. (2021) demonstrated in cell free system that NOX5, used as a model enzyme for the NOX subgroup of NOX/DUOX enzyme family, catalyzes the formation of NAADP from NAADPH. Formation of NAADP takes part at the cytosolic side of the plasma membrane and requires almost neutral pH (pH optimum at approx. 7.5). Km and vmax values are very much comparable between the substrates NAADPH and NADPH (Gu et al. 2021). Also the dual NADPH oxidases (DUOX) DUOX1 and DUOX2 oxidized NAADPH to NAADP; for DUOX2, the enzymatic activity was similar for the substrates NADPH or NAADPH, while DUOX1 preferentially oxidized NADPH (Gu et al. 2021). The substrate NAADPH was synthesized by chemical reduction of NAADP and characterized by photometry at 340 nm. As a technical note, NAADPH is not very stable under oxidizing conditions; thus, buffers used for storage or the enzyme assays should by free of dissolved O2 and control samples without enzyme/protein fraction should always be run in parallel to correct the enzymatic activity for non-enzymatic degradation (oxidation) of NAADPH.
NAADP formation by (dual) NADPH oxidases (NOX/DUOX). (a) Classical view of NADPH oxidase activity focusing on formation of reactive oxygen species as main product(s). NADPH acts as co-substrate providing electrons to be transferred to oxygen. (b) Alternative view of NADPH oxidase activity with NAADPH as major substrate that is converted to Ca2+ mobilizing second messenger NAADP in the cytosol below the plasma membrane. Oxygen acts as co-substrate on the extracellular side of the plasma membrane by accepting electrons from NAADPH. NOX5 used as example here
Though NAADP production by membranes overexpressing NOX5, DUOX1, or DUOX2 was clearly demonstrated, the question remained whether the oxidation of NAADPH to NAADP would play any role in intact cells. As in many similar settings, knockout models were employed. T cells express mainly NOX1, NOX2, and to a minor extent also DUOX1 and DUOX2 (Gu et al. 2021). Despite relatively high expression levels of NOX1 and NOX2, no phenotype regarding TCR/CD3-evoked Ca2+ signaling was observed in T cells from Nox1−/− or mCybb−/−mice (mCybb = gene name for gene of DUOX2) (Gu et al. 2021). In contrast, a functional double knockout of DUOX1 and DUOX2 showed a very clear Ca2+ phenotype: almost complete lack of Ca2+ microdomains over the first 15 s upon TCR/CD3 stimulation and delayed onset of global Ca2+ signaling combined with attenuated Ca2+ peak and plateau data (Gu et al. 2021); this functional double knockout of DUOX1 and DUOX2 is due to gene deletion of the maturation factors DUOXA1 and DUOXA2 (Grasberger and Refetoff 2006) resulting in lack of expression and lack of correct trafficking of DUOX1 and DUOX2 (Gunaratne et al. 2021). To clarify the role of each isozyme, DUOX1 or DUOX2, individual gene deletions were made in rat effector T cells. Whereas Duox1−/− T cells showed Ca2+ microdomains almost identical to wild-type T cells over the first 15 s, in Duo2−/− T cells Ca2+ microdomains were diminished, in particular over the first 10s of stimulation (Gu et al. 2021). These data suggested that DUOX2 plays the major role for NAADP production leading to initial Ca2+ microdomains in T cells; however, from approx. 20s on the effect of single gene deletion of Duox2 vanished, while in the functional double knockout of DUOX2 and DUOX1 no Ca2+ microdomains were observed above background at this time point. This indicates that DUOX1 may substitute for DUOX2 in that period of time. However, this intricate relation clearly requires further work in the future.
As mentioned above NOX and DUOX enzymes produce ROS while oxidizing NADPH, but this is of course not restricted to this coenzyme, but also holds true for NAADPH as substrate. It is well known that ROS, e.g. H2O2, interferes with Ca2+ signaling. Certain Ca2+ channels, e.g. transient receptor potential melastatin 2, are activated by H2O2 (Wehage et al. 2002). The underlying mechanism is not fully clarified, but likely is an indirect one evoked by an increase in cytosolic adenosine diphosphoribose (ADPR) concentration, as discussed recently in Fliegert et al. (2018). Further, it was reported that ROS-dependent sulfonylation inhibits sarcoplasmic/endoplasmic reticular Ca2+ ATPase 2 (SERCA2) (reviewed by Roscoe and Sevier 2020). In both examples, ROS generation would also increase the free cytosolic Ca2+ concentration. However, at this point it is necessary to take a closer look to the concentrations and topology involved. Whereas TRPM2 activation or SERCA2 inhibition requires H2O2 concentration in the micromolar range, the concentration of the by-product H2O2 during NAADP formation in T cells is in the low nanomolar range (approx. 40 nM). Further, while formation of NAADP by DUOX2 takes place just below the inner leaflet of the plasma membrane, equimolar H2O2 is produced at the extracellular space, just above the plasma membrane. Extracellular H2O2 likely rapidly diffuses away from the plasma membrane and may, likely only partially, be taken up by H2O2-transporting aquaporins 3 or 8 (da Silva and Soveral 2021), if such an uptake happens at all at such low H2O2 concentrations.
Nevertheless, no evidence for critical H2O2 formation/uptake in the first 15 s of T cell activation was obtained (Gu et al. 2021). However, at longer stimulation periods, H2O2 generation by DUOX1 that depends mainly (or exclusively) on NADPH as electron donating coenzyme was shown to elevate the free cytosolic Ca2+ concentration by increased activation of the d-myo-inositol 1,4,5-trisphosphate (IP3) signaling pathway (Kwon et al. 2010).
Oxidation of NAADPH to NADP by NOX/DUOX enzymes only constitutes one half of the newly described redox cycle as hub for NAADP metabolism. The second half consists of reduction of NAADP to NAADPH. That this reaction works not only for NADP, but also for NAADP, was shown by Genazzani’s group using glucose 6-phosphate dehydrogenase (G6P-DH) as catalyzing enzyme (Billington et al. 2004). Other major NADP-dependent dehydrogenases, e.g. 6-phosphogluconate dehydrogenase, isocitrate dehydrogenase, or malate dehydrogenase did not convert NAADPH to NAADP (Gu et al. 2021). Thus, at least so far the novel redox cycle employs NOX/DUOX enzymes for oxidation and G6P-DH for reduction (Fig. 2). This redox cycle allows not only for very rapid formation of NAADP, but also for a similarly rapid backward reaction to the inactive (regarding Ca2+ release activity) NAADPH. Nevertheless, since NAADP can also be degraded by CD38 (Schmid et al. 2011) or by alkaline phosphatase (Schmid et al. 2012), the NAADP/NAADPH redox cycle requires fill-up reactions for either NAADP or NAADPH that need to be defined in the future.
3 Novel NAADP Receptors Coupling to Ca2+ Channels
Ligand-activated ion channels form a significant group among ion channels. Since IP3, the first Ca2+ mobilizing second messenger discovered, directly binds as activating ligand to its target Ca2+ channel, the IP3 receptor, researchers expected a similar situation also for NAADP. Thus, it came as a big surprise when photoaffinity labeling experiments using NAADP modified at the 5′-position of the nicotinic acid moiety resulted in labeling of small cytosolic proteins, but not in labeling of the candidate Ca2+ channels RYR1 or TPC (Walseth et al. 2012a,b; Lin-Moshier et al. 2012). Since an NAADP binding protein was proposed earlier by Petersen’s group (Gerasimenko et al. 2003), a unifying hypothesis to harmonize the different models of NAADP’s mode of action was developed (Guse 2012). Central idea of the unifying hypothesis is that NAADP binds to a receptor/binding protein which is not an ion channel, but when bound to NAADP, activates an ion channel (Guse 2012). Though specific labeling of proteins by the photoaffinity probes developed by Walseth and Slama (Jain et al. 2010; Lin-Moshier et al. 2012; Walseth et al. 2012a, b; Ali et al. 2014; Trabbic et al. 2015; Gunaratne et al. 2019; Asfaha et al. 2019; Su et al. 2021) was already possible in 2012, it took another 9 years until identification of HN1L/JPT2 (Roggenkamp et al. 2021; Gunaratne et al. 2021), and briefly thereafter of Lsm12 (Zhang et al. 2021) as NAADP receptors/binding proteins.
HN1L/JPT2 was identified independently in two different cell systems, human Jurkat T-lymphoma cells (Roggenkamp et al. 2021) and human erythrocytes (Gunaratne et al. 2021). However, in both projects classical column chromatography for enrichment of NAADP binding proteins and detection by photoaffinity labeling were employed. Gunaratne et al. (2021) in addition used an affinity purification approach in which pre-purified cytosolic protein was first photoaffinity labeled by the bifunctional photoprobe alkyne–“all-in-one-clickable” (AIOC)–NAADP, which in a second step was biotinylated by copper-catalyzed azide-alkyne cycloaddition. Then, this adduct was bound to neutravidin agarose beads for purification from unbound proteins. After another chromatography step, HN1L/JPT2 was identified by mass spec as highly enriched candidate (Gunaratne et al. 2021). In contrast, Roggenkamp et al. (2021) relied on a series of classical column chromatography steps: anion exchange, cation exchange, and hydrophobic interaction resulting in three candidates. A small molecular weight protein, approx. 22/23 kDa, was submitted to mass spectrometry and HN1L/JPT2 (molecular mass 20.1 kDa) was detected as one of the four most abundant candidates (Roggenkamp et al. 2021). However, HN1L/JPT2 was actually the only protein among these four proteins for which a fully defined function was not available.
While both studies used comparable purification and detection approaches, different strategies for evaluating HN1L/JPT’s potential role in NAADP signaling were pursued. Roggenkamp et al. (2021) used Crispr/CAS for gene deletion of Hn1l/Jpt2 in human Jurkat T-lymphoma cells and global Ca2+ signaling upon TCR/CD3 stimulation was similarly amended as for knockout of Duox2 (Gu et al. 2021): delayed signal onset and decreased Ca2+ peak and plateau values (Roggenkamp et al. 2021). By transient re-expression of HN1L/JPT2 in Hn1l/Jpt2−/− Jurkat T cells, this phenotype was at least partially compensated. A more direct effect was expected for NAADP-dependent Ca2+ microdomains in Hn1l/Jpt2−/− T cells. In fact, TCR/CD3-evoked Ca2+ microdomains were almost absent in the first 15 s upon stimulation. These results were confirmed in rat effector T cells; here, in addition, it was shown that the changes of global Ca2+ signaling upon TCR/CD3 stimulation of Hn1l/Jpt2−/− T cells were almost identical to NAADP antagonism by BZ194 (Dammermann et al. 2009) and that both NAADP antagonism and knockout of Hn1l/Jpt2 were not different from each single intervention (Roggenkamp et al. 2021). These cell biology data were backed up by showing that HN1L/JPT2 produced recombinantly in E. coli was specifically photoaffinity labeled (Roggenkamp et al. 2021), using [32P]-azide-AIO-NAADP (Asfaha et al. 2019).
In contrast, Gunaratne et al. (2021) used gene silencing in HEK293 and U2OS cell lines and found significantly diminished photoaffinity labeling of an approx. 23 kDa band. Further, immunoprecipitation of HN1L/JPT2 from either erythrocyte or U2OS cell lysates resulted in almost selective photoaffinity labeling of the approx. 23 kDa band (Gunaratne et al. 2021). Also, a classical binding assay on PVDF plates showed specific binding of HN1L/JPT2 for NAADP in the low nanomolar range (Gunaratne et al. 2021).
Next question in both studies was the identity of the Ca2+ channel involved in HN1L/JPT2 signaling, as predicted by the unifying hypothesis (Guse 2012). Co-immunoprecipitation experiments in HEK293 cells resulted in HN1L/JPT2–TPC1 interaction, but not in HN1L/JPT2-TPC2 interaction (Gunaratne et al. 2021). In Jurkat T-lymphoma cells, evidence for interaction of HN1L/JPT2 and RYR1 was provided by (1) co-localization studies using STED super-resolution microscopy at approx. 40 nm spatial resolution and (2) co-immunoprecipitation of HN1L/JPT2 with RYR1 (Roggenkamp et al. 2021). Further, a TCR/CD3 stimulation-dependent re-localization of HN1L/JPT2 toward the plasma membrane was observed, indicating that upon NAADP binding, HN1L/JPT2 re-localizes to plasma membrane–ER junctions where RYR is localized in very close proximity to Orai1 (Diercks et al. 2018).
The current situation for HN1L/JPT2 activity is schematically shown in Fig. 2 where HN1L/JPT2 may interact with both RYR1 and/or TPC1. Further characterization of this process is certainly necessary to better understand why NAADP in some cell types appears to signal through RYR1 and in others through TPCs. This becomes further complicated by a second NAADP binding protein, Lsm12 (Zhang et al. 2021). Lsm12 was found in a screen as interaction partner of TPC2, but not TPC1. It is currently unknown whether Lsm12 may also interact with other Ca2+ channels. The situation appears even more complex since a third NAADP binding protein was detected, namely aspartate dehydrogenase domain-containing protein (He et al. 2022). In contrast to HN1L/JPT2 and Lsm12, aspartate dehydrogenase domain-containing protein showed a lower affinity for NAADP (Kd 455 nM; He et al. 2022), as compared to HN1L/JPT2 (IC50 20 ± 3.6 nM; Gunaratne et al. 2021), indicating that aspartate dehydrogenase domain-containing protein may not be involved in NAADP signaling.
4 NAADP in Immunity and Inflammation
The chapters above are related to T cell activation, one of the central processes of the adaptive immune system. Thus, NAADP signaling can be considered as one central cytosolic process important for T cell activation. But what about inflammation? Is there experimental evidence for a role of NAADP signaling in inflammatory processes?
In the central nervous system (CNS) NAADP is a regulator of autoimmunity. Effector T cells analyzed in the rat model of multiple sclerosis, the experimental autoimmune encephalomyelitis (EAE), were affected in several aspects by NAADP antagonism (Cordiglieri et al. 2010). NAADP antagonism markedly inhibited (1) migration of T cells toward the blood–brain barrier, (2) re-activation of T cells in the CNS, (3) antigen-evoked T-cell proliferation, and (4) secretion of pro-inflammatory cytokines interferon-g and interleukin-17. As a result, EAE clinical store was reduced significantly in animals treated with NAADP antagonist BZ194 (Cordiglieri et al. 2010). CNS inflammation often leads to damage of neurons resulting in paralysis. One mechanism discussed to be involved in neuronal damage is excitotoxicity by glutamate (reviewed in Mandolesi et al. 2015). Recently, initial evidence was presented that NAADP signaling may play a role in glutamate signaling via metabotropic glutamate receptors; NAADP antagonists BZ194 or Ned-19 resulted in a decrease of glutamate-evoked Ca2+ signaling in cultured hippocampal neurons (Hermann et al. 2020). However, details of NAADP signaling and its role in excitotoxicity in neurons await further clarification.
IL17 is a pro-inflammatory cytokine that regulates neutrophil migration to sites of inflammation. As mentioned above, IL-17 levels in the CNS were decreased upon NAADP antagonism in EAE (Cordiglieri et al. 2010). In a recent study of gut inflammation, NAADP antagonist Ned-19 evoked transdifferentiation of IL-17-producing Th17 cells into IL-10-producing T regulatory type 1 cells in vitro and in vivo (Nawrocki et al. 2021). Thus, pharmacological intervention in NAADP signaling appears as a novel way to control plasticity of CD4+ T cells. When duodenitis was induced in mice by anti-CD3 stimulation, NAADP antagonism resulted in reduced systemic inflammation (Nawrocki et al. 2021).
5 Conclusion
Research on NAADP signaling has suffered from the fact that major proteins involved were unknown. This situation changed dramatically with newly identified NAADP receptors/binding proteins and a redox cycle for oxidation and reduction of NAADPH/NAADP. Both HN1L/JPT2 and Lsm12 are bona fide NAADP receptors/binding proteins and likely constitute the connection between NAADP and NAADP-sensitive Ca2+ channels. The NAADPH/NAADP redox cycle allows for very rapid NAADP formation upon receptor stimulation and reduction to inactive NAADPH to rapidly terminate the signal. This fits to the rapid, but transient increases of NAADP observed upon receptor stimulation in some cell systems (Yamasaki et al. 2005; Gasser et al. 2006).
Still, open questions remain, e.g. how the NAADPH/NAADP redox cycle is kept sufficiently filled for proper function of NAADP as Ca2+ mobilizing second messenger, or how exactly NAADP-sensitive Ca2+ channels RYR1 and TPC are activated by which NAADP receptor/binding protein in which cell type.
References
Aarhus R, Graeff RM, Dickey DM, Walseth TF, Lee HC (1995) ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J Biol Chem 270(51):30327–30333
Albrieux M, Lee HC, Villaz M (1998) Calcium signaling by cyclic ADP-ribose, NAADP, and inositol trisphosphate are involved in distinct functions in ascidian oocytes. J Biol Chem 273(23):14566–14574
Ali RA, Zhelay T, Trabbic CJ, Walseth TF, Slama JT, Giovannucci DR, Wall KA (2014) Activity of nicotinic acid substituted nicotinic acid adenine dinucleotide phosphate (NAADP) analogs in a human cell line: difference in specificity between human and sea urchin NAADP receptors. Cell Calcium 55(2):93–103
Asfaha TY, Gunaratne GS, Johns ME, Marchant JS, Walseth TF, Slama JT (2019) The synthesis and characterization of a clickable-photoactive NAADP analog active in human cells. Cell Calcium 83:102060
Berg I, Potter BVL, Mayr GW, Guse AH (2000) Nicotinic acid adenine dinucleotide phosphate NAADP+ is an essential regulator of T-lymphocyte Ca2+-signaling. J Cell Biol 150(3):581–588
Billington RA, Thuring JW, Conway SJ, Packman L, Holmes AB, Genazzani AA (2004) Production and characterization of reduced NAADP (nicotinic acid-adenine dinucleotide phosphate). Biochem J 378(Pt 1):275–280
Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, Patel S (2009) Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol 186(2):201–209
Buvelot H, Jaquet V, Krause KH (2019) Mammalian NADPH oxidases. Methods Mol Biol 1982:17–36
Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang K-T, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX (2009) NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459(7246):596–600. https://doi.org/10.1038/nature08030
Cancela JM, Churchill GC, Galione A (1999) Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature 398(6722):74–76
Clapper DL, Walseth TF, Dargie PJ, Lee HC (1987) Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J Biol Chem 262(20):9561–9568
Cordiglieri C, Odoardi F, Zhang B, Nebel M, Kawakami N, Klinkert WE, Lodygin D, Lühder F, Breunig E, Schild D, Ulaganathan VK, Dornmair K, Dammermann W, Potter BV, Guse AH, Flügel A (2010) Nicotinic acid adenine dinucleotide phosphate-mediated calcium signalling in effector T cells regulates autoimmunity of the central nervous system. Brain 133(Pt 7):1930–1943
Dammermann W, Zhang B, Nebel M, Cordiglieri C, Odoardi F, Kirchberger T, Kawakami N, Dowden J, Schmid F, Dornmair K, Hohenegger M, Flugel A, Guse AH, Potter BVL (2009) NAADP-mediated Ca2+ signaling via type 1 ryanodine receptor in T cells revealed by a synthetic NAADP antagonist. Proc Natl Acad Sci U S A 106(26):10678–10683
da Silva IV, Soveral G (2021) Aquaporins in immune cells and inflammation: new targets for drug development. Int J Mol Sci 22(4):1845
DiAntonio A, Milbrandt J, Figley MD (2021) The SARM1 TIR NADase: mechanistic similarities to bacterial phage defense and toxin-antitoxin systems. Front Immunol 12:752898
Diercks BP, Werner R, Weidemüller P, Czarniak F, Hernandez L, Lehmann C, Rosche A, Krüger A, Kaufmann U, Vaeth M, Failla AV, Zobiak B, Kandil FI, Schetelig D, Ruthenbeck A, Meier C, Lodygin D, Flügel A, Ren D, Wolf IMA, Feske S, Guse AH (2018) ORAI1, STIM1/2, and RYR1 shape subsecond Ca2+ microdomains upon T cell activation. Sci Signal 11(561):eaat0358
Fang C, Li T, Li Y, Xu GJ, Deng QW, Chen YJ, Hou YN, Lee HC, Zhao YJ (2018) CD38 produces nicotinic acid adenosine dinucleotide phosphate in the lysosome. J Biol Chem 293(21):8151–8160. Erratum in: J Biol Chem. 2019 Dec 13;294(50):19447
Fliegert R, Hölzer HT, Guse AH (2018) TRPM2 activation: paradigm shifted? Cell Calcium 76:132–134
Galione A, Davis LC, Martucci LL, Morgan AJ (2022) NAADP-mediated Ca2+ signalling. Handb Exp Pharmacol. https://doi.org/10.1007/164_2022_607
Gasser A, Bruhn S, Guse AH (2006) Second messenger function of nicotinic acid adenine dinucleotide phosphate revealed by an improved enzymatic cycling assay. J Biol Chem 281(25):16906–16913
Gerasimenko JV, Maruyama Y, Yano K, Dolman NJ, Tepikin AV, Petersen OH, Gerasimenko OV (2003) NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J Cell Biol 163(2):271–282
Grasberger H, Refetoff S (2006) Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem 281(27):18269–18272
Gu F, Krüger A, Roggenkamp HG, Alpers R, Lodygin D, Jaquet V, Möckl F, Hernandez CLC, Winterberg K, Bauche A, Rosche A, Grasberger H, Kao JY, Schetelig D, Werner R, Schröder K, Carty M, Bowie AG, Huber S, Meier C, Mittrücker HW, Heeren J, Krause KH, Flügel A, Diercks BP, Guse AH (2021) Dual NADPH oxidases DUOX1 and DUOX2 synthesize NAADP and are necessary for Ca2+ signaling during T cell activation. Sci Signal 14(709):eabe3800
Gunaratne GS, Su P, Marchant JS, Slama JT, Walseth TF (2019 Jul) 5-Azido-8-ethynyl-NAADP: a bifunctional, clickable photoaffinity probe for the identification of NAADP receptors. Biochim Biophys Acta Mol Cell Res 1866(7):1180–1188
Gunaratne GS, Brailoiu E, He S, Unterwald EM, Patel S, Slama JT, Walseth TF, Marchant JS (2021) Essential requirement for JPT2 in NAADP-evoked Ca2+ signaling. Sci Signal 14(675):eabd5605
Guse AH (2012) Linking NAADP to ion channel activity: a unifying hypothesis. Sci Signal 5(221):pe18
Guse AH (2015) Calcium mobilizing second messengers derived from NAD. Biochim Biophys Acta 1854(9):1132–1137
Guse AH, Diercks B-P (2018) Integration of nicotinic acid adenine dinucleotide phosphate (NAADP)-dependent calcium signalling. J Physiol 596(14):2735–2743. https://doi.org/10.1113/JP275974
Hohenegger M, Suko J, Gscheidlinger R, Drobny H, Zidar A (2002) Nicotinic acid-adenine dinucleotide phosphate activates the skeletal muscle ryanodine receptor. Biochem J 367(Pt 2):423–431
He X, Kang Y, Chen L (2022) Identification of ASPDH as a novel NAADP-binding protein. Biochem Biophys Res Commun 621:168–175
Hermann J, Bender M, Schumacher D, Woo MS, Shaposhnykov A, Rosenkranz SC, Kuryshev V, Meier C, Guse AH, Friese MA, Freichel M, Tsvilovskyy V (2020) Contribution of NAADP to glutamate-evoked changes in Ca2+ homeostasis in mouse hippocampal neurons. Front Cell Dev Biol 8:496
Jain P, Slama JT, Perez-Haddock LA, Walseth TF (2010) Nicotinic acid adenine dinucleotide phosphate analogues containing substituted nicotinic acid: effect of modification on Ca2+ release. J Med Chem 53(21):7599–7612
Kwon J, Shatynski KE, Chen H, Morand S, de Deken X, Miot F, Leto TL, Williams MS (2010) The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Sci Signal 3(133):ra59
Lee HC, Aarhus R (1995) A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J Biol Chem 270(5):2152–2157
Lin-Moshier Y, Walseth TF, Churamani D, Davidson SM, Slama JT, Hooper R, Brailoiu E, Patel S, Marchant JS (2012) Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J Biol Chem 287(4):2296–2307
Mandolesi G, Gentile A, Musella A, Fresegna D, De Vito F, Bullitta S, Sepman H, Marfia GA, Centonze D (2015) Synaptopathy connects inflammation and neurodegeneration in multiple sclerosis. Nat Rev Neurol 11(12):711–724
Nam TS, Park DR, Rah SY, Woo TG, Chung HT, Brenner C, Kim UH (2020) Interleukin-8 drives CD38 to form NAADP from NADP+ and NAAD in the endolysosomes to mobilize Ca2+ and effect cell migration. FASEB J 34(9):12565–12576
Nawrocki M, Lory N, Bedke T, Stumme F, Diercks BP, Guse AH, Meier C, Gagliani N, Mittrücker HW, Huber S (2021) Trans-ned 19-mediated antagonism of nicotinic acid adenine nucleotide-mediated calcium signaling regulates Th17 cell plasticity in mice. Cell 10(11):3039
Rautenberg S, Keller M, Leser C, Chen CC, Bracher F, Grimm C (2022) Expanding the toolbox: novel modulators of endolysosomal cation channels. Handb Exp Pharmacol. https://doi.org/10.1007/164_2022_605
Roggenkamp HG, Khansahib I, Hernandez CL, Zhang Y, Lodygin D, Krüger A, Gu F, Möckl F, Löhndorf A, Wolters V, Woike D, Rosche A, Bauche A, Schetelig D, Werner R, Schlüter H, Failla AV, Meier C, Fliegert R, Walseth TF, Flügel A, Diercks BP, Guse AH (2021) HN1L/JPT2: a signaling protein that connects NAADP generation to Ca2+ microdomain formation. Sci Signal 14(675):eabd5647
Roscoe JM, Sevier CS (2020) Pathways for sensing and responding to hydrogen peroxide at the endoplasmic reticulum. Cell 9(10):2314
Santella L, Kyozuka K, Genazzani AA, De Riso L, Carafoli E (2000) Nicotinic acid adenine dinucleotide phosphate-induced Ca2+ release. Interactions among distinct Ca2+ mobilizing mechanisms in starfish oocytes. J Biol Chem 275(12):8301–8306
Schmid F, Bruhn S, Weber K, Mittrücker HW, Guse AH (2011) CD38: a NAADP degrading enzyme. FEBS Lett 585(22):3544–3548
Schmid F, Fliegert R, Westphal T, Bauche A, Guse AH (2012) Nicotinic acid adenine dinucleotide phosphate (NAADP) degradation by alkaline phosphatase. J Biol Chem 287(39):32525–32534
Soares S, Thompson M, White T, Isbell A, Yamasaki M, Prakash Y, Lund FE, Galione A, Chini EN (2007) NAADP as a second messenger: neither CD38 nor base-exchange reaction are necessary for in vivo generation of NAADP in myometrial cells. Am J Physiol Cell Physiol 292(1):C227–C239
Su P, Bretz JD, Gunaratne GS, Marchant JS, Walseth TF, Slama JT (2021) Chemo-enzymatic synthesis of adenine substituted nicotinic acid adenine dinucleotide phosphate (NAADP) analogs. Bioorg Med Chem 30:115901
Trabbic CJ, Zhang F, Walseth TF, Slama JT (2015) Nicotinic acid adenine dinucleotide phosphate analogues substituted on the nicotinic acid and adenine ribosides. Effects on receptormediated Ca2+ release. J Med Chem 58(8):3593–3610
Walseth TF, Lin-Moshier Y, Jain P, Ruas M, Parrington J, Galione A, Marchant JS, Slama JT (2012a) Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide phosphate (NAADP)-binding proteins in sea urchin egg. J Biol Chem 287(4):2308–2315
Walseth TF, Lin-Moshier Y, Weber K, Marchant JS, Slama JT, Guse AH (2012b) Nicotinic acid adenine dinucleotide 2′-phosphate (NAADP) binding proteins in T-lymphocytes. Messenger 1(1):86–94
Wehage E, Eisfeld J, Heiner I, Jüngling E, Zitt C, Lückhoff A (2002) Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem 277(26):23150–23156
Wolf IM, Diercks BP, Gattkowski E, Czarniak F, Kempski J, Werner R, Schetelig D, Mittrucker HW, Schumacher V, von Osten M, Lodygin D, Flugel A, Fliegert R, Guse AH (2015) Frontrunners of T cell activation: initial, localized Ca2+ signals mediated by NAADP and the type 1 ryanodine receptor. Sci Signal 8(398):ra102
Yamasaki M, Thomas JM, Churchill GC, Garnham C, Lewis AM, Cancela JM, Patel S, Galione A (2005) Role of NAADP and cADPR in the induction and maintenance of agonist-evoked Ca2+ spiking in mouse pancreatic acinar cells. Curr Biol 15(9):874–878
Zhang J, Guan X, Shah K, Yan J (2021) Lsm12 is an NAADP receptor and a two-pore channel regulatory protein required for calcium mobilization from acidic organelles. Nat Commun 12(1):4739
Zhao ZY, Xie XJ, Li WH, Liu J, Chen Z, Zhang B, Li T, Li SL, Lu JG, Zhang L, Zhang LH, Xu Z, Lee HC, Zhao YJ (2019) A cell-permeant mimetic of NMN activates SARM1 to produce cyclic ADP-ribose and induce non-apoptotic cell death. iScience 15:452–466
Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rötzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C (2009) The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch 458(5):891–899
Acknowledgments
We gratefully acknowledge fruitful discussions with members of the Calcium Signalling Group, University Medical Center Hamburg-Eppendorf, and members of SFB1328. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) (project number 335447717; SFB1328, project A01 to AHG), EU project INTEGRATA DLV-813284 (to AHG), and by NCL-Stiftung Hamburg (to AHG).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Guse, A.H. (2023). NAADP-Evoked Ca2+ Signaling: The DUOX2–HN1L/JPT2–Ryanodine Receptor 1 Axis. In: Wahl-Schott, C., Biel, M. (eds) Endolysosomal Voltage-Dependent Cation Channels. Handbook of Experimental Pharmacology, vol 278. Springer, Cham. https://doi.org/10.1007/164_2022_623
Download citation
DOI: https://doi.org/10.1007/164_2022_623
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-31522-0
Online ISBN: 978-3-031-31523-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)