Abstract
Pain impacts the lives of billions of people around the world – both directly and indirectly. It is complex and transcends beyond an unpleasant sensory experience to encompass emotional experiences. To date, there are no successful treatments for sufferers of chronic pain. Although opioids do not provide any benefit to chronic pain sufferers, they are still prescribed, often resulting in more complications such as hyperalgesia and dependence. In order to develop effective and safe medications to manage, and perhaps even treat pain, it is important to evaluate novel contributors to pain pathologies. As such, in this chapter we review the role of Toll-like receptor 4, a receptor of the innate immune system, that continues to gain substantial attention in the field of pain research. Positioned in the nexus of the neuro and immune systems, TLR4 may provide one of the missing pieces in understanding the complexities of pain. Here we consider how TLR4 enables a mechanistical understanding of pain as a multidimensional biopsychosocial state from molecules to cells to systems and back again.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- A20
- Biased signalling
- Biopsychosocial pain
- Chronic pain
- Genetics
- GPCRs
- Neuroimmunology
- Pain
- Toll-like receptors
- TRPV1
1 Introduction
Pain is complex, occurring at multiple levels from the molecular and cellular to psychological and behavioural, with impact on both the individual and society. Hence a biopsychosocial model of pain is the most compelling (Raja et al. 2020). But the biopsychosocial pain nexus (the interface of these inputs that leads to pain experiences) is largely unexplored from the perspective of the cellular and molecular networks that can connect this multidimensional state. When this important protective physiological response transcends from acute physical pain to chronic pain it encompasses emotional states of helplessness, anxiety and depression. The manifestation and severity of chronic pain in individuals cannot yet be predicted, nor can it be completely evaluated due to the use of subjective assessments and the dire lack of objective measures. Subjective measures of pain are prone to manipulation and can be completely ineffective in some cases, such as for young children, non-verbal patients or those with neurodegenerative diseases. With chronic pain impacting the quality of life of 1 in 5 individuals worldwide, it is critical to understand the complex molecular mechanisms behind the diverse symptoms of pain. Such a mechanistic understanding will inform a future of precision medicine and precision pain management, evolving us beyond the current empirical practices.
In 2020, the International Association for the Study of Pain revised its definition of pain (Raja et al. 2020) as follows: ‘An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage’. This definition is augmented by six additional contextual clarifications of critical importance: pain is a personal experience influenced to varying degrees of biopsychosocial factors. Pain and nociception are different. Pain cannot be inferred solely from activity in sensory neurons. Throughout life, individuals learn the concept of pain. A person’s report of pain should be respected. While pain can be adaptive, it may adversely affect function and social and psychological wellbeing. Verbal description is only one of several behaviours to express pain; inability to communicate does not negate the possibility that humans or non-human animals experience pain. This pain definition highlights the complexities that we face in defining what pain is, even in humans with whom we generally can communicate.
Although much is known about nociceptive pathways (the transmission of a noxious stimulus to the brain) resulting in the pain experience (see von Hehn et al. (2012) for review) the transition from acute to chronic pain remains poorly understood. What is clear though is the emerging role of the innate immune system, in particular Toll-like receptors (TLRs), in the detection, creation, transition and maintenance stages that facilitate chronic pain (Lacagnina et al. 2018). In this chapter we will provide an overview of TLR4 in pain; an important member of the TLR family that has been implicated in chronic pain. Importantly, embodied within this greater understanding of the involvement of TLR4 in pain is the ability for us to begin to appreciate the biopsychosocial nature of pain in its full complexity. This step of appreciating the true complexity of pain must not be overlooked across the multidimensionality of pain as it is cultivated and experienced in time (seconds to years), distance (nanometres to metres) and endogenous to exogenous environments.
1.1 Conceptualising Pain as Multidimensional States
We are challenged to address pain in more complex ways, as exemplified by the complexity of the revised IASP pain definition. For pain to be appreciated simultaneously from the top down at a systems level as a biopsychosocial multidimensional state, and from the bottom up as events occurring at the nanoscale in a complex web of molecular and cellular events, we must define mechanisms that can mediate these profound events over vast time and length scales. Much of our research and development focus over the past two decades has been on interrogating the roles that the innate immune system, and specifically TLR4, might play in this complex multidimensional pain state.
Such challenges of conceptualisation, like we face in understanding pain, have been explored in mathematics and theoretical physics. In these disciplines, models that help us conceptualise complex systems are designed from their foundations to operate in real-world scenarios. This is distinct from the classical Cartesian approaches currently adopted in the field of pain research that necessitate operations between two binary states of homeostasis and an altered state. For the pain field, this would mean only including injured and uninjured states in experimental settings. Or considering only neuronal action potentials in nociceptive fibres as ‘pain’. In contrast, the evolved view of biological conditions acknowledges that real-world systems are unstable and continuously compensating to reach some form of new steady state condition, termed allostasis. This establishment of synchrony within complex systems has been set forth in examples like the Kuramoto model (Dattani and Barahona 2017) and dynamical systems models. The real world is dominated by open systems owing to their co-existence with their environment and this is at the foundation of viewing pain as a complex biopsychosocial state. These open systems are constantly changing and adapt to new conditions but do so constrained to some form of inherent order or synchrony related to that specific system. These models force us to connect time and space with defined molecular and cellular mechanisms (Fig. 1). This is crucial for the field to acknowledge when we consider pain.
A field that has embraced this multidimensionality and open systems biological approach is psychoneuroimmunology. Psychoneuroimmunology conceptualises health as a system involving interactions between the body and mind. From a psychoneuroimmunology model perspective becoming sick is a complex molecular pattern-to-cells-to-systems response that is initiated by our innate immune system (Dantzer and Kelley 2007). Inherently this is a multidimensional opportunity and challenge. Hence, the innate pattern recognition system and receptors like Toll-Like Receptor 4 (TLR4) are key molecular connectors of mind and body (Frank et al. 2015). Our innate immune system is the ultimate integrated surveillance and response system because it is the first responder to the detection of threats from diverse origins. When we get sick with an illness, our innate immune system mounts a protective inflammatory response within minutes of exposure, which helps to control and limit the negative impact of the invading pathogen (Dantzer and Kelley 2007). These events are triggered by our molecular pattern recognition capabilities at the nanoscale, a crucial one being TLR4. The resulting inflammatory response recruits specific molecular systems to mitigate the threat and occurs so rapidly that we are not consciously aware of a change in our health status, even though profound molecular events are occurring in our blood and tissues. These molecular nanoscale responses in turn have an immediate impact on neural activity at macroscales to elicit functionally adaptive behaviours by the organism that prioritise personal health and protect the community (Lasselin 2021). Here psychoneuroimmunology acknowledges that the system can respond to endogenous and exogenous factors, scaling and focusing the response to these as needed (Fig. 1). This allows for the innate immune system to undertake information down sampling at the receptor level as it scales the immune signalling. Moreover, some brain networks are more sensitive to these molecular changes, resulting in specific functional adaptations observed early in discrete immune responses (Wegner et al. 2014; Hutchinson 2014). A crucial and long appreciated adaptation is illness-induced pain states. However, the historic association of these illness events and the modern understanding of TLR4 in hypernociception and complex multidimensional biopsychosocial pain states are only just beginning to be appreciated.
2 The Shared Toll-Like Receptor Language of Sensory Neurons and Immune Cells
As a receptor family, TLRs play a sentinel surveillance role as pattern recognition receptors that can identify molecular patterns as ‘non-self’ or ‘danger’ signals (Buchanan et al. 2010). Within the CNS, these receptors are mainly found in the innate immune system on endothelial cells, microglia, some astrocytes and sensory neurons (Bsibsi et al. 2002; Goethals et al. 2010; Nagyoszi et al. 2010). Whilst neurons have specific, ligand selective receptors for neurotransmitters, TLRs have evolved to unequivocally recognise a vast array of threats via pathogen-associated molecular patterns (PAMPs; microbial pathogens) and danger-associated molecular patterns (DAMPs; cellular signals of danger or stress) (Akira and Takeda 2004). In response to detection, TLRs then coordinate signal transduction to modulate the inflammatory state of the host. Importantly, TLRs are also expressed on peripheral immune cells. As the immune and sensory nervous systems communicate via shared mediators and networks, this places TLRs in the nexus of both neuro and immune systems (neuroimmune interface) where they are perfectly positioned to influence nociceptive processing and pain (Lacagnina et al. 2018). This is a crucial molecules-to-systems capacity of TLRs enabling a common molecular language to span the biopsychosocial divide. Importantly this influence and communication is bidirectional between neuro and immune systems, making TLRs a critical connector of the biological, psychological and environmental state of the organism. Hence, this multidimensionality of TLRs and the cells that express them enables a molecular understanding of the biopsychosocial pain condition.
2.1 The Molecular Origins of TLR4 in Acute Pain
The illness response is a coordinated set of molecular drivers, to behaviourally presented adaptations, which develop during the course of an infection (Dantzer 2001). During an infection multiple systems are adapted (Kelley et al. 2003) including sensory disturbances such as increased sensitivity to pain (Yirmiya et al. 2006). Experimentally these behaviours and sensory changes can be recreated using either the exogenous bacterial endotoxin or the endogenous-mimicking effector agents like recombinant proinflammatory cytokines, such as Interleukin-1 beta (IL-1β). These data demonstrate that the innate immune response to molecular threat and the generation of molecular mediators like IL-1β is sufficient to change neuroimmune function (Kelley et al. 2003; Dantzer and Kelley 2007). The identification and naming of TLR4 as an innate immune detection system for endotoxin entwines two key threads; that innate immune function and sensory changes are associated with illness.
The specific use of lipopolysaccharide (LPS), the classical TLR4 ligand, to induce hyperalgesia and induction of inflammatory mediators identified the importance of TLR4 in pathology (Wicrtelak et al. 1994). This identification of exogenous agent driven TLR4 pain behaviours led to the implication of endogenously derived factors in nociceptive hypersensitivity. Clinically, systemic LPS results in anatomically dependent altered mechanical sensitivity, which correlates with peripheral immune activation, specifically circulating IL-6 (Wegner et al. 2014). There is a clear role for TLR4 in pain sensitisation as part of an adaptive illness response, likewise, changes in expression can be associated with transition to maladaptive, pathological pain states.
2.2 Why Is the Role of TLR4 in Persistent Pain Important?
De Leo and colleagues were the first to demonstrate that changes in TLR4 expression are important for generating a pathological pain state. They demonstrated TLR4 upregulation in spinal microglia is associated with cytokine expression and establishment of thermal and mechanical sensitivity following spinal nerve ligation, a model of neuropathic pain (Tanga et al. 2005). Importantly both behavioural and spinal molecular changes were attenuated in genetically modified (TLR4 knockout (KO) and point mutation) mice and rats with spinally administered TLR4 antisense oligodeoxynucleotide (Tanga et al. 2005). Likewise, Yaksh and colleagues show that TLR4 mediates transition to pathological pain in a mouse serum-transferred arthritis model. Like De Leo et al., they correlated spinal microglial reactivity with mechanical hypersensitivity, inhibited by TLR4 genetic manipulation (KO) and antagonism (LPS from Rhodobacter sphaeroides [LPS-RS]). Interestingly, they demonstrated administration of LPS-RS following onset of mechanical hypersensitivity had no behavioural effect, alluding to TLR4 being an important component of generation, but not maintenance, of the molecular pathological pain phenotype (Christianson et al. 2011). This contrasts earlier studies which report LPS-RS attenuation of established chronic constriction injury (CCI)-induced neuropathic pain in rats (Hutchinson et al. 2008). Collectively these studies highlight non-neuronal changes in TLR4 expression influence the inflammatory environment leading to pathological pain. Additionally, changes to neuronal TLR4 expression must also be considered in the context of the molecular pathological pain phenotype. Moreover, it is important to note that TLR4 does not act alone, as blockade of TLR2 has also been reported to attenuate neuropathic pain in rodent models and TLR3 has also been implicated in neuropathic pain (Jurga et al. 2016).
This implication of the nanoscale events of TLR4 in pain needs to then be connected to what TLR4 expressing cell systems are critical to pain. To this end TLR4 expression has been reported in rat trigeminal (TG) and dorsal root ganglia (DRG) by Wadachi and Hargreaves. Upregulation of TLR4 was also observed on neurons within inflamed human dental pulp (Wadachi and Hargreaves 2006). The authors reported co-expression with neuronal-ion channel, transient receptor potential cation channel subfamily V member 1 (TRPV1), an important nociceptive detector of noxious stimuli. The authors later demonstrated that TLR4 antagonist LPS-RS alters the activity of neuronal TRPV1 following activation of TLR4 with LPS (Diogenes et al. 2011). The relationship between TLR4 and TRPV1 is an important neuroimmune interaction discussed in greater detail later in this chapter. TLR4 expression has also been observed in rat and human DRG neurons in a study investigating chemotherapy-induced peripheral neuropathy (CIPN) (Li et al. 2015). TLR4 can therefore be considered more than an initiator of immune signalling, but a receptor with a direct role in nociceptor detection of injury, danger and infection by neurons, underwriting its critical multidimensionality. The numerous examples of TLR4-dependent pathological pain models suggest an evolved response to illness and injury as opposed to nociceptive pathways hijacked by immune infrastructure. As a result, TLRs have been brought forward as key contributors to pain pathology and novel targets for modifying pain processing.
3 With Immense Power Must Come Profound Controls
3.1 Overview of TLR Signalling
TLR4 relays critical information about the presence of danger/damage signals to intracellular adapter proteins critical for initiating a cellular response (please see Fitzgerald and Kagan (2020) for recent review on TLRs). This TLR4 signalling is achieved by a multi-receptor complex, which includes a TLR4 dimer and its co-receptors myeloid differentiation factor 2 (MD-2) and Cluster of differentiation 14 (CD14) (Núñez Miguel et al. 2007). Shimazu and co-workers highlighted the critical role of MD-2 in TLR4 signalling (Shimazu et al. 1999). MD-2 functions by recognising LPS and dimerising TLR4 monomers, which in turn allows for receptor interaction with intracellular adapter proteins. CD14 has been reported to enhance LPS recognition and due to its sensitivity is capable of binding picomolar concentrations of LPS (Gioannini et al. 2004). Highlighting the importance of understanding the contributions of other proteins in TLR4 signalling, and hence potential targets for pain management, is the evidence from Cao et al. demonstrating that CD-14 knockout mice display significantly decreased behavioural sensitivity to pain following L5 nerve transection compared to wild-type injured mice (Cao et al. 2009). The TLR4 multi-protein signalling complex assembly further demonstrates the multidimensionality of the system. Here we see that extracellular environments may be conditioned by paracrine or autocrine pre-signalling events to influence the potential TLR4 signalling capacity. Importantly, this complexity of TLR4’s non-membrane bound co-factors, like MD-2, is crucial to incorporate into in vitro systems. For example, the use of non-biologically relevant culture media that does not have the necessary co-factors can result in several orders of magnitude change in the TLR4 ligand responsivity, causing the apparent loss of function and may explain differences in reported functions of TLR4 (Hutchinson et al. 2010).
Once this complex signalling unit is formed following binding of a ligand, TLR4 is the only member of the TLR family capable of activating two major intracellular signalling pathways: the myeloid differentiation primary response 88 (MyD88)-dependent pathway and the TIR-domain-containing adapter-inducing interferon (IFN)-β (TRIF) pathway (also commonly referred to as MyD88-independent). The MyD88 pathway is activated by the recruitment of two adapter proteins MyD88 and Mal (also known as TIR Domain Containing Adaptor Protein [TIRAP]) to TLR4 at the plasma membrane. This results in the rapid activation of transcription factor NF-κB, MAPKs, activator protein-1 (AP-1), and IFN regulatory factor 5 (IRF5) and the ultimately the secretion of proinflammatory cytokines such as interleukin (IL-)1β, IL-6, tumour necrosis factor (TNF) and chemokines like monocyte chemoattractant protein 1 (MCP-1) and IL-8 (Arthur and Ley 2013; Medzhitov et al. 1998; Takaoka et al. 2005). This complex signalling system can also lead to the production of nitric oxide synthase (iNOS) and anti-inflammatory IL-1R antagonist and IL-10 (Lacagnina et al. 2018). Evidence that the MyD88 pathway is implicated in pain pathophysiology has been presented. Following chronic constriction injury, an increase in MyD88 protein levels is observed in nociceptive pathways including the DRG, dorsal horn and related signalling components (Lacagnina et al. 2018). In studies where MyD88 signalling is blocked, as in the Liu et al. study with the use of an inhibitory peptide, an attenuation of mechanical allodynia and thermal hyperalgesia was reported (Liu et al. 2017). It should be noted that much of this intracellular signalling knowledge has been derived from classical immune cell studies. Therefore, the heterogeneity in secondary signalling pathways across different cell systems like glia and neurons may be profound. For example, differences in TLR4 signalling capacity have been noted between macrophages and dendritic cells under specific conditions (Tsukamoto et al. 2013).
Parallel or instead of the MyD88 pathway, TRIF signalling can occur once TLR4 has internalised and recruited adapter proteins TRIF and TRAM. This leads to the activation of interferon regulatory factor 3 (IRF3) and the release of type 1 interferons such as beta interferon (IFN-β) and IL-10 (Yamamoto et al. 2003). There is some evidence that the expression of IFNs is implicated in the inhibition of nociceptive transmission (Liu et al. 2016). The activation of the TRIF pathway also results in the activation of NF-κB, albeit delayed (Sakai et al. 2017). The kinetic profile of each signalling event may play a fundamental role in the ultimate physiological response. This complexity of one receptor with multiple downstream potential signalling partners underscores the information down sampling that the TLR4 signalling system can compute at the molecular level. However, we are yet to fully appreciate what determines this molecular computation and how it contributes to pathological pain.
Much of the characterisation of TLR4 signalling has been conducted with LPS. Interestingly, depending on the bacterial species LPS is isolated from, activation of both pathways simultaneously or selective activation of either the MyD88 dependent or TRIF pathway is possible (Stephens et al. 2021). This molecular computation is termed ‘biased signalling’ and has been well studied in the context of G protein-coupled receptor (GPCR) signalling. As the roles of TLR4’s signalling pathways have yet to be fully deciphered, ‘biased’ LPS provides a valuable tool to further interrogate TLR4 function in pain. Excitingly, the concept of biased signalling presents a tantalising opportunity to pharmacologically modulate TLR4 signalling with biased ligands allowing potential therapies to move beyond complete antagonism to selectively activating/deactivating MyD88-dependent and -independent pathways.
3.2 Regulation of TLR4 Signalling
Innate immune signalling is fundamental in maintaining homeostasis, therefore its regulation at molecular, cell and systems levels is crucial in preventing a detrimental inflammatory response. At the molecular level, TLR4 is critical to the innate immune response, thus, tightly tuned regulation of its signalling is pertinent. As with many aspects of human biology, there is high redundancy in TLR4 regulation, with multiple mechanisms beyond transcription factor control.
3.2.1 TLR4 Epigenetics
Previous research into epigenetic regulation of innate immune responses has focused primarily on epigenetic mechanisms downstream of TLR activation, with less focus on the contribution of epigenetic modifications to variable TLR4 expression in primary tissue, which is somewhat justified based on the general hypomethylation status of the human TLR4 promoter (Xie et al. 2018). Reflecting this, in the sole study to date in the context of pain, TLR4 promoter methylation was not significantly associated with persistent pain after breast cancer surgery (Kringel et al. 2019). Whilst there are many reports of case-control differences in TLR4 epigenetics for other diseases/conditions, a key limitation of these studies has been that they have either not quantified TLR4 expression or not reported on the correlation between epigenetics and expression. Consequently, whilst experimental (e.g., in vitro) modification of TLR4 promoter methylation, histone trimethylation and/or acetylation can alter TLR4 expression (Du et al. 2019; Kim et al. 2016; Takahashi et al. 2011), it remains unclear the extent to which epigenetics determine clinical variability in TLR4 expression in or between individuals (Poole et al. 2020), or between tissues/cell types. The field would advance more quickly if clinical epigenetics and expression were quantified and reported (i.e., their correlation) in tandem.
3.2.2 Post-Transcriptional/Translational Regulation
TLR4 splice variants have been identified in multiple species, some of which may act as negative regulators of TLR4 signalling (Vaure and Liu 2014). In addition, human and rodent TLR4 are targeted by multiple miRNAs, with strong evidence (reporter assay, Western blot and/or qPCR) for effects on TLR4 expression (Huang et al. 2019). Tissue expression of these miRNAs changes in response to TLR4 pathway activation and in different pain states/pathologies, and they may play important negative-feedback roles in the control of inflammation. For example, miR-124, miR-146a and miR-451 target TLR4 directly, as well as other genes in the TLR4 signalling pathway (Wang et al. 2021; Yang et al. 2011; Sun and Zhang 2018; Lu et al. 2015; Taganov et al. 2006; Ma et al. 2014). Their expression changes in response to TLR activation in vitro, and in animal models of inflammatory and neuropathic pain, with the administration of miR-124 and miR-146a mimics able to attenuate pain in those models (Ma et al. 2014; Ponomarev et al. 2011; Taganov et al. 2006; Willemen et al. 2012; Lu et al. 2015; Kynast et al. 2013; Grace et al. 2018; Sun and Zhang 2018).
Glycosylation is a form of co- and post-translational modification that modifies proteins by the addition of specific glycans. We now know that the presence, absence or even the pattern of glycosylation plays a key role in biological processes such as cellular communication, differentiation and intracellular signal transduction (Ohtsubo and Marth 2006). Examples in literature suggest that polysialic acid (PSA), a cell surface glycan, is involved in a number of plasticity-related responses including cellular adaptations to pain (Rutishauser 2008). In 2006, Weber and co-workers reported an increase in PSA neural adhesion molecule expression in the hippocampus of heroin addicts (Weber et al. 2006). At the receptor level, there is evidence to implicate an important role for glycosylation in regulating TLR4 signalling. For example, da Silva and colleagues have demonstrated that N-linked glycosylation sites are important for TLR4 activity and that removal of glycosylation sites (N-linked) on both TLR4 and its accessory protein MD-2 inhibits LPS-induced activation (da Silva Correia and Ulevitch 2002). In line with this, it has been shown cleavage of sialic acid and endogenous sialidase activity by neuraminidase 1 facilitates signal transduction of TLR4 (Feng et al. 2012; Amith et al. 2010). Furthermore, it has recently been reported that endogenous neuraminidase is critically involved in TLR4 mediated microglial reactivity (Allendorf et al. 2020). Taken together, these studies demonstrate the need to investigate the role of glycosylation in pain as they may provide novel targets for the management of pain and addiction.
Following TLR4 activation at the cell surface, there are also mechanisms in place to regulate signalling at the level of intracellular protein cascades. Here, the predominant mechanisms modify ubiquitin structures. Ubiquitin is a small protein which links together forming chains. These ubiquitin chains are fundamental in linking proteins to form intracellular signalling cascades which facilitate signal transduction. This is regulated by specialised Deubiquitinating enzymes (DUBs), which cleave the ubiquitin chains to prevent further downstream protein interaction, thus arresting signalling (Das et al. 2020). In the context of TLR4, the most characterised DUB is A20 (encoded by TNFAIP3).
A20 is recognised as a crucial regulator of numerous inflammatory signalling pathways (TLR4, IL1R, TNFR1 and 2, NLRs, IL-17R, TCR) (Catrysse et al. 2014). A20 is particularly unique owing to its dual enzymatic function both ligating and cleaving ubiquitin motifs (Coornaert et al. 2009). Despite this complex dual function, literature to date suggests it is the K63-linked DUB activity of A20 that regulates TLR4 signalling. Specifically, in the canonical MyD88 dependant pathway A20 binds to adaptor protein TRAF6 and cleaves the K63 ubiquitin chain to prevent further signalling (Boone et al. 2004). The extent of regulation exerted by A20 on the MyD88-independent pathway is less clear.
Beyond its direct regulation of TLR4 signalling, A20 also exerts regulatory action on the mechanisms by which TLR4 exerts a biological effect. For example, A20 regulates the signalling of various cytokines (such as TNF-α and IL-1) produced following TLR4 activation. This is important as it means A20 is strongly positioned to regulate the action of TLR4 signalling at multiple points. As a result, dysfunction of A20 is associated with a range of TLR4- linked pathologies (Ma and Malynn 2012). Endotoxic shock, also known as septic shock, is a well-characterised pathology driven by TLR4. Early work demonstrates that A20 is fundamental in preventing endotoxic shock via a TLR specific mechanism (Boone et al. 2004). Furthermore, TLR4 signalling has been implicated in pathologies of the central nervous system (CNS) which has led to the subsequent exploration of A20 in this domain. It has recently been shown that A20 is critical in the regulation of inflammatory signalling in the CNS and that its ablation is associated with uncontrolled inflammation, including infiltration by peripheral immune cells, and poor prognosis of experimental autoimmune encephalitis (a rodent model of multiple sclerosis) (Mohebiany et al. 2020; Voet et al. 2018). Despite extensive literature implicating TLR4 signalling in pain and addiction, the role of A20 is yet to be established here.
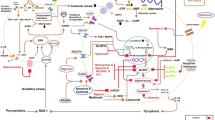
In outlining the three predominant post-transcriptional/translational mechanisms by which TLR4 is regulated (Fig. 2), it is apparent there is a gaping hole in our understanding of how immune regulation mediates pain and addiction. By exploring these further, novel therapeutic targets and strategies may be developed, helping to improve the quality of life of individuals impacted by pain.
Post-transcriptional/translational regulation of TLR4 signalling. Schematic illustrating three broad areas of post-transcriptional/translational TLR4 regulation focusing on the MyD88 dependent pathway. (a) Modifications to receptor glycan residues at the cell surface serve to regulate signal transduction. (b) Intracellular mechanisms such as de-ubiquitination by deubiquitinating enzymes (DUBs) arrest signal transduction by inhibiting intracellular signalling cascades. (c) Post-transcriptional regulation of TLR4 signalling occurs through via MicroRNA (MiR) targeting of TLR4 and various proteins involved in its signalling. Created with BioRender.com
4 Molecular Interactions Amplify Pain Complexity: TLR Interactions with Other Proteins
4.1 Ion Channels Like TRPV1 Also ‘Talk’ to TLR4
Transient receptor potential cation channel subfamily V member 1 (TRPV1) has been extensively studied in the context of pain as it allows control of nociceptive activation. TRPV1 is a non-selective neuronal-ion channel first classified in 1997 by Caterina and colleagues as vanilloid receptor 1 (VR1); a receptor for capsaicin, the pungent ingredient found in chilli peppers (Caterina et al. 1997). In addition to capsaicin, TRPV1 is activated by noxious heat, protons and a myriad of exogenous and endogenous chemicals including piperine (black pepper), spider toxins and endocannabinoids (e.g., anandamide) (Liu and Simon 1996; Siemens et al. 2006; Zygmunt et al. 1999). TRPV1 is expressed throughout the nervous system on small and medium diameter, C- and Aδ-fibre nociceptors, spinal cord dorsal horn, hypothalamus, hippocampus, cortex and microglia (Kunert-Keil et al. 2006; Mezey et al. 2000; Szallasi et al. 2007). TRPV1 therefore presents as an ideal multimodal detector for noxious stimuli and an important component of the nociceptive system.
This Cartesian mode of study has now established that activation of TRPV1 on primary afferents results in action potential generation and nociceptive firing, leading to an initial ‘spontaneous’ pain response followed by a period of hypersensitivity (Caterina and Julius 2001). However, it is now clear that there is a multidimensionality to TRPV1 function as LPS application results in potentiation of capsaicin-induced calcitonin gene related peptide (CGRP) content, correlating with increased CGRP release from excised rat trachea (Hua et al. 1996). Interestingly this effect was blocked by IL-1β and cyclooxygenase (COX) inhibitors (Hua et al. 1996), suggesting a more complex neuroimmune involvement in the system. A headache model also observed an increase in capsaicin-induced behaviours when animals were subjected to a 5 h LPS treatment (Kemper et al. 1998). These studies demonstrate the ability for inflammatory responses to modulate the function of TRPV1.
It is now known that cytokines (IL-6, IL-1β, TNFα) and nerve growth factor (NGF) are associated with TRPV1 upregulation and membrane trafficking, while prostaglandins (PGE2, PI2), bradykinin and NGF sensitise the receptor by initiating receptor phosphorylation and dephosphorylation events via protein kinase A and C (PKA/C), phospholipase C (PLC), Src tyrosine kinase (Src) and phosphoinositide 3-kinase (PI3K) (Cesare et al. 1999; Ebbinghaus et al. 2012; Fang et al. 2015; Hensellek et al. 2007; Stein et al. 2006; Zhang et al. 2005; Moriyama et al. 2005; Numazaki et al. 2003; Stratiievska et al. 2018). This has been complemented by recent studies which have investigated the TRPV1/TLR4 relationship specifically. It is now clear that potentiated TRPV1 responses are created following comparatively short LPS exposure. Short-term activation (15 min) with LPS can potentiate capsaicin responses including increased inward current amplitude and calcium accumulation in TLR4/TRPV1 expressing HEK cells (Min et al. 2014).
These results are replicated in primary cells, with 15 min LPS stimulation of rat trigeminal neurons capable of producing potentiation of capsaicin evoked calcium accumulation and CGRP release, which can be attenuated by TLR4 antagonist LPS-RS (Diogenes et al. 2011; Ferraz et al. 2011). Primary cells excised from a rat CIPN model reveal chemotherapy agent paclitaxel increases TRPV1 sensitisation in a TLR4 dependent manner (Li et al. 2015). In excised rat DRG neurons, 10 min application of paclitaxel potentiates capsaicin-induced intercellular calcium accumulation, an effect blocked by co-treatment with LPS-RS. Excised DRG neurons from rats with paclitaxel-induced CIPN show potentiated capsaicin-induced calcium accumulation compared to vehicle treated rats. The effect is therefore seen after both acute and long-term paclitaxel exposure (Li et al. 2015). Paclitaxel not only induces primary-nociceptor-specific changes, but spinal changes; ex vivo studies show an increased rate of miniature excitatory post synaptic currents (mEPSCs) to a second application of capsaicin following paclitaxel treatment, an effect blocked by LPS-RS (Li et al. 2015).
Comparable findings are observed in a 2,4,6-trinitrobenzine sulphate (TNBS)-induced colitis model, where capsaicin-induced currents were significantly potentiated in colitis animals, an effect attenuated in TLR4 KO mice (Wu et al. 2019). Interestingly TLR4 KO mice show significantly reduced inward currents compared to WT animals, suggesting activation of TLR4 is not necessary for this functional interaction between the receptors (Wu et al. 2019). The exact mechanism of potentiated TRPV1 responses following short-term TLR4 agonism is yet to be identified, although as above, kinase activity is a strong candidate.
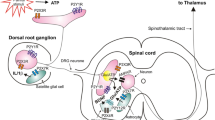
TLR4 mediated increases in PKA and PKC, and calcium dependent increases in phospholipase C (PLC) and phosphoinositide 3-kinase (PI3K) following LPS have been reported (Cabral et al. 2015; Kim et al. 2015). Activation of Src is reported to last between 5 and 60 min following LPS administration in human lung microvascular epithelial cells, reversed by TLR4 small interfering (si)RNA-induced knockdown (Gong et al. 2008). These kinases were previously referenced for their TRPV1 modulating effects following cellular exposure to proinflammatory mediators. Therefore, they provide outstanding candidates for indirect, rapid, TLR4-dependent TRPV1 sensitisation (Fig. 3).
Proposed mechanisms of direct and indirect TLR4-induced TRPV1 sensitisation. (a) LPS-induced increase in enzymatic activity (PKC, PKA, PI3K) results in increased trafficking of TRPV1 to the cell membrane. (b) LPS-induced increase in enzymatic activity (Src, PKA, PKC, PLC) alters TRPV1 sensitisation via receptor phosphorylation events. (c) Direct interaction proposed between the TIR domain of TLR4 and TRPV1. Created with BioRender.com
There is growing evidence to suggest just the presence of TLR4 alone alters TRPV1 mediated responses. It is important to note that multiple studies report co-expression in vivo; in trigeminal ganglia (TG), and DRG in rats and humans (Ferraz et al. 2011; Li et al. 2015; Wadachi and Hargreaves 2006; Wu et al. 2019). In vitro studies co-expressing TRPV1 and TLR4 report altered response amplitude and calcium accumulation in HEK overexpression systems when compared to cells expressing TRPV1 only (Min et al. 2014). One proposed link involves the TIR domain of TLR4, whereby the interaction with TIR prevents desensitisation of and internalisation of TRPV1 (Min et al. 2018). This potential interaction requires further study, as does the proposed mechanism, as studies contradict this theory, suggesting TLR4 may facilitate receptor desensitisation from repeated capsaicin doses (Li et al. 2015). Min et al. acknowledge unidentified intermediary adaptors may be involved in the observed TIR domain potentiation of TRPV1 activity (Min et al. 2018).
One potential intermediary factor is cytoplasmic scaffolding protein A-kinase anchoring protein 79 (AKAP79), which is important to the sensitising effect of PKA and PKC on TRPV1 (Faux and Scott 1997; Jeske et al. 2008, 2009; Zhang et al. 2008). TLR-TRP interactions have been observed previously; co-immunoprecipitation revealed an interaction between TRPA1 and TLR7 which potentiates TRPA1 induced inward current, suggesting a physical interaction (Park et al. 2014). Further investigation of a direct protein–protein link is required. While detection of unmodified protein interactions in tissues remains elusive due to the unreliability of available TLR4 antibodies, advances in protein–protein interaction assays such as bioluminescence resonance energy transfer (BRET) have the potential to improve our understanding using overexpression models (Dimri et al. 2016; McCarthy et al. 2017).
Importantly, Hutchinson et al. demonstrated the human clinical relevance of a TLR4/TRPV1 relationship. In this case, where capsaicin and endotoxin were co-administered to healthy individuals, a potentiation of capsaicin-induced mechanical allodynia and hyperalgesia was observed at 3 h, but not 2 h or directly following intravenous low dose (0.4 ng/kg) LPS (Hutchinson et al. 2013). Interestingly this effect was anatomically variable, observed on the forearm but not the forehead. Further, the timing of potentiation correlated with peak levels of serum IL-6 (Hutchinson et al. 2013). Importantly, the nature of the measurement of mechanical allodynia demonstrates central sensitisation was created by this combined neuro and immune nociceptive challenges. Therefore, the mechanism of action here appears to be one of the inflammatory mediated sensitisation and/or ascending sensitisation events, rather than the rapid changes observed in vitro in primary DRG, TG and HEK293FT cultures. There is significant evidence that the interaction between TLR4 and TRPV1 has the potential to effect pain outcomes. The next steps need to clarify the events or residues that link the two receptors and identify those that may be important for generation and/or maintenance of pathological pain states.
4.2 GPCRs Do Not Operate in Isolation
GPCRs are the largest and most researched class of cell surface receptors highlighting the important role they play in human physiology and disease. Their widespread expression allows them to influence diverse biological outcomes by transducing a range of extracellular signals to intracellular mediators (G proteins and β-arrestin). Importantly many GPCR ligands have been implicated in the regulation of inflammatory responses through the modulation of immune cell functions such as the production of inflammatory mediators. However, their widespread expression and recognition of diverse ligands is not sufficient to explain their influence on the myriad of physiological and pathophysiological states. It is now appreciated that GPCRs achieve many of their biological actions through ‘receptor crosstalk’; a process by which GPCRs can influence the signalling outcomes of other GPCRs and unrelated receptors. This is achieved primarily either via heterologous desensitisation or direct receptor interaction (heteromerisation).
Desensitisation is an important regulatory mechanism which has evolved to prevent the overstimulation of receptors in the presence of continuous agonist stimulation (Lefkowitz et al. 1992; Lefkowitz and Shenoy 2005). When an agonist activated GPCR is, itself, desensitised to prevent further signal transduction, this is described as homologous desensitisation. In the case where the continuous activation of one GPCR results in the desensitisation of another, often inactivated, GPCR or unrelated receptor this is termed as heterologous desensitisation. Heterologous desensitisation can be viewed as an indirect modulation of a third-party receptor signalling system. Heterologous desensitisation is mediated by intracellular signalling mediators such as second messenger-dependent GPCR kinases (GRKs) and β-arrestin regulatory/signalling proteins and/or by altering the expression of receptor proteins. β-arrestin was originally discovered in the context of GPCR signalling regulation; it is responsible for attenuating G protein-dependent GPCR signalling and modulating GPCR endocytosis. However, β-arrestin is now appreciated as a key signalling protein. It functions as a molecular scaffold for pathways including MAPK (Shukla et al. 2011). Beyond its recognition as a GPCR signalling protein, it has been implicated in the negative regulation of TLR-mediated signalling. β-arrestin 2 has been demonstrated to interact with and prevent post-translational modification of TRAF6 (Wang et al. 2006) and IKBα (Gao et al. 2004) and therefore prevent the activation of NF-Kβ in both cases (Fig. 4). Further demonstrating the important regulatory role of β-arrestin 2 on TLR4 signalling, mice lacking β-arrestin 2 were reported to attenuate IL-10, a key cytokine which inhibits the production of proinflammatory cytokines following TLR stimulation (Li et al. 2014). These mice when treated with LPS were shown to be more susceptible to LPS-induced septic shock. Li and co-workers determined β-arrestin 2 negatively regulated TLR4-mediated inflammatory responses via regulation of p38 MAPK and resulting IL-10 expression. In this context, β-arrestin 2 can be viewed as an important player in preventing excessive inflammation. Therapies targeting the activity and/or expression of β-arrestin 2 in specific cells critical for the transition from acute to persistent pain may prove to be fruitful in preventing chronic disease.
Proposed mechanisms of direct and indirect TLR4-GPCR interactions. Consequences of TLR4 activation (green arrows) include the generation of proinflammatory cytokines, Type 1 IFNs and activation of inflammatory regulators such as A20. A20 activation results in the regulation of β-arrestin recruitment, and therefore, receptor function and trafficking. Consequences of GPCR signalling proteins and receptor activation (blue arrows) include negative regulation and activation of signalling pathways common to those downstream of TLR4 activation (black arrows). Adapted from ‘TLR4/5/7/8 Signalling Cascade’, by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates
GPCR heteromerisation is a more direct approach requiring close proximity of receptor proteins. Heteromerisation requires at least two functional receptor units to form a macromolecular complex, either by directly interacting with each other or through a ‘bridging’ protein. Critically, a heteromer is defined as such if it demonstrates biochemical properties that are different from those of its individual components (Ferré et al. 2009). Although GPCR-TLR heteromers have yet to be definitively identified, this is a very exciting and active area of research.
Many GPCRs have been implicated in pain, with the opioid receptors being one of the most studied in this respect. GPCRs have also been reported to be implicated in the creation of microglial reactive states – a role often attributed to TLRs (Gu et al. 2021). Taken together with their inherent ability to ‘collaborate’ with other receptors and signalling systems, it is no surprise that GPCR signalling has been implicated in TLR function in the context of pain. It is important to highlight though that this modulation/communication is bidirectional. A 2008 study by Loniewski was the first study to report a role for TLRs in the regulation of GRKs and arrestins in macrophages. This study reported the activation of TLR4 selectively decreased β-arrestin 1 and GRK5/6 protein expression but increased GRK2 protein expression in in vitro studies (Loniewski et al. 2008). Interestingly, it was demonstrated the localisation of the TLRs (plasma membrane or intracellular) as well as choice of signalling pathway (MyD88 dependent or independent) determined which proteins were regulated. This highlights both the precise nature and the complexity in the regulation of signalling pathways. In the next section, we will highlight two GPCRs, chemokine and opioid receptors, and their relationship with TLR4 function and signalling in pain as an example of how the multidimensionality of pain spans receptor families and classes.
4.2.1 Chemokine Receptors Talk to TLR4
Chemokines are a family of small proinflammatory cytokines that transduce their actions via the G protein-coupled chemokine receptors. Although both chemokines and their receptors have been implicated in pain for over 20 years (Oh et al. 2001), their role in the neuroimmune mechanisms responsible for persistent pain is relatively recent (Knerlich-Lukoschus et al. 2011). C-X-C motif receptor 4 (CXCR4) and its ligand CXCL12 have been extensively investigated for their role in the neuromodulation of pathological pain. This discovery has led to investigations into the relationship between these chemokine receptors and TLRs in pain. Early studies of TLR4 and CXCR4 co-localisation and receptor crosstalk provided support for further investigations (Hajishengallis et al. 2008; Triantafilou et al. 2008). Furthermore, a 2016 study reported TLR4 to be co-localised with CXCL12 and CXCR4 in the spinal dorsal horn of rats with ischemia-reperfusion-induced inflammatory pain but not in control animals (Li et al. 2016). This demonstrated that the relationship between TLR4 and CXCR4 receptor protein expression is dynamic, but no conclusions can be made whether co-expression is a result or cause of pain pathology. Interestingly, the attenuation of CXCL12/CXCR4 expression demonstrated a reduction in the sensation of inflammatory pain in a mechanism similar to direct antagonism of TLR4, by the TLR4 antagonist TAK-242 (Li et al. 2016). Although this was attributed to the downregulation of cytokines IL-1β and TNF-α, there was no investigation of TLR4/CXCR4 heterodimerisation. However, in a 2012 study reporting high mobility group box protein 1 (HMGB1), a nuclear protein and known TLR4 activator, can not only activate CXCR4 but also form a complex with CXCL12 (Schiraldi et al. 2012) the authors concluded that this could not be explained by heterodimersation of TLR4-CXCR4 as the effect was also observed in cells isolated from TLR4 knockout mice. However, it is not implausible to suggest this lack of detection of a heterodimer may be specific to this ligand combination and does not rule out receptor interaction completely. Nevertheless, a sophisticated relationship between the TLR4 and chemokine receptor signalling pathways exists (likely through downstream signalling pathway such as NF-kβ, MAPK and a series of signal transducers and activators of transcription pathways) and may be open for exploitation for novel pain therapies (Fig. 4).
4.2.2 Opioid Receptors and TLR4 Communicate
To date, opioid agonists are considered the gold standard for acute pain management and are well known to act on the G protein-coupled opioid receptors. However, although acute pain is effectively managed by opioid agonists there is little success or patient benefit in managing chronic pain with opioids. Paradoxically, the use of opioids in persistent pain can enhance the sensitisation of neuronal and immune cells resulting in opioid-induced hyperalgesia (Hutchinson et al. 2010). Multiple systems are at play with the complete multidimensional mechanism yet to be fully understood (King et al. 2005; Kovelowski et al. 2000; Ossipov et al. 2003, 2004, 2005). A contemporary neuroimmune hypothesis has immerged with TLR4-induced microglial reactivity and inflammatory signalling negatively impacting the beneficial opioid analgesia pharmacodynamics.
Hutchinson and co-workers have previously reported that the antagonism of TLR4 increases the magnitude and duration of morphine analgesia (Hutchinson et al. 2007). Furthermore, it has been reported in mice lacking TLR4 there was a significant lack of analgesic tolerance to morphine compared to wild-type and MyD88 knockout mice (Thomas et al. 2022; Liu et al. 2011). Together these suggest a role for TLR4 in analgesic tolerance, for which there is also opposing data (Ferrini et al. 2013; Fukagawa et al. 2013; Mattioli et al. 2014). For example, in a study comparing mutant TLR4 to wild-type mice, no difference in morphine induced hyperalgesia was observed (Ferrini et al. 2013). These discrepancies underscore how complex the biopsychosocial state must be, as it is clear that the absence of TLR4 can be compensated by other systems (Thomas et al. 2022). Further refinement of these models capturing principles of the Kuramoto model and the Fröhlich condensate may help reconcile such challenges.
There is much evidence supporting a connection between the opioid receptor and TLR4 signalling systems including their co-expression in several non-neuronal cell types including glia and macrophages (Maduna et al. 2019; Franchi et al. 2012) and receptor crosstalk via common downstream signalling pathways such as MAPK, PKC and NF-Kβ as described earlier. For example, A20 (a key regulator of TLR4 signalling introduced earlier) has been described by Shao and colleagues as in inhibitor of β-arrestin 2 recruitment to μ opioid receptor (Fig. 4) (Shao et al. 2020). It is now widely accepted that many of the unwanted side effects of opioids are due to the μ opioid engagement of the β-arrestin 2 signalling pathway (Bohn et al. 2000). In this study, it was demonstrated A20 plays a key role in enhancing the analgesic effects of morphine at the μ opioid receptor by interacting with β-arrestin 2 and inhibiting its interaction with the μ opioid receptor (Shao et al. 2020). This type of multidimensionality of TLR4 actions may be exploited as a feasible target for pain management via A20 modulation.
4.2.3 From Single Receptor Systems to Complex Receptor Systems
The complex relationship between opioids and cytokines in the context of pain has been reviewed previously in detail (Thomas et al. 2015). Briefly, there is evidence in literature supporting the hypothesis chemokines can influence the perception of pain and inhibit opioid-induced analgesia via heterologous desensitisation. Szabo and co-workers demonstrated that DAMGO (μ opioid receptor agonist) treatment of rats pre-treated with a CXCR4 receptor ligand (CXCL12) exhibited a dose-dependent reduction in analgesic responses compared to saline pre-treated control rats (Szabo et al. 2002). Taken together with reports that CXCR4 forms a complex with δ opioid receptor (Pello et al. 2008), it is no surprise that heterodimerisation between CXCR4 and μ opioid receptors has been investigated as a possible mechanism for receptor crosstalk. A very recent study by Ma and co-workers has investigated the existence of putative CXCR4 and μ opioid receptor heteromers by developing a bivalent ligand that has the capability to interact with both receptor units simultaneously (Ma et al. 2022). This technique has been reported previously for the investigation of other GPCR heteromers including CXCR4 and μ opioid receptor heteromers (Akgün et al. 2015; Ma et al. 2020). Although this provides some insight into the proximity of the ligand binding sites of the receptors, further demonstration of the effect on receptor heteromer specific signalling profiling and co-internalisation such as provided for the GPCR heteromer 1A-adrenoceptor-CXCR2 would add further weight (Mustafa et al. 2012). It is also important to consider heterodimerisation may only represent one of many mechanisms by which CXCR4 and μ opioid receptors crosstalk. It is likely a more complex relationship between these receptor systems exists. Furthermore, as it has been established that CXCR4 and μ opioid receptors both co-localise with TLR4 in neuroanatomical structures important for pain processing, such as dorsal root ganglion and spinal cord dorsal horn (Li et al. 2016), it is not inconceivable that these receptor protein signalling systems work in concert, regulating each other’s signalling outcomes to achieve the complexity seen in chronic pain patients. In order to further our knowledge of pain and the development of effective analgesics, future research approaches should examine the ‘holistic signalling system’ and not receptor signalling pathways in isolation. Such an approach will enable the true complexity of the biopsychosocial contributors to pain to be appreciated from molecules-to-cells-to-systems. Of course, this raises the issues of nature versus nurture and the profound heritability of pain states. As such, an examination of genomic determinates of health and disease is warranted.
5 Human TLR4 Genetic Polymorphisms
Given the important roles of TLR4 signalling in acute and chronic pain and non-classical opioid pharmacology outlined above, polymorphic variability in TLR4 has the potential to contribute to interindividual variability in pain and treatment response. Likely reflecting the crucial role of our innate immune system in survival, human TLR genes display relatively little polymorphic variability. For example, only 3 missense or protein truncating polymorphisms with a global minor allele frequency (MAF) greater than 1% have so far been identified in TLR4 (Howe et al. 2021). The most common of these is rs4986790 (c.896A>G, Asp299Gly), with a global MAF of only 6%, but large inter-population variability in frequency (e.g., 0% in East Asian, but up to 14% in South Asian, populations) (Barratt et al. 2021). It is in strong linkage disequilibrium with the next most common missense SNP, rs4986791 (c.1196C>T, Thr399Ile) (global MAF = 4%). However, functional consequences of the rs49867910-rs4986791 haplotype appear primarily due to rs4986790, which has been associated with reduced TLR4 signalling in vitro, ex vivo and in vivo, without significant effects on expression (Long et al. 2014; Lundberg et al. 2008; Arbour et al. 2000).
Regarding pain specifically, rs4986790 variant genotypes have been associated with lower pain tolerance and higher post-surgical morphine requirements (Barratt et al. 2021), as well as increased risk of endometriosis (Latha et al. 2011). This suggests that, at least in some cases, the TLR4 rs4986790 SNP may result in a dysregulated, rather than simply hypo-responsive, in vivo/clinical phenotype. Other, more common, non-coding TLR4 SNPs (e.g., rs1927911, rs2770150, rs2149356) have been associated with altered immunophenotypes. However, they have not yet been investigated for associations with variability in pain phenotypes specifically.
Though in its relatively early stages, research on TLR-related genetics and pain has so far revealed large inter-population variability in frequencies of TLR gene polymorphisms that may contribute to observed ‘interethnic’ differences in pain. In addition, it has demonstrated that genotypes associated with increased or decreased TLR function in vitro do not necessarily translate to increased or decreased pain, respectively, in vivo/clinically. This may be due to the penetrance of genetic effects in general likely being influenced by life history and clinical context (Somogyi et al. 2016; Rafiei et al. 2012; Barratt et al. 2021) as well as the complexity of systems in which TLRs function, where both increased or decreased function might lead to dysregulation and pathology. Further research on the combined effects of TLR4 signalling pathway genetic polymorphisms on pain, psychoneuroimmune and drug response phenotypes as part of a ‘signalling ecosystem’ has the potential to lead to a better understanding of key in vivo system regulators for the identification of new drug targets and greater precision in pain management.
6 Pharmacological Modulation of TLR4 Signalling
6.1 Understanding How Opioid Ligands Modulate TLR4 Function Will Inform Drug Design
Literature suggests that opioid ligands can modulate TLR4 expression. Separate in vitro and ex vivo studies have observed both the up and downregulation of TLR4 protein following morphine exposure (Chen et al. 2021; Franchi et al. 2012). Furthermore, there is evidence that opioids can promote the internalisation of TLR4 from the cell surface (Liang et al. 2016). As discussed earlier, many of these actions can be attributed to receptor crosstalk between the opioid receptors and TLR4. However, others may be due to the direct activation of the TLR4 receptor.
The discovery that the opioid receptor antagonists, naltrexone and naloxone, blocked the biological effects of LPS (Liu et al. 2000; Das et al. 1995; Yirmiya et al. 1994) and literature reporting that opioid agonists may competitively bind and activate TLR4 (Wang et al. 2012; Watkins et al. 2009) have raised interesting questions around the actions of opioid ligands on TLR4 and their own role in compromising their analgesic effects via opioid receptors. Opioids including morphine and morphine-3-glucuronide (M3G) have been reported to non-stereoselectively activate TLR4 signalling (Wang et al. 2012). The opioid-dependent activation of TLR4 results in the release of proinflammatory mediators such as nitric oxide, reactive oxygen species, prostaglandin E2, interleukins, interferons and various chemokines which exacerbate nociception and can lead to hyperalgesia. Despite the (−)-isomers of naltrexone and naloxone displaying the same potencies as (+)- isomers (Wang et al. 2016; Hutchinson et al. 2008), (+)-isomers of opioids may have particular application on TLR4 signalling modulation without effecting the beneficial effect of endogenous (−) opioid isomers on opioid receptors (Wang et al. 2020).
Wang and colleagues have demonstrated that (+)-Naltrexone and (+)-naloxone inhibit the LPS-induced activation of IRF3 and prevent IFN-β (Wang et al. 2016). However, they do not inhibit TLR4 dependent activation of either NF-κB or MAPKs. This suggests (+)-naltrexone and (+)-naloxone are TRIF-IRF3-biased TLR4 antagonists (Wang et al. 2016). In vivo studies also showed (+)-naltrexone and (+)-naloxone decrease opioid, cocaine and methamphetamine-induced dependence and addiction (Wang et al. 2019; Hutchinson et al. 2012; Brown et al. 2018; Hutchinson et al. 2010). Based on this data the TRIF-biased (+)-opioid TLR4 antagonists may be suitable targets for future development for opioid addiction and dependence.
To understand the details of the molecular interactions of (+)-naltrexone and its derivatives with TLR4/MD-2 and extend the ligand-based drug discovery, Zhang and co-workers performed in silico and in vitro assays (Zhang et al. 2018). These studies elucidated the innate immune recognition of the opioid inactive (+)-isomers. The calculated binding free energies of (+)-naltrexone and its derivatives in complex with MD-2 via molecular dynamics simulations correlated well with their experimental binding affinities and TLR4 antagonistic activities. It indicated that the binding free energies would be an excellent criterion to evaluate the antagonistic activities during rational drug design. Increasing the hydrophobicity of substituted group at N-17 improved its TLR4 antagonistic activity, while charged groups disfavoured the binding with MD-2 (Zhang et al. 2018). This provided molecular insight into the innate immune recognition of opioid inactive (+)-isomers and has the potential to aid the development of new (+)-opioid based TLR4 antagonists.
Although TLR4/MD-2 cannot stereoselectively recognise naltrexone isomers, whether TLR4/MD-2 is enantioselective is still unclear. By linking 2 naltrexone units through a rigid pyrrole spacer, three bivalent ligands ((+)-norbinaltorphimine [(+)-1], (−)-norbinaltorphimine [(−)-1] and the meso isomer of norbinaltorphimine [2]) were formed. Surprisingly, (+)-1 showed ∼25 times better TLR4 antagonist activity than naltrexone in the microglial BV-2 cell line, whereas (−)-1 lost its TLR4 activity (Zhang et al. 2019). The enantioselectivity of norbinaltorphimine was further confirmed in primary microglia, astrocytes and macrophages (Zhang et al. 2019). By rebuilding the binding energy profile, molecular dynamic simulations further uncovered the mechanism of enantioselectivity: the stereochemistry of (+)-1 is derived from the (+)-naltrexone pharmacophore. This is the first report showing enantioselective modulation of the innate immune TLR signalling and is an exciting development for future pain targeted therapies.
6.2 Biased Signalling and the Future of TLR4 Signalling Modulation for Pain Management
As discussed earlier, evidence in literature suggests blockade of TLR4 signalling provides beneficial outcomes in pain pathologies and opioid dependence (Hutchinson et al. 2008; Bettoni et al. 2008). For example, following treatment with TLR4 antagonist FP-1, Bettoni and colleagues reported increased morphine analgesia together with reduced hyperalgesia in mice with painful neuropathy. However, complete antagonism of TLR4 may have detrimental impacts on the body’s immunity and inflammation status. There is now increasing evidence supporting the role of the MyD88 pathway in pain pathophysiology (Lacagnina et al. 2018; Liu et al. 2017). Exploiting the potential of TLR4 to signal via two distinct signalling pathways (biased signalling) presents exciting opportunities to intelligently modulate TLR4 signalling and function to manage pain, without impacting its role in immunity and inflammation, recently reviewed (Lin et al. 2021).
Studies investigating the signalling properties of LPS isolated from different bacterial species have highlighted that biased ligands have the capability to selectively activate/deactivate MyD88 or TRIF-dependent signalling pathways (Stephens et al. 2021). Similar to the literature in the GPCR field, where biased signalling and biased ligands have been extensively researched (Kenakin 2011), it is clear that the interaction between a ligand/receptor protein results in the stabilisation of different receptor conformations. Each receptor conformation then either reveals or uncovers specific binding sites for specific intracellular signalling adapter proteins resulting in the activation/deactivation of the chosen pathway. Although biased ligands have the power to influence the signalling and ultimately biological outcome of TLR4 signalling, it is important to note that the microenvironment of the endosome is very different to that of the plasma membrane (for example, lipid composition and the pH). By promoting unique TLR4 dimer conformations, the microenvironment may also play a role in biased signalling.
Examples of how TLR4 biased signalling can be exploited already exist where TLR4 activators are included as adjuvants in vaccines for their immunostimulatory properties (Mata-Haro et al. 2007; Bowen et al. 2012; Richard et al. 2020). In the study by Mata-Haro and co-workers, it was demonstrated that monophosphoryl lipid A (MPLA), a low-toxic derivative of LPS used as an adjuvant in human vaccines, displays reduced MyD88-dependent signalling activity, but strong TRIF-dependent signalling (Mata-Haro et al. 2007). In a study where the vaccine adjuvant CRX-547 was utilised, a minor structural modification to the carboxyl bioisostere corresponding to the 1-phosphate group on most lipid A types resulted in a TRIF-selective signal (Bowen et al. 2012). Much can be learnt from these examples when identifying and designing TLR4 biased ligands for chronic pain management.
Our understanding of biased signalling has been increased by investigating the impact of the varying length of lipid A chains from LPS isolated from different bacterial species and human pathogens (Maeshima and Fernandez 2013; Stephens et al. 2021). Structure-activity relationship (SAR) studies have provided evidence that structure of the LPS can result in varying degrees of inflammation due to the preferential activation of the signalling pathways (Maeshima and Fernandez 2013). However, it can be argued that an important approach to identify biased ligands is structure-based drug design (SBDD). Molecular dynamics is an important tool, which has the power to move beyond ligand docking to biased receptor structures of TLR4, for predicting the stable interactions between ligands and biased TLR4 receptor states. Unfortunately, due to the lack structural data representing the ‘biased’ conformations of TLR4 receptor, this is not currently possible.
Therefore, another approach to identifying biased ligands is to screen compound libraries. The downstream factors of TLR4 signalling are essential markers for the screening and discovery of biased modulators of TLR4. The secretion of IFN-β and CXCL10 are often used for the discovery of TRIF-biased TLR4 small molecule modulators as they are only dependent on TLR4-induced TRIF signalling (Bowen et al. 2012; Richard et al. 2020). Given that the internalisation of TLR4 into endosomes is crucial for the activation of TRIF-dependent signalling (Park et al. 2009), TLR4 internalisation can also be used as a specific marker of TRIF-biased signalling. An antibody described by Zanoni and co-workers, SA15-21, is reported to recognise both the monomer and dimer forms of TLR4. Although laborious and not high throughput, this antibody could be potentially used to examine the endocytosis of TLR4 during compound screening (Zanoni et al. 2011). However, among the downstream proteins of TLR4 signalling, no cytokine or chemokine has been found to be strictly dependent on the MyD88 signalling pathway. A lack of tools to identify TLR4-induced MyD88 signalling may explain why there are no reported MyD88-biased small molecule modulators of TLR4 signalling (Lin et al. 2021). This obstacle can be overcome by utilising proximity-based assays to directly identify the interaction of TLR4 protein with signalling adapter proteins MyD88 or TRIF (Lin et al. 2021). This technology has the capability to identify in real-time, which signalling pathway has been activated. Alternatively, the ubiquitination of IL-1R-associated kinase-1 (IRAK1) is specific to the MyD88-dependent signalling (Conze et al. 2008). Therefore, the measurement of the polyubiquitination of IRAK1 by in-cell Western assay would be an excellent way to screen and identify MyD88-biased small molecule modulators, potentially for novel pain indications in the future.
7 Conclusion
We have provided an overview of TLR4 signalling and regulation in pain. Importantly, this review highlights the need to examine and appreciate the role of TLR4 signalling and regulation as part of a ‘signalling ecosystem’. This takes into consideration the many other proteins and signalling pathways which modulate TLR4 signalling or are themselves modulated by TLR4 and its signalling partners. By doing so, only then we may be able to understand the deep and complex mechanisms that result in the biopsychosocial nature of pain. Although the complex interactions may be challenging and take time to decipher fully, it is clear that TLR4 receptor signalling provides multiple mechanisms for modulating the unwanted effects of opioid analgesics and the management of complex pain pathologies. Optimistically, TLR4 may provide one of the missing ‘bridges’ to the future of precision medicine and pain management.
References
Akgün E, Javed MI, Lunzer MM, Powers MD, Sham YY, Watanabe Y, Portoghese PS (2015) Inhibition of inflammatory and neuropathic pain by targeting a mu opioid receptor/chemokine Receptor5 Heteromer (MOR-CCR5). J Med Chem 58:8647–8657
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499–511
Allendorf DH, Franssen EH, Brown GC (2020) Lipopolysaccharide activates microglia via neuraminidase 1 desialylation of toll-like receptor 4. J Neurochem 155:403–416
Amith SR, Jayanth P, Franchuk S, Finlay T, Seyrantepe V, Beyaert R, Pshezhetsky AV, Szewczuk MR (2010) Neu1 desialylation of sialyl alpha-2,3-linked beta-galactosyl residues of TOLL-like receptor 4 is essential for receptor activation and cellular signaling. Cell Signal 22:314–324
Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA (2000) TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet 25:187–191
Arthur JS, Ley SC (2013) Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 13:679–692
Barratt DT, Sia AT, Tan EC, Somogyi AA (2021) Innate immune and neuronal genetic markers are highly predictive of postoperative pain and morphine patient-controlled analgesia requirements in Indian but not Chinese or Malay hysterectomy patients. Pain Med 22:2648–2660
Bettoni I, Comelli F, Rossini C, Granucci F, Giagnoni G, Peri F, Costa B (2008) Glial TLR4 receptor as new target to treat neuropathic pain: efficacy of a new receptor antagonist in a model of peripheral nerve injury in mice. Glia 56:1312–1319
Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG (2000) Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408:720–723
Boone DL, Turer EE, Lee EG, Ahmad R-C, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A (2004) The ubiquitin-modifying enzyme A20 is required for termination of toll-like receptor responses. Nat Immunol 5:1052–1060
Bowen WS, Minns LA, Johnson DA, Mitchell TC, Hutton MM, Evans JT (2012) Selective TRIF-dependent signaling by a synthetic toll-like receptor 4 agonist. Sci Signal 5:ra13
Brown KT, Levis SC, O'Neill CE, Northcutt AL, Fabisiak TJ, Watkins LR, Bachtell RK (2018) Innate immune signaling in the ventral tegmental area contributes to drug-primed reinstatement of cocaine seeking. Brain Behav Immun 67:130–138
Bsibsi M, Ravid R, Gveric D, van Noort JM (2002) Broad expression of toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol 61:1013–1021
Buchanan MM, Hutchinson M, Watkins LR, Yin H (2010) Toll-like receptor 4 in CNS pathologies. J Neurochem 114:13–27
Cabral JM, Grácio D, Soares-da-Silva P, Magro F (2015) Short- and long-term regulation of intestinal Na+/H+ exchange by toll-like receptors TLR4 and TLR5. Am J Physiol Gastrointest Liver Physiol 309:G703–G715
Cao L, Tanga FY, Deleo JA (2009) The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain. Neuroscience 158:896–903
Caterina MJ, Julius D (2001) The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24:487–517
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824
Catrysse L, Vereecke L, Beyaert R, van Loo G (2014) A20 in inflammation and autoimmunity. Trends Immunol 35:22–31
Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA (1999) Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron 23:617–624
Chen J, Wang G, Sun T, Ma C, Huo X, Kong Y (2021) Involvement of TCF7L2 in generation of morphine-induced antinociceptive tolerance and hyperalgesia by modulating TLR4/ NF-κB/NLRP3 in microglia. Toxicol Appl Pharmacol 416:115458
Christianson CA, Dumlao DS, Stokes JA, Dennis EA, Svensson CI, Corr M, Yaksh TL (2011) Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain 152:2881–2891
Conze DB, Wu CJ, Thomas JA, Landstrom A, Ashwell JD (2008) Lys63-linked polyubiquitination of IRAK-1 is required for interleukin-1 receptor- and toll-like receptor-mediated NF-kappaB activation. Mol Cell Biol 28:3538–3547
Coornaert B, Carpentier I, Beyaert R (2009) A20: central gatekeeper in inflammation and immunity. J Biol Chem 284:8217–8221
da Silva Correia J, Ulevitch RJ (2002) MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J Biol Chem 277:1845–1854
Dantzer R (2001) Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun 15:7–24
Dantzer R, Kelley KW (2007) Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun 21:153–160
Das KP, McMillian MK, Bing G, Hong JS (1995) Modulatory effects of [Met5]-enkephalin on interleukin-1 beta secretion from microglia in mixed brain cell cultures. J Neuroimmunol 62:9–17
Das T, Shin SC, Song EJ, Kim EE (2020) Regulation of deubiquitinating enzymes by post-translational modifications. Int J Mol Sci 21:428
Dattani J, Barahona M (2017) Stochastic models of gene transcription with upstream drives: exact solution and sample path characterization. J R Soc Interface 14:20160833
Dimri S, Basu S, De A (2016) Use of BRET to study protein-protein interactions in vitro and in vivo. Methods Mol Biol 1443:57–78
Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM (2011) LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res 90:759–764
Du P, Gao K, Cao Y, Yang S, Wang Y, Guo R, Zhao M, Jia S (2019) RFX1 downregulation contributes to TLR4 overexpression in CD14(+) monocytes via epigenetic mechanisms in coronary artery disease. Clin Epigenetics 11:44
Ebbinghaus M, Uhlig B, Richter F, von Banchet GS, Gajda M, Brauer R, Schaible HG (2012) The role of interleukin-1beta in arthritic pain: main involvement in thermal, but not mechanical, hyperalgesia in rat antigen-induced arthritis. Arthritis Rheum 64:3897–3907
Fang D, Kong LY, Cai J, Li S, Liu XD, Han JS, Xing GG (2015) Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: roles in the development of bone cancer pain in a rat model. Pain 156:1124–1144
Faux MC, Scott JD (1997) Regulation of the AKAP79-protein kinase C interaction by Ca2+/Calmodulin. J Biol Chem 272:17038–17044
Feng C, Stamatos NM, Dragan AI, Medvedev A, Whitford M, Zhang L, Song C, Rallabhandi P, Cole L, Nhu QM, Vogel SN, Geddes CD, Cross AS (2012) Sialyl residues modulate LPS-mediated signaling through the toll-like receptor 4 complex. PLoS One 7:e32359
Ferraz CC, Henry MA, Hargreaves KM, Diogenes A (2011) Lipopolysaccharide from Porphyromonas gingivalis sensitizes capsaicin-sensitive nociceptors. J Endod 37:45–48
Ferré S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR, Javitch JA, Lohse MJ, Mackie K, Milligan G, Pfleger KD, Pin JP, Volkow ND, Waldhoer M, Woods AS, Franco R (2009) Building a new conceptual framework for receptor heteromers. Nat Chem Biol 5:131–134
Ferrini F, Trang T, Mattioli TA, Laffray S, Del'Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, De Koninck Y (2013) Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl− homeostasis. Nat Neurosci 16:183–192
Fitzgerald KA, Kagan JC (2020) Toll-like receptors and the control of immunity. Cell 180:1044–1066
Franchi S, Moretti S, Castelli M, Lattuada D, Scavullo C, Panerai AE, Sacerdote P (2012) Mu opioid receptor activation modulates toll like receptor 4 in murine macrophages. Brain Behav Immun 26:480–488
Frank MG, Weber MD, Watkins LR, Maier SF (2015) Stress sounds the alarmin: the role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav Immun 48:1–7
Fukagawa H, Koyama T, Kakuyama M, Fukuda K (2013) Microglial activation involved in morphine tolerance is not mediated by toll-like receptor 4. J Anesth 27:93–97
Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G (2004) Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell 14:303–317
Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, Weiss JP (2004) Isolation of an endotoxin-MD-2 complex that produces toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci 101:4186–4191
Goethals S, Ydens E, Timmerman V, Janssens S (2010) Toll-like receptor expression in the peripheral nerve. Glia 58:1701–1709
Gong P, Angelini DJ, Yang S, Xia G, Cross AS, Mann D, Bannerman DD, Vogel SN, Goldblum SE (2008) TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula Adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J Biol Chem 283:13437–13449
Grace PM, Strand KA, Galer EL, Maier SF, Watkins LR (2018) MicroRNA-124 and microRNA-146a both attenuate persistent neuropathic pain induced by morphine in male rats. Brain Res 1692:9–11
Gu C, Chen Y, Chen Y, Liu C-F, Zhu Z, Wang M (2021) Role of G protein-coupled receptors in microglial activation: implication in Parkinson’s disease. Front Aging Neurosci 13:768156
Hajishengallis G, Wang M, Liang S, Triantafilou M, Triantafilou K (2008) Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc Natl Acad Sci 105:13532–13537
Hensellek S, Brell P, Schaible HG, Brauer R, Segond von Banchet G (2007) The cytokine TNFalpha increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol Cell Neurosci 36:381–391
Howe KL, Achuthan P, Allen J, Allen J, Alvarez-Jarreta J, Amode MR, Armean IM, Azov AG, Bennett R, Bhai J, Billis K, Boddu S, Charkhchi M, Cummins C, Da Rin Fioretto L, Davidson C, Dodiya K, El Houdaigui B, Fatima R, Gall A, Garcia Giron C, Grego T, Guijarro-Clarke C, Haggerty L, Hemrom A, Hourlier T, Izuogu OG, Juettemann T, Kaikala V, Kay M, Lavidas I, Le T, Lemos D, Gonzalez Martinez J, Marugán JC, Maurel T, McMahon AC, Mohanan S, Moore B, Muffato M, Oheh DN, Paraschas D, Parker A, Parton A, Prosovetskaia I, Sakthivel MP, Salam AIA, Schmitt BM, Schuilenburg H, Sheppard D, Steed E, Szpak M, Szuba M, Taylor K, Thormann A, Threadgold G, Walts B, Winterbottom A, Chakiachvili M, Chaubal A, De Silva N, Flint B, Frankish A, Hunt SE, GR II, Langridge N, Loveland JE, Martin FJ, Mudge JM, Morales J, Perry E, Ruffier M, Tate J, Thybert D, Trevanion SJ, Cunningham F, Yates AD, Zerbino DR, Flicek P (2021) Ensembl 2021. Nucleic Acids Res 49:D884–d891
Hua XY, Chen P, Fox A, Myers RR (1996) Involvement of cytokines in lipopolysaccharide-induced facilitation of CGRP release from capsaicin-sensitive nerves in the trachea: studies with interleukin-1beta and tumor necrosis factor-alpha. J Neurosci 16:4742–4748
Huang H-Y, Lin Y-C-D, Li J, Huang K-Y, Shrestha S, Hong H-C, Tang Y, Chen Y-G, Jin C-N, Yu Y, Xu J-T, Li Y-M, Cai X-X, Zhou Z-Y, Chen X-H, Pei Y-Y, Hu L, Su J-J, Cui S-D, Wang F, Xie Y-Y, Ding S-Y, Luo M-F, Chou C-H, Chang N-W, Chen K-W, Cheng Y-H, Wan X-H, Hsu W-L, Lee T-Y, Wei F-X, Huang H-D (2019) miRTarBase 2020: updates to the experimentally validated microRNA–target interaction database. Nucleic Acids Res 48:D148–D154
Hutchinson MR (2014) Want more pain? Just add a dash of endotoxin to enhance your clinical pain model. Brain Behav Immun 41:44–45
Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR (2007) Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. Sci World J 7:98–111
Hutchinson MR, Buijs M, Tuke J, Kwok YH, Gentgall M, Williams D, Rolan P (2013) Low-dose endotoxin potentiates capsaicin-induced pain in man: evidence for a pain neuroimmune connection. Brain Behav Immun 30:3–11
Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR (2012) Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci 32:11187–11200
Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR (2008) Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). Eur J Neurosci 28:20–29
Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR (2010) Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun 24:83–95
Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM (2008) A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain 138:604–616
Jeske NA, Patwardhan AM, Ruparel NB, Akopian AN, Shapiro MS, Henry MA (2009) A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1. Pain 146:301–307
Jurga AM, Rojewska E, Piotrowska A, Makuch W, Pilat D, Przewlocka B, Mika J (2016) Blockade of toll-like receptors (TLR2, TLR4) attenuates pain and potentiates buprenorphine analgesia in a rat neuropathic pain model. Neural Plast 2016:1–12
Kelley KW, Bluthé RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR (2003) Cytokine-induced sickness behavior. Brain Behav Immun 17(Suppl 1):S112–S118
Kemper RH, Spoelstra MB, Meijler WJ, Ter Horst GJ (1998) Lipopolysaccharide-induced hyperalgesia of intracranial capsaicin sensitive afferents in conscious rats. Pain 78:181–190
Kenakin T (2011) Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther 336:296–302
Kim KS, Jang JH, Lin H, Choi SW, Kim HR, Shin DH, Nam JH, Zhang YH, Kim SJ (2015) Rise and fall of Kir2.2 current by TLR4 signaling in human monocytes: PKC-dependent trafficking and PI3K-mediated PIP2 decrease. J Immunol 195:3345–3354
Kim TW, Lee SJ, Oh BM, Lee H, Uhm TG, Min JK, Park YJ, Yoon SR, Kim BY, Kim JW, Choe YK, Lee HG (2016) Epigenetic modification of TLR4 promotes activation of NF-κB by regulating methyl-CpG-binding domain protein 2 and Sp1 in gastric cancer. Oncotarget 7:4195–4209
King T, Ossipov MH, Vanderah TW, Porreca F, Lai J (2005) Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance? Neurosignals 14:194–205
Knerlich-Lukoschus F, von der Ropp-Brenner B, Lucius R, Mehdorn HM, Held-Feindt J (2011) Spatiotemporal CCR1, CCL3(MIP-1α), CXCR4, CXCL12(SDF-1α) expression patterns in a rat spinal cord injury model of posttraumatic neuropathic pain. J Neurosurg Spine 14:583–597
Kovelowski CJ, Ossipov MH, Sun H, Lai J, Malan TP Jr, Porreca F (2000) Supraspinal cholecystokinin may drive tonic descending facilitation mechanisms to maintain neuropathic pain in the rat. Pain 87:265–273
Kringel D, Kaunisto MA, Kalso E, Lötsch J (2019) Machine-learned analysis of global and glial/opioid intersection-related DNA methylation in patients with persistent pain after breast cancer surgery. Clin Epigenetics 11:167
Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H (2006) Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics 7:159
Kynast KL, Russe OQ, Möser CV, Geisslinger G, Niederberger E (2013) Modulation of central nervous system-specific microRNA-124a alters the inflammatory response in the formalin test in mice. Pain 154:368–376
Lacagnina MJ, Watkins LR, Grace PM (2018) Toll-like receptors and their role in persistent pain. Pharmacol Ther 184:145–158
Lasselin J (2021) Back to the future of psychoneuroimmunology: studying inflammation-induced sickness behavior. Brain Behav Immun Health 18:100379
Latha M, Vaidya S, Movva S, Chava S, Govindan S, Govatati S, Banoori M, Hasan Q, Kodati VL (2011) Molecular pathogenesis of endometriosis; toll-like receptor-4 A896G (D299G) polymorphism: a novel explanation. Genet Test Mol Biomarkers 15:181–184
Lefkowitz RJ, Inglese J, Koch WJ, Pitcher J, Attramadal H, Caron MG (1992) G-protein-coupled receptors: regulatory role of receptor kinases and arrestin proteins. Cold Spring Harb Symp Quant Biol 57:127–133
Lefkowitz RJ, Shenoy SK (2005) Transduction of receptor signals by beta-arrestins. Science 308:512–517
Li H, Hu D, Fan H, Zhang Y, Lesage GD, Caudle Y, Stuart C, Liu Z, Yin D (2014) β-Arrestin 2 negatively regulates toll-like receptor 4 (TLR4)-triggered inflammatory signaling via targeting p38 MAPK and interleukin 10. J Biol Chem 289:23075–23085
Li X-Q, Zhang Z-L, Tan W-F, Sun X-J, Ma H (2016) Down-regulation of CXCL12/CXCR4 expression alleviates ischemia-reperfusion-induced inflammatory pain via inhibiting glial TLR4 activation in the spinal cord. PLoS One 11:e0163807
Li Y, Adamek P, Zhang H, Tatsui CE, Rhines LD, Mrozkova P, Li Q, Kosturakis AK, Cassidy RM, Harrison DS, Cata JP, Sapire K, Zhang H, Kennamer-Chapman RM, Jawad AB, Ghetti A, Yan J, Palecek J, Dougherty PM (2015) The cancer chemotherapeutic paclitaxel increases human and rodent sensory neuron responses to TRPV1 by activation of TLR4. J Neurosci 35:13487–13500
Liang Y, Chu H, Jiang Y, Yuan L (2016) Morphine enhances IL-1β release through toll-like receptor 4-mediated endocytic pathway in microglia. Purinergic Signal 12:637–645
Lin C, Wang H, Zhang M, Mustafa S, Wang Y, Li H, Yin H, Hutchinson MR, Wang X (2021) TLR4 biased small molecule modulators. Pharmacol Ther 228:107918
Liu B, Du L, Kong LY, Hudson PM, Wilson BC, Chang RC, Abel HH, Hong JS (2000) Reduction by naloxone of lipopolysaccharide-induced neurotoxicity in mouse cortical neuron-glia co-cultures. Neuroscience 97:749–756
Liu CC, Gao YJ, Luo H, Berta T, Xu ZZ, Ji RR, Tan PH (2016) Interferon alpha inhibits spinal cord synaptic and nociceptive transmission via neuronal-glial interactions. Sci Rep 6:34356
Liu F, Wang Z, Qiu Y, Wei M, Li C, Xie Y, Shen L, Huang Y, Ma C (2017) Suppression of MyD88-dependent signaling alleviates neuropathic pain induced by peripheral nerve injury in the rat. J Neuroinflammation 14:70
Liu L, Coller JK, Watkins LR, Somogyi AA, Hutchinson MR (2011) Naloxone-precipitated morphine withdrawal behavior and brain IL-1β expression: comparison of different mouse strains. Brain Behav Immun 25:1223–1232
Liu L, Simon SA (1996) Similarities and differences in the currents activated by capsaicin, piperine, and zingerone in rat trigeminal ganglion cells. J Neurophysiol 76:1858–1869
Long H, O'Connor BP, Zemans RL, Zhou X, Yang IV, Schwartz DA (2014) The toll-like receptor 4 polymorphism Asp299Gly but not Thr399Ile influences TLR4 signaling and function. PLoS One 9:e93550
Loniewski K, Shi Y, Pestka J, Parameswaran N (2008) Toll-like receptors differentially regulate GPCR kinases and arrestins in primary macrophages. Mol Immunol 45:2312–2322
Lu Y, Cao DL, Jiang BC, Yang T, Gao YJ (2015) MicroRNA-146a-5p attenuates neuropathic pain via suppressing TRAF6 signaling in the spinal cord. Brain Behav Immun 49:119–129
Lundberg A, Wikberg LA, Ilonen J, Vaarala O, Böttcher MF (2008) Lipopolysaccharide-induced immune responses in relation to the TLR4(Asp299Gly) gene polymorphism. Clin Vaccine Immunol 15:1878–1883
Ma A, Malynn BA (2012) A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol 12:774–785
Ma C, Li Y, Li M, Deng G, Wu X, Zeng J, Hao X, Wang X, Liu J, Cho WC, Liu X, Wang Y (2014) microRNA-124 negatively regulates TLR signaling in alveolar macrophages in response to mycobacterial infection. Mol Immunol 62:150–158
Ma H, Li M, Pagare PP, Wang H, Nassehi N, Santos EJ, Stevens Negus S, Selley DE, Zhang Y (2022) Novel bivalent ligands carrying potential antinociceptive effects by targeting putative mu opioid receptor and chemokine receptor CXCR4 heterodimers. Bioorg Chem 120:105641
Ma H, Wang H, Li M, Barreto-de-Souza V, Reinecke BA, Gunta R, Zheng Y, Kang G, Nassehi N, Zhang H, An J, Selley DE, Hauser KF, Zhang Y (2020) Bivalent ligand aiming putative mu opioid receptor and chemokine receptor CXCR4 dimers in opioid enhanced HIV-1 entry. ACS Med Chem Lett 11:2318–2324
Maduna T, Audouard E, Dembélé D, Mouzaoui N, Reiss D, Massotte D, Gaveriaux-Ruff C (2019) Microglia express mu opioid receptor: insights from transcriptomics and fluorescent reporter mice. Front Psychiatry 9:726
Maeshima N, Fernandez RC (2013) Recognition of lipid a variants by the TLR4-MD-2 receptor complex. Front Cell Infect Microbiol 3:3
Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC (2007) The vaccine adjuvant monophosphoryl lipid a as a TRIF-biased agonist of TLR4. Science 316:1628–1632
Mattioli TA, Leduc-Pessah H, Skelhorne-Gross G, Nicol CJB, Milne B, Trang T, Cahill CM (2014) Toll-like receptor 4 mutant and null mice retain morphine-induced tolerance, hyperalgesia, and physical dependence. PLoS One 9:e97361
McCarthy GM, Bridges CR, Blednov YA, Harris RA (2017) CNS cell-type localization and LPS response of TLR signaling pathways. F1000Res 6:1144
Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA Jr (1998) MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 2:253–258
Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A (2000) Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A 97:3655–3660
Min H, Cho WH, Lee H, Choi B, Kim YJ, Lee HK, Joo Y, Jung SJ, Choi SY, Lee S, Lee SJ (2018) Association of TRPV1 and TLR4 through the TIR domain potentiates TRPV1 activity by blocking activation-induced desensitization. Mol Pain 14:1744806918812636
Min H, Lee H, Lim H, Jang YH, Chung SJ, Lee CJ, Lee SJ (2014) TLR4 enhances histamine-mediated pruritus by potentiating TRPV1 activity. Mol Brain 7:59
Mohebiany AN, Ramphal NS, Karram K, Di Liberto G, Novkovic T, Klein M, Marini F, Kreutzfeldt M, Härtner F, Lacher SM, Bopp T, Mittmann T, Merkler D, Waisman A (2020) Microglial A20 protects the brain from CD8 T-cell-mediated immunopathology. Cell Rep 30:1585–1597.e6
Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M (2005) Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain 1:3
Mustafa S, See HB, Seeber RM, Armstrong SP, White CW, Ventura S, Ayoub MA, Pfleger KD (2012) Identification and profiling of novel α1A-adrenoceptor-CXC chemokine receptor 2 heteromer. J Biol Chem 287:12952–12965
Nagyoszi P, Wilhelm I, Farkas AE, Fazakas C, Dung NT, Haskó J, Krizbai IA (2010) Expression and regulation of toll-like receptors in cerebral endothelial cells. Neurochem Int 57:556–564
Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M (2003) Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci U S A 100:8002–8006
Núñez Miguel R, Wong J, Westoll JF, Brooks HJ, O'Neill LA, Gay NJ, Bryant CE, Monie TP (2007) A dimer of the toll-like receptor 4 cytoplasmic domain provides a specific scaffold for the recruitment of signalling adaptor proteins. PLoS One 2:e788
Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ (2001) Chemokines and Glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci 21:5027–5035
Ohtsubo K, Marth JD (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126:855–867
Ossipov MH, Lai J, King T, Vanderah TW, Malan TP Jr, Hruby VJ, Porreca F (2004) Antinociceptive and nociceptive actions of opioids. J Neurobiol 61:126–148
Ossipov MH, Lai J, King T, Vanderah TW, Porreca F (2005) Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers 80:319–324
Ossipov MH, Lai J, Vanderah TW, Porreca F (2003) Induction of pain facilitation by sustained opioid exposure: relationship to opioid antinociceptive tolerance. Life Sci 73:783–800
Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO (2009) The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458:1191–1195
Park CK, Xu ZZ, Berta T, Han Q, Chen G, Liu XJ, Ji RR (2014) Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 82:47–54
Pello OM, Martínez-Muñoz L, Parrillas V, Serrano A, Rodríguez-Frade JM, Toro MJ, Lucas P, Monterrubio M, Martínez AC, Mellado M (2008) Ligand stabilization of CXCR4/delta-opioid receptor heterodimers reveals a mechanism for immune response regulation. Eur J Immunol 38:537–549
Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL (2011) MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat Med 17:64–70
Poole A, Song Y, O'Sullivan M, Lee KH, Metcalfe J, Guo J, Brown H, Mullins B, Loh R, Zhang GB (2020) Children with nut allergies have impaired gene expression of toll-like receptors pathway. Pediatr Allergy Immunol 31:671–677
Rafiei A, Abedini M, Hosseini SH, Hosseini-Khah Z, Bazrafshan B, Tehrani M (2012) Toll like receptor-4 896A/G gene variation, a risk factor for migraine headaches. Iran J Immunol 9:159–167
Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K (2020) The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 161:1976–1982
Richard K, Perkins DJ, Harberts EM, Song Y, Gopalakrishnan A, Shirey KA, Lai W, Vlk A, Mahurkar A, Nallar S, Hawkins LD, Ernst RK, Vogel SN (2020) Dissociation of TRIF bias and adjuvanticity. Vaccine 38:4298–4308
Rutishauser U (2008) Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci 9:26–35
Sakai J, Cammarota E, Wright JA, Cicuta P, Gottschalk RA, Li N, Fraser IDC, Bryant CE (2017) Lipopolysaccharide-induced NF-κB nuclear translocation is primarily dependent on MyD88, but TNFα expression requires TRIF and MyD88. Sci Rep 7:1428
Schiraldi M, Raucci A, Muñoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, Thelen M, Varani L, Mellado M, Proudfoot A, Bianchi ME, Uguccioni M (2012) HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 209:551–563
Shao S, Sun Y, Zhang Y, Tian X, Li Y, Tan B, Su R (2020) A20 enhances mu-opioid receptor function by inhibiting beta-arrestin2 recruitment. Biochem Biophys Res Commun 528:127–133
Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M (1999) MD-2, a molecule that confers lipopolysaccharide responsiveness on toll-like receptor 4. J Exp Med 189:1777–1782
Shukla AK, Xiao K, Lefkowitz RJ (2011) Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci 36:457–469
Siemens J, Zhou S, Piskorowski R, Nikai T, Lumpkin EA, Basbaum AI, King D, Julius D (2006) Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature 444:208–212
Somogyi AA, Sia AT, Tan EC, Coller JK, Hutchinson MR, Barratt DT (2016) Ethnicity-dependent influence of innate immune genetic markers on morphine PCA requirements and adverse effects in postoperative pain. Pain 157:2458–2466
Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE (2006) Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol 128:509–522
Stephens M, Liao S, Von Der Weid P-Y (2021) Ultra-purification of lipopolysaccharides reveals species-specific signalling bias of TLR4: importance in macrophage function. Sci Rep 11:1335
Stratiievska A, Nelson S, Senning EN, Lautz JD, Smith SE, Gordon SE (2018) Reciprocal regulation among TRPV1 channels and phosphoinositide 3-kinase in response to nerve growth factor. elife 7:e38869
Sun X, Zhang H (2018) miR-451 elevation relieves inflammatory pain by suppressing microglial activation-evoked inflammatory response via targeting TLR4. Cell Tissue Res 374:487–495
Szabo I, Chen XH, Xin L, Adler MW, Howard OMZ, Oppenheim JJ, Rogers TJ (2002) Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci 99:10276–10281
Szallasi A, Cortright DN, Blum CA, Eid SR (2007) The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov 6:357–372
Taganov KD, Boldin MP, Chang KJ, Baltimore D (2006) NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 103:12481–12486
Takahashi K, Sugi Y, Nakano K, Tsuda M, Kurihara K, Hosono A, Kaminogawa S (2011) Epigenetic control of the host gene by commensal bacteria in large intestinal epithelial cells. J Biol Chem 286:35755–35762
Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S, Honda K, Ohba Y, Mak TW, Taniguchi T (2005) Integral role of IRF-5 in the gene induction programme activated by toll-like receptors. Nature 434:243–249
Tanga FY, Nutile-McMenemy N, DeLeo JA (2005) The CNS role of toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A 102:5856–5861
Thomas J, Mustafa S, Johnson J, Nicotra L, Hutchinson M (2015) The relationship between opioids and immune signalling in the spinal cord. Springer, Berlin, pp 207–238
Thomas JHL, Lui L, Abell A, Tieu W, Somogyi AA, Bajic JE, Hutchinson MR (2022) Toll-like receptors change morphine-induced antinociception, tolerance and dependence: studies using male and female TLR and Signalling gene KO mice. Brain Behav Immun 102:71–85
Triantafilou M, Lepper PM, Briault CD, Ahmed MA, Dmochowski JM, Schumann C, Triantafilou K (2008) Chemokine receptor 4 (CXCR4) is part of the lipopolysaccharide “sensing apparatus”. Eur J Immunol 38:192–203
Tsukamoto H, Fukudome K, Takao S, Tsuneyoshi N, Ohta S, Nagai Y, Ihara H, Miyake K, Ikeda Y, Kimoto M (2013) Reduced surface expression of TLR4 by a V254I point mutation accounts for the low lipopolysaccharide responder phenotype of BALB/c B cells. J Immunol 190:195–204
Vaure C, Liu Y (2014) A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol 5:316
Voet S, Mc Guire C, Hagemeyer N, Martens A, Schroeder A, Wieghofer P, Daems C, Staszewski O, Vande Walle L, Jordao MJC, Sze M, Vikkula H-K, Demeestere D, Van Imschoot G, Scott CL, Hoste E, Gonçalves A, Guilliams M, Lippens S, Libert C, Vandenbroucke RE, Kim K-W, Jung S, Callaerts-Vegh Z, Callaerts P, de Wit J, Lamkanfi M, Prinz M, van Loo G (2018) A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation. Nat Commun 9:2036
von Hehn AC, Baron R, Woolf JC (2012) Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73:638–652
Wadachi R, Hargreaves KM (2006) Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J Dent Res 85:49–53
Wang X, Loram LC, Ramos K, De Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H (2012) Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci 109:6325–6330
Wang X, Northcutt AL, Cochran TA, Zhang X, Fabisiak TJ, Haas ME, Amat J, Li H, Rice KC, Maier SF, Bachtell RK, Hutchinson MR, Watkins LR (2019) Methamphetamine activates toll-like receptor 4 to induce central immune signaling within the ventral tegmental area and contributes to extracellular dopamine increase in the Nucleus Accumbens Shell. ACS Chem Neurosci 10:3622–3634
Wang X, Zhang Y, Peng Y, Hutchinson MR, Rice KC, Yin H, Watkins LR (2016) Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br J Pharmacol 173:856–869
Wang Y, Dai G, Jiang L, Liao S, Xia J (2021) Microarray analysis reveals an inflammatory transcriptomic signature in peripheral blood for sciatica. BMC Neurol 21:50
Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G (2006) Association of beta-arrestin and TRAF6 negatively regulates toll-like receptor-interleukin 1 receptor signaling. Nat Immunol 7:139–147
Wang Y, Zhang S, Li H, Wang H, Zhang T, Hutchinson MR, Yin H, Wang X (2020) Small-molecule modulators of toll-like receptors. Acc Chem Res 53:1046–1055
Watkins LR, Hutchinson MR, Rice KC, Maier SF (2009) The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci 30:581–591
Weber M, Modemann S, Schipper P, Trauer H, Franke H, Illes P, Geiger KD, Hengstler JG, Kleemann WJ (2006) Increased polysialic acid neural cell adhesion molecule expression in human hippocampus of heroin addicts. Neuroscience 138:1215–1223
Wegner A, Elsenbruch S, Maluck J, Grigoleit JS, Engler H, Jager M, Spreitzer I, Schedlowski M, Benson S (2014) Inflammation-induced hyperalgesia: effects of timing, dosage, and negative affect on somatic pain sensitivity in human experimental endotoxemia. Brain Behav Immun 41:46–54
Wicrtelak EP, Smith KP, Furness L, Mooney-Heiberger K, Mayr T, Maier SF, Watkins LR (1994) Acute and conditioned hyperalgesic responses to illness. Pain 56:227–234
Willemen HL, Huo XJ, Mao-Ying QL, Zijlstra J, Heijnen CJ, Kavelaars A (2012) MicroRNA-124 as a novel treatment for persistent hyperalgesia. J Neuroinflammation 9:143
Wu Y, Wang Y, Wang J, Fan Q, Zhu J, Yang L, Rong W (2019) TLR4 mediates upregulation and sensitization of TRPV1 in primary afferent neurons in 2,4,6-trinitrobenzene sulfate-induced colitis. Mol Pain 15:1744806919830018
Xie Z, Huang G, Wang Z, Luo S, Zheng P, Zhou Z (2018) Epigenetic regulation of toll-like receptors and its roles in type 1 diabetes. J Mol Med (Berl) 96:741–751
Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S (2003) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640–643
Yang K, He YS, Wang XQ, Lu L, Chen QJ, Liu J, Sun Z, Shen WF (2011) MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett 585:854–860
Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, Shavit Y, Ovadia H, Weidenfeld J, Morag A, Newman ME, Pollmächer T (2006) Illness, cytokines, and depression. Ann N Y Acad Sci 917:478–487
Yirmiya R, Rosen H, Donchin O, Ovadia H (1994) Behavioral effects of lipopolysaccharide in rats: involvement of endogenous opioids. Brain Res 648:80–86
Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC (2011) CD14 controls the LPS-induced endocytosis of toll-like receptor 4. Cell 147:868–880
Zhang X, Cui F, Chen H, Zhang T, Yang K, Wang Y, Jiang Z, Rice KC, Watkins LR, Hutchinson MR, Li Y, Peng Y, Wang X (2018) Dissecting the innate immune recognition of opioid inactive isomer (+)-naltrexone derived toll-like receptor 4 (TLR4) antagonists. J Chem Inf Model 58:816–825
Zhang X, Huang J, McNaughton PA (2005) NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J 24:4211–4223
Zhang X, Li L, McNaughton PA (2008) Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 59:450–461
Zhang X, Peng Y, Grace PM, Metcalf MD, Kwilasz AJ, Wang Y, Zhang T, Wu S, Selfridge BR, Portoghese PS, Rice KC, Watkins LR, Hutchinson MR, Wang X (2019) Stereochemistry and innate immune recognition: (+)-norbinaltorphimine targets myeloid differentiation protein 2 and inhibits toll-like receptor 4 signaling. FASEB J 33:9577–9587
Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400:452–457
Acknowledgements
This work was supported by the Australian Research Council Centre of Excellence for Nanoscale BioPhotonics (CE140100003), Australian Postgraduate Award, an Australian Research Council Future Fellowship (FT180100565) and the University of Adelaide Davies Livestock Research Centre.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mustafa, S. et al. (2022). Toll-Like Receptor 4 in Pain: Bridging Molecules-to-Cells-to-Systems. In: Kumar, V. (eds) Toll-like Receptors in Health and Disease. Handbook of Experimental Pharmacology, vol 276. Springer, Cham. https://doi.org/10.1007/164_2022_587
Download citation
DOI: https://doi.org/10.1007/164_2022_587
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-06511-8
Online ISBN: 978-3-031-06512-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)