Abstract
Complex wounds represent a challenge for the wound care specialist. The aim of treatment of electrical burn wounds is to achieve skin cover of the soft-tissue defects, prevent infection, and allow a good function of the affected anatomical segment. Surgical reconstructive techniques for soft-tissue defects covering, such as muscle flaps, free flap transfers, and cross-leg techniques, are frequently used in treating sequelae after electrical burns. In some cases, when the patient cannot be a candidate for free flap surgery the use of negative-pressure wound therapy (NPWT) is an effective alternative that can minimize the traditional reconstructive surgery methods and can reduce the surface of the soft-tissue defect by filling it with new formed granulation tissue, creating a skin graft receptor bed.
This newer and simpler technique used for covering of exposed bone tissue can question the gold standard of plastic reconstructive surgery that utilizes muscle flaps as the only way to cover these defects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
1.1 Electricity and Electrical Burn
Electrical burns are relatively uncommon; in adults they usually occur in occupational settings, whereas in children they occur accidentally [1]. In the United States approximately 1000 deaths per year are caused by electrical injuries, the mortality rate being around 3–5% [2].
Electricity is the movement of electrons, which comprise the current, from atom to atom, across a potential gradient from high to low concentration through a conductive material. The voltage represents the magnitude of this potential difference. Amperage measures the volume of electrons flowing across the potential gradient.
Resistance is a measure of how difficult it is for the electrons to pass through a material [3]. The resistance of the human body to electricity is relatively high on the outside and low on the inside; it varies depending on the electrolyte and water content of the tissue through which the electrical current is being conducted. Skin resistance varies on the moisture content, thickness, and cleanliness. All internal tissues offer low resistance, excluding the bone which is a poor conductor of energy. Muscles, nerves, and blood vessels have low resistance due to their high electrolyte and water content and are good electricity conductors. Bones, tendons, and fat have higher resistance. Electricity creates heat, following the path of least resistance through the body (Table 1) [4, 5].
The severity of an electrical burn depends on many factors and can be classified depending on the type of the circuit (electrical current can flow in direct (DC) or alternating current (AC)), duration, resistance of tissues, voltage (low or high), amperage, and pathway of the current [3, 4].
High-voltage direct current (DC) often causes a single-muscle contraction, throwing the victim away from the source, while the same voltage of alternating current is considered to be more dangerous because the cyclic flow of electrons causes muscle tetany and prolongs the exposure to the electrical source [4, 6]. The degree of tissue destruction is directly proportional with the duration of contact with high-voltage current [4].
Contact with high-voltage current can be associated with an electric arc, which is formed between two bodies of sufficiently different potential that are not in direct contact (e.g., a highly charged source and the ground). The arc consists of ionized particles. The temperature of these particles and their surroundings can be as high as 4000 °C [7]. When portions of the arc touch the patient, deep thermal burns occur, the electric arc remaining the cause of most high-voltage injuries.
“Entry” and “exit” are commonly used terms to describe electrical injuries and the pathway that the current takes can determine the severity of the injury and the tissues at risk: disruption of cardiac rhythm, direct myocardial injury, respiratory arrest, paralysis, sensory and motor deficits, seizures, memory loss, cataract, strong muscle contractions resulting in scapular fractures or shoulder dislocations, flash burns, and blood vessel, nerve, and muscle destruction [4, 5, 7].
The incidence of low-voltage burns is currently declining but high-voltage injuries, particularly in adolescent males, remain an unsolved problem [8].
2 Sequelae After Electrical Burn
“There are 2 possible consequences of electrical injury: the person either survives or dies” [5]. Efforts are directed towards preventing additional tissue loss, managing a potential compartment syndrome, or handling the necrotic tissue. Electrical injury often results in high rates of morbidity [4, 5]. The long-term sequelae after electrical burn can be neurologic injuries, psychological trauma, ocular deficiencies, pain, etc.
Viable surgical reconstructive techniques for soft-tissue defect covering, such as muscle flaps, free flap transfers, and cross-leg techniques, are frequently used in treating sequelae after electrical burns. Free flaps are considered to be the gold standard when the treatment of leg wounds is needed, because of their ability to cover large defects. Reverse-flow flaps are useful to cover defects of the lower leg and the ankle. When this type of flaps are not available to use, cross-leg flap can be a useful technique (Fig. 1) [9].
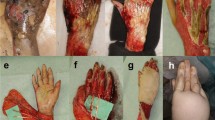
A 15-year-old male, victim of a high-voltage electrical injury, suffered major third- and fourth-degree burns on 60% of the body surface. After repetitive excision of the necrotic tissue, the distal extremity of the right leg—lower half of the tibial bone, lower half of the fibula, and internal and external malleoli—became exposed circularly due to the massive, circumferential soft-tissue defect [10]
In some cases, traditional reconstructive surgical techniques can be insufficient when covering a complex soft-tissue defect. The muscle flap can prove unsuccessful in managing a large defect due to nonhealing of the flap, flap necrosis, infection, hematoma, inadequate debridement of the necrotic tissue, use of a traumatized muscle graft, or unrealistic objectives for the muscle flap coverage (Fig. 2) [10].
Soft-tissue defects that are difficult to cover by muscle flaps and free tissue transfer are often a challenge for the practitioner. In some cases, when the patient cannot be a candidate for free flap surgery the use of negative-pressure wound therapy (NPWT) is an effective alternative that can minimize the traditional reconstructive surgery methods and can reduce the surface of the soft-tissue defect by filling it with new formed granulation tissue, creating a skin graft receptor bed [11, 12].
In their retrospective study over a period of 12 years, published in 2006, Parret et al. [13], found out that the free flap use decreased from 42% during the first period to 11% in the last 4 years of the study, when NPWT started to be extensively used. NPWT can reduce both the need for flap transfer and the size of the flap (Fig. 3).
3 Negative-Pressure Wound Therapy
NPWT, also referred to as VAC therapy (vacuum-assisted closure) or micro deformational wound therapy (MDWT), has begun to play an increasingly important role in the global landscape of wound treatment. For the last 15 years, this type of therapy intends to augment and improve the traditional methods of approaching these pathologies bringing numerous benefits on morbidity, mortality, as well as aesthetic benefits [12, 14].
The VAC therapy applies subatmospheric pressure to the wound bed, using a computerized device that produces controlled suction, via a connective port. The subatmospheric pressure helps the wound healing through mechanisms that ultimately result in wound contraction, fluid drainage, prevention of bacterial growth, and granulation tissue formation [15,16,17].
The negative-pressure wound therapy aims to create a perfect environment for wound healing. The mechanisms of action include macro deformation of the wound, micro deformation at the wound-wound filler surface, fluid drainage—thus reducing edema, improvement in local blood flow, creating a moist environment that facilitates wound healing [18], reduced inflammation, improvement in cell proliferation [19], influence in hemostasis, stimulation of angiogenesis, granulation tissue formation, alteration in bacterial burden, and affecting of cellular responses in division, migration, and differentiation (Table 2) [14, 20, 21].
The macro deformation of the wound refers to the contraction and size reduction of the wound due to the centripetal forces that NPWT induce, shrinkage that is also caused by the collapse of the foam pores. Micro deformation refers to the interaction between wound bed and NPWT contact layer, the undulated wound surface induced by the porous material of the foam [11].
The mechanical deformation starts a signaling cascade, leading to wound healing [22]. Fluid removal optimizes tissue perfusion by reducing the compression on the capillaries, thus allowing increased blood flow to the wound area [23, 24].
4 Technique
4.1 NPWT System
The negative-pressure wound therapy system has four major components: a wound filler material, an airtight vacuum seal, a connecting tube, and a vacuum pump [14]. The vacuum device has an incorporated canister, where the fluid is collected and which is equipped with an alarm system that notifies the practitioner when the canister is full.
Contraindications for the use of NPWT include eschar with the presence of necrotic tissue, untreated osteomyelitis, malignant cells in the wound, direct use on exposed blood vessels and nerves, nonenteric and unexplored fistulas, exposed anastomoses, and exposed organs. Some characteristics to consider before using this treatment include high risk of hemorrhage (including patients on anticoagulants or platelet aggregation inhibitors), infected wounds, friable vessels and infected blood vessels, sharp edges in the wound, spinal cord injuries, circumferential dressing application, proximity of the foam to vagus nerve, and patient weight and size [14, 25].
4.1.1 Sequence of Procedure (Table 3)
-
1.
Wound Bed Preparation (WBP)
For wound bed preparation, the necessary supplies can be organized into five categories: anesthetic, sterile field, irrigation, debridement, and dressing (Table 4).
The management of any traumatic wound starts with thorough irrigation using sterile saline, in order to clean the wound and facilitate inspection. Lavage intends to clear the debris from the wound and lower the bacterial burden. Bacterial clearance can be improved by early irrigation. On his study on an animal model, Owens concluded that earlier irrigation in a contaminated wound resulted in a superior bacterial removal: irrigation within 3 h lowered the bacterial load by 70%, within 6 h 52%, and 37% at 12 h (Fig. 4) [10, 26].
A couple of months later, the patient was transferred to our department presenting a chronic wound. Clinical examination of the right leg revealed two soft-tissue defects, tibial bone exposed on an area of 15/3 cm in the lower half, the peroneal malleolus exposed and had a surface of 7/2.5 cm [10]
A proper debridement of the devitalized, necrotic tissue must be obtained, to facilitate healing and decrease the risk of infection at the wound site, with due regard to vital anatomic structures, hemostasis, and wound hygiene. NPWT cannot be applied over necrotic, devitalized, or infected tissue. A devitalized tissue, by absent or tenuous blood supply, is poorly penetrated by systemic antibiotics and provides a good environment for bacterial proliferation [10, 27].
Profuse lavage of the wound is recommended each time the change of the dressing is performed. If required, a swab culture for microbiology should be taken before saline lavage (Fig. 5) [28].
-
2.
Placement of Contact Layer and Foam
The VAC therapy requires a contact material that enables the negative pressure to reach the wound bed. The wound filler material, as part of the commercial NPWT systems, is available as foam wound filler and gauze wound filler [29].
The foam wound filler is custom cut by the practitioner to fit the wound. Several types of foam are available:
-
(a)
Polyurethane (PU) black foam, hydrophobic and reticulated, made of highly interconnected cells [14], allows even distribution of the negative pressure across the wound bed [30] and improves fluid drainage [14] and wound contraction [31]. This type of foam is often used in wounds with large fluid drainage and when stimulation of the granulation tissue formation is wanted [14].
-
(b)
Silver-coated foam that can be used in the surgical therapy of infected wounds because of the antimicrobial effects of silver nanoparticles that can destroy bacterial cell walls and inhibit enzymes for bacterial cell replication [32].
-
(c)
Polyvinyl alcohol white foam (PVA), hydrophilic, with higher tensile strength than the PU foam [30]: This type of foam can be used when growth of granulation tissue is less needed [31], because of the increased density and the smaller pores. It is recommended in wound with delicate underlying structures (e.g., tendons, blood vessels) that need to be protected [14, 33].
-
(a)
Wound bed preparation: The aspect of the leg after wound-edge excision and debridement of devitalized bone tissue: removal of the outer layer of the anterior cortex and the whole anterior cortex in some regions, causing the bone to bleed [10]
The foam can be used in association with a silver nanoparticle contact layer that has the ability to reduce wound infection rates, decrease the frequency of dressing changes, diminish pain levels, and promote wound healing. Also, a silicone wound layer may be used to reduce trauma and pain at dressing changes, prevent the ingrowth of the new formed tissue in the foam reticules, protect the delicate wound structures, and facilitate the formation of granulation tissue (Figs. 6 and 7) [12].
-
3.
Creating an Airtight Seal
The second component of the negative-pressure wound therapy is creating an airtight seal over the wound and the wound filler, thus facilitating the suction to the wound bed. This can be done with an adhesive occlusive dressing. Depending on the anatomical location of the wound, this process can be sometimes difficult and application of skin adhesive to maintain the seal is needed [34]. The sealing dressing must entirely cover the wound filler and the wound. Special care must be taken for the wound edges, ensuring that these are clean and dry (Fig. 8).
-
4.
Application of NPWT
Third component, the non-collapsible tube is embedded in the foam through an incision made in the sealing dressing, geometrically fitted for the connecting device.
The vacuum pump is a computerized device that creates negative pressure at the wound site via the canister and the tube. There are several types of vacuum pumps. The traditional pump is usually portable and the canister in use with this pump can hold from 300 to 1000 mL wound exudate. It usually incorporates a computerized alarm system that detects inadequate seal, excessive fluid drainage, blockages of the tube, etc. [15]. It is electrically powered and has a rechargeable battery. From this device the practitioner can choose what type of pressure to use, continuous, intermittent, and variable, and the amount of pressure applied to the wound.
The NPWT pump delivers negative pressure to the entire wound bed. The amount of pressure applied can vary between 25 and 200 mmHg depending on the wound and the wound filler type. In clinical practice the amount of pressure that is usually used is 125 mmHg.
After the application of NPWT, the dressing must be changed in an interval of 2–4 days. Dressing changing can be a painful maneuver; local or general anesthesia is recommended. Avivement (surgical trimming of wound edges before suturing them) of the wound and lavage must be performed each time (Fig. 9).
Stages in granulation tissue formation during NPWT, final wound coverage, and wound healing. (a) On the sixth day of using the NPWT the contraction of the wound edges could be observed. (b) After 15 days of using the NPWT granulation tissue covered the proximal and the distal ends of the wound. (c) Day 25 of NPWT. (d) Day 33 of NPWT. (e) Split skin grafting. (f) Healed wound
5 Discussion
Compared to conventional burns, high-voltage burns are characterized by an increased morbidity and worse potential for rehabilitation. During the early posttraumatic period, the surgical management of these particular burns is represented by repetitive debridements and necrectomies. Mortality in this type of injury is remarkably high, even with the aggressive approach to remove necrotic tissue.
In burns, conventional surgical debridement is the gold standard. Although full excision was historically standard practice in excision of burns, nowadays tangential excision, removal of necrotic tissue by sequential layered excision of devitalized tissue until the level of healthy, bleeding, vitalized tissue, has replaced full excision. Timing of debridement is an important aspect as well; tangential excision facilitates early debridement by intraoperatively determining the depth of the burn. In electrical burn wounds the intraoperative determination of the burn depth is hard to obtain [10].
Muscle flaps (reverse flow flaps, cross-leg, free microvascular flaps, etc.) can be used in an attempt for limp salvage [35].
The normal healing process includes hemostasis, inflammation, cell proliferation, and cell maturation. These phenomenons that appear in the healing process of an acute wound do not apply entirely in the processes involved in the healing of chronic wounds. The delayed healing of chronic wounds is due to a failure to progress through these phases, the sequence of events becoming disrupted at one or more of the steps of the healing process. Usually chronic wounds are “stuck” in the inflammation phase of healing as a barrier defect that has not healed in 3 months [36, 37].
Usually chronic wounds include, but are not limited to, diabetic, venous, pressure foot and leg ulcers. These type of chronic wounds have a different pathophysiology than an acute wound or a traumatic chronic wound and the modalities of treatment and means of healing differ. A good understanding of the differences between different types of chronic wounds should lead to better healing rates and improve treatment management. The primary challenge in treatment of a chronic wound is to overcome the factors that sustain a delayed healing and to have a comprehensive approach to wound care. Chronic lower extremity wounds include leg and foot ulcers due to a vascular disease, diabetes, neurological foot, chronic venous insufficiency, arterial disease, neuropathy, and prolonged pressure [13, 38].
Some of the factors that contribute to the delayed healing include prolonged and massive inflammation, unremitting infection with drug-resistant microbes, and lack of epithelialization. A major step forward in managing the problems of wound healing is concerned by the wound bed preparation, allowing the practitioner to identify hypoxia, increased bacterial load, presence of necrotic tissue, and alteration of the matrix. Wound bed preparation can accelerate the endogenous healing of the wound or facilitate the effectiveness of other therapeutical strategies [36, 37].
NPWT protocol when treating electrical burn sequelae wounds is determined by the goal of treatment (granulation tissue growth to cover a soft-tissue defect, fluid drainage and edema removal, flap or graft immobilization), pressure values, pressure modes, and type of dressing.
The duration of the negative-pressure wound therapy depends on the goals of treatment. When the device is used to stimulate the formation of the granulation tissue, for preparing a skin graft receptor bed, the therapy can continue until the soft-tissue defect has been covered and the granulation tissue has reached the skin level so the skin graft can be safely placed.
The amount of pressure applied to the wound can vary, as well as the modes of pressure available. A series of basic animal studies demonstrated that the blood flow levels increased when 125 mmHg negative pressure was applied [39]. Other animal studies concluded that wound contraction and fluid removal are directly proportional with the level of negative pressure until reaching a steady state. Maximum wound contraction was observed at a pressure of −75 mmHg and the maximum fluid drainage from the wound was at −125 mmHg [40].
NPWT can be delivered in continuous, intermittent, or variable modes. The continuous pressure mode is the most commonly used, during which the pressure level is constant. In the intermittent mode the negative pressure is switched on and off repeatedly. Studies suggest that the intermittent pressure therapy results in faster healing by stimulating the formation of granulation tissue (by mechanically stimulating the wound bed and increasing blood flow to the wound edges), but is painful. Variable pressure was introduced to decrease the amount of pain by creating a smooth transition between the two modes of negative pressure [41]. Variable pressure is the most indicated pressure mode when formation of granulation tissue is needed as well as the management of pain. Special attention concerning pain management during treatment and dressing changes must be given.
The placement of the foam and contact layer is an important aspect for successful NPWT. The foam must be cut to size to fit the wound and the contact layer, if used, must be inserted into all undermined areas and must fill all irregularities of the wound. Placing the foam directly on top of intact skin should be avoided.
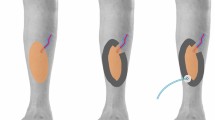
In some cases there are more than one soft-tissue defects in the same anatomic region. In order to use a single-vacuum port a foam bridge that connects both areas can be created. The healthy skin between them must be protected using a seal drape or a hydrocolloid dressing (Fig. 10).
The adverse effects when using this type of treatment are represented by pain and discomfort, skin irritations caused by allergies from the adhesive sealing drapes, excoriation of the skin if the foam is not correctly cut to size, and sometimes odor from the dressings or the canister. Common complications of NPWT include bleeding, infection, foam-tissue adherence, and foam retention in the wound [28].
A potential and serious complication of NPWT is bleeding from the wound site. When preparing the wound for applying the negative-pressure wound therapy, removal of the devitalized tissue must be done, until healthy, bleeding tissue is revealed. When removing devitalized bone, coagulation in the remaining bone during suction can be sometimes difficult and severe hemorrhage can occur. Special care should be taken concerning this aspect. Also, removal of devitalized bone tissue can affect the mechanical resistance of the remaining bone. Considering this aspect, one of the complications that can occur after debridement of the necrotic bone tissue can be the fracture of the bone at the wound site (Fig. 11). This situation needs special care, immobilization of the leg, and therefore a more difficult access to the wound that can endanger wound’s healing.
Conclusions
The care of traumatic electrical burn wounds requires prompt evaluation, pain management, irrigation, debridement, and application of appropriate dressings. Initial management of electrical burn wounds should intend to optimize function and minimize long-term scarring.
The treatment of delayed deep and extensive soft-tissue defects is a challenge for the practitioners, especially if the patient’s overall condition is poor. In some cases, by using the NPWT it is possible to cover major soft-tissue defects, thus avoiding the amputation of the limp. Considering these results, there is a trend in using fewer muscle flaps and more delayed closures, using skin grafts on a receptor bed created by VAC therapy.
This newer and simpler technique used for covering of exposed bone tissue can question the gold standard of plastic reconstructive surgery that utilizes muscle flaps as the only way to cover these defects.
References
Gajbhiye AS, Meshram MM, Gajaralwar RS, Kathod AP (2013) The management of electrical burn. Indian J Surg 75(4):278–283
Haberal MA (1995) An eleven-year survey of electrical burn injuries. J Burn Care Rehabil 16(1):43–48
Dalziel CF (1956) Effects of electric shock on man. IRE Trans Med Electron 5:44–62
Price T, Cooper MA, Marx J, Hockberger R, Walls R (2002) Electrical and lighting injuries Rosen's emergency medicine, 5th edn. Mosby, New York, pp 2010–2020
Wesner ML, Hickie J (2013) Long-term sequelae of electrical injury. Canadian Fam Phys 59(9):935–939
Lee RC (1997) Injury by electrical forces: pathophysiology, manifestations, and therapy. Curr Probl Surg 34(9):677–764
Fish RM (1999) Electric injury, part I: treatment priorities, subtle diagnostic factors, and burns. J Emerg Med 17(6):977–983
Rai J, Jeschke MG, Barrow RE, Herndon DN (1999) Electrical injuries: a 30-year review. J Trauma 46(5):933–936
Bajantri B, Bharathi RR, Sabapathy SR (2012) Wound coverage considerations for defects of the lower third of the leg. Indian J Plast Surg 45(2):283–290
Block L, King TW, Gosain A (2015) Debridement techniques in pediatric trauma and burn-related wounds. Adv Wound Care 4(10):596–606
Verbelen J, Hoeksema H, Pirayesh A, Van Landuyt K, Monstrey S (2016) Exposed tibial bone after burns: flap reconstruction versus dermal substitute. Burns 42(2):e31–e37
Tevanov I, Enescu DM, Bălănescu R, Sterian G, Ulici A (2016) Negative Pressure Wound Therapy (NPWT) to treat complex defect of the leg after electrical burn. Chirurgia (Bucur) 111(2):175–179
Parrett BM, Matros E, Pribaz JJ, Orgill DP (2006) Lower extremity trauma: trends in the management of soft-tissue reconstruction of open tibia-fibula fractures. Plast Reconstr Surg 117(4):1315–1322
Huang C, Leavitt T, Bayer LR, Orgill DP (2014) Effect of negative pressure wound therapy on wound healing. Curr Probl Surg 51(7):301–331
Siqueira MB, Ramanathan D, Klika AK, Higuera CA, Barsoum WK (2016) Role of negative pressure wound therapy in total hip and knee arthroplasty. World J Orthop 7(1):30–37
Glass GE, Murphy GF, Esmaeili A, Lai LM, Nanchahal J (2014) Systematic review of molecular mechanism of action of negative-pressure wound therapy. Br J Surg 101(13):1627–1636
Webb LX, Pape HC (2008) Current thought regarding the mechanism of action of negative pressure wound therapy with reticulated open cell foam. J Orthop Trauma 22(10 Suppl):S135–S137
Orgill DP, Manders EK, Sumpio BE, Lee RC, Attinger CE, Gurtner GC, Ehrlich HP (2009) The mechanisms of action of vacuum assisted closure: more to learn. Surgery 146(1):40–51
Scherer SS, Pietramaggior G, Mathews JC, Prsa MJ, Huang S, Orgill DP (2008) The mechanism of action of the vacuum-assisted closure device. Plast Reconstr Surg 122:786–797
Hasan MY, Teo R, Nather A (2015) Negative-pressure wound therapy for management of diabetic foot wounds: a review of the mechanism of action, clinical applications. Diabet Foot Ankle 6:27618
Mouës CM, Heule F, Hovius SER (2011) A review of topical negative pressure therapy in wound healing: sufficient evidence? Am J Surg 201:544–556
Borgquist O, Gustafsson L, Ingemansson R, Malmsjö M (2010) Micro- and macromechanical effects on the wound bed of negative pressure wound therapy using gauze and foam. Ann Plast Surg 64(6):789–793
Argenta LC, Morykwas MJ (1997) Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 38:563–576
Adamkova M, Tymonova J, Zamecnikova I, Kadlcik M, Klosova H (2005) First experience with the use of vacuum assisted closure in the treatment of skin defects at the burn center. Acta Chir Plast 47:24–27
FDA Safety Communication: UPDATE on serious complications associated with negative pressure wound therapy systems (2017) http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm244211.htm. Accessed 15 Feb 2017
Owens BD, Wenke JC (2007) Early wound irrigation improves the ability to remove bacteria. J Bone Joint Surg Am 89(8):1723–1726
Nather A (2011) Role of negative pressure wound therapy in healing of diabetic foot ulcers. J Surg Tech Case Rep 3(1):10–11
Pham CT, Middleton P, Maddern G (2003) Vacuum-assisted closure for the management of wounds: an accelerated systematic review. ASERNIP-S Report No 37
Birke-Sorensen H, Malmsjo M, Rome P, Hudson D, Krug E, Berg L, Bruhin A, Caravaggi C, Chariker M, Depoorter M, Dowsett C, Dunn R, Duteille F, Ferreira F, Francos Martínez JM, Grudzien G, Ichioka S et al (2011) Evidence-based recommendations for negative pressure wound therapy: treatment variables (pressure levels, wound filler and contact layer) – steps towards an international consensus. J Plast Reconstr Aesthet Surg 64(Suppl):S1–S16
Excell ET (2009) Use of negative pressure wound therapy for abdominal wounds: a review of recent literature. School of Physician Assistant Studies. Paper, p 187
Baranoski S, Ayello EA (2008) Wound care essentials: practice principles, 2nd edn. Lippincott Williams &Wilkins, Philadelphia, p 152
Sachsenmaier S, Peschel A, Ipach I, Kluba T (2013) Antibacterial potency of V.A.C. GranuFoam Silver(®) dressing. Injury 44(10):1363–1367
Malmsjö M, Ingemansson R, Martin R, Huddleston E (2009) Negative-pressure wound therapy using gauze or open-cell polyurethane foam: similar early effects on pressure transduction and tissue contraction in an experimental porcine wound model. Wound Repair Regen 17(2):200–205
Karadsheh MJ, Nelson J, Wilcox R (2015) The application of skin adhesive to maintain seal in negative pressure wound therapy. Wounds 27(9):244–248
Handschin AE, Jung FJ, Guggenheim M, Moser V, Wedler V, Contaldo C, Kuenzi W, Giovanoli P (2007) Surgical treatment of high-voltage electrical injuries. Handchir Mikrochir Plast Chir 39(5):345–349
Panuncialman J, Falanga V (2009) The science of wound bed preparation. Surg Clin North Am 89(3):611–626
Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W (2003) Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 11(Suppl 1):S1–28
Frykberg RG, Banks J (2015) Challenges in the treatment of chronic wounds. Adv Wound Care 4(9):560–582
Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W (1997) Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 38:553–562
Borgquist O, Ingemansson R, Malmsjö M (2011) The influence of low and high pressure levels during negative-pressure wound therapy on wound contraction and fluid evacuation. Plast Reconstr Surg 127:551–559
Malmsjö M, Gustafsson L, Lindstedt S, Gesslein B, Ingemansson R (2012) The effects of variable, intermittent, and continuous negative pressure wound therapy, using foam or gauze, on wound contraction, granulation tissue formation, and ingrowth into the wound filler. Eplasty 12:e5
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Ulici, A., Tevanov, I., Enescu, D.M., Ulici, A. (2018). The Use of NPWT in Treating Electrical Burn Wounds. In: Shiffman, M., Low, M. (eds) Pressure Injury, Diabetes and Negative Pressure Wound Therapy. Recent Clinical Techniques, Results, and Research in Wounds, vol 3. Springer, Cham. https://doi.org/10.1007/15695_2017_51
Download citation
DOI: https://doi.org/10.1007/15695_2017_51
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-10700-0
Online ISBN: 978-3-030-10701-7
eBook Packages: MedicineMedicine (R0)