Abstract
The aim of the present chapter is to confirm the clinical expediency of utilization of particular type of biological skin substitute—porcine skin xenograft in the treatment of scald partial-thickness burns. Among 109 patients, 78 (71%) patients healed within 14 days with a mean time 9.6 days. One sample t-test which compared mean healing times achieved within 14 days with the value of 14 days proved to be significant. Four patients received split-thickness skin grafts. The data obtained were compared with similar studies, and other treatment options in this indication were discussed. The retrospective study proved the clinical efficiency of utilization of skin xenograft for the treatment of partial-thickness scald burns. This method with its treatment effect is fully comparable with the other ones, which are using biological or another type of skin substitutes in given indication.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Burns are primarily damaging the skin and nearby structures. Secondarily, they are able to endanger all the systems in the human body. From this point of view, the management of burns represents a part of the medicine, where multidisciplinary approach is needed. Nevertheless, the basic and inevitable prerequisite of successful treatment of burns represents a treatment of the burn wound, where the skin substitutes are still holding an irreplaceable position.
Burn wound is like an opened door. Through this opened door, not invited guests are coming (causing bacterial contamination/colonization/infection). On the contrary, inhabitants which wished to stay (body heat, wound fluid, electrolytes, proteins) are leaving. The more the door is opened (the larger extent of burn and depth are) and the longer the time period of opening is (length of time delay between injury and wound cover/closure), the greater negative impact they are likely to have. Except the burn depth, burn extent, and time delay of the treatment, many other factors can influence the speed and quality of wound closure (burn etiology and localization, age, associated illnesses/injuries), so it is not always easy to close the door (perform wound coverage/closure) securely and in time.
The main requirement in the management of partial-thickness burn wounds is an economical, easy-to-apply, readily available dressing or method of coverage that would provide pain relief, protect the wound from infection, induce healing, prevent heat and fluid loss, and provide other beneficial activities while waiting for spontaneous epithelialization.
According to epidemiological studies [1,2,3], scald injuries are the most frequent cause of burns afflicting lower age groups of the patients (<15 years of age). Scald injuries are an important public health issue and cause considerable morbidity. Mainly the partial-thickness scald burns are creating one of the frequent indications for the utilization of biological skin substitute—porcine skin xenograft at the authors’ workplace. In regard to the high incidence of this type of injury—43% of the whole amount of burned patients admitted in the years 1989–1993 in Bratislava [4], their reasonable and efficacious treatment is of great importance. To prove the beneficial effects of skin xenograft as biological skin substitute, the retrospective study had been created and performed [5].
By describing the preparation method of skin xenografts, wound management protocol, clinical results of the retrospective study, their comparison with other methods of wound coverage used at other clinical workplaces, and discussion, it would be possible for the reader to gain comprehensive approach to the wound management of partial-thickness burns.
2 Technique
The retrospective study was performed in 109 patients with fresh superficial and deeper partial-thickness burns, who were hospitalized at the Teaching Department of Burns and Reconstructive Surgery of the University Hospital Bratislava, Ruzinov Hospital, Slovakia, in the years 2005–2007. The study was approved by the Ethics Committee of the Ruzinov Hospital.
2.1 Xenograft Preparation Method
Skin xenografts (SXG) have been used as biological skin substitutes at the Department of Burns and Reconstructive Surgery since 1987. They have been prepared for clinical use in the Central Tissue Bank (CTB), a part of the Teaching Department of Burns and Reconstructive Surgery Bratislava, Slovakia, where SXG preparation method has been performed according to the original method of Moserová (1974) [6] with some minor modifications. Porcine skin removed from veterinary-certified slaughtered pigs, stored at the temperature of 4 °C, has been transported from the slaughterhouse to CTB. Within 24 h after the slaughter, under sterile conditions, the retrieval of the dermoepidermal grafts has been done by electrical dermatome (Fig. 1). After the washing of grafts retrieved in a solution of chloramine, lavage in antibiotic solution, and lavage in cryoprotective agent (Fig. 2), four small samples (0.5 × 0.5 cm each) have been taken from each graft for the bacteriological control. After then, each graft has been put on the sterile gauze with the dermal side up, folded up to create four layers at maximum (Fig. 3), and sealed in the sterile plastic bag. Prepared SXG have been placed into the deep-freezer appliance and stored at the temperature of −80 °C. After obtaining negative results of the sterility tests according to Czech-Slovak Pharmacopoeia, 4th Ed., the SXG could have been released for the clinical use.
2.2 Wound Management Protocol
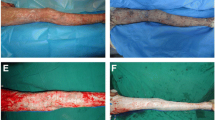
During the admission of the patient, initial assessment of the depth and extent of burns has been performed. For estimation of an extent of burns, body surface area of burns (BSAB), the Lund-Browder chart has been used. The depth of the burn has been expressed in degrees. When indicated for the xenograft coverage, after the disinfection of the wounds with Betadine, all patients underwent complete debridement of the nonviable epidermis using blunt debridement (gauze) under systemic analgesia, by children with greater extent of burns under general anesthesia. After obtaining the clean surface of the wound with the dermis exposed (Fig. 4), the cryopreserved porcine xenografts (defrosted rapidly in saline immediately before the usage) were applied (Fig. 5). Treated areas were then covered by tulle dressing impregnated with Vaseline, gauze with silver sulfadiazine, dry gauze layers, and elastic bandage. In the case of placement of xenografts over the joints of the limbs mainly by children, Kramer splints were used. Systemic antibiotic prophylaxis was provided by intravenous administration of penicillin in standard doses in cases with negative history of penicillin allergy. Wound cultures were taken by initial treatment and also regularly during the hospital stay. If the wound dressings were not displaced and if no complications occurred, the dressing changes were performed every 48 h. The xenografts were left to adhere to the wound surface till their separation by means of transepithelial elimination (Fig. 6). The residual defects (if present) were treated by application of topical antimicrobial agents within a scope of the methods for the wound healing during the hospital stay or on an outpatient basis.
Inclusion Criteria
-
The inclusion criteria were as follows:
-
1.
Patients were hospitalized during the 3-year period (from the beginning of 2005 to the end of 2007).
-
2.
All patients sustained partial-thickness (superficial and deep dermal) scald burns.
-
3.
All patients were admitted and treated within the first 24 h after injury.
-
4.
All patients were indicated for wound coverage by biological skin substitutes.
-
5.
Patients of every age, extent of burn, and associated illness were included.
Evaluation Criteria
-
1.
Total number of patients healed within 14 days postburn and their mean healing time
-
2.
Mean healing time of all patients included to the study (total healing time)
-
3.
Mean discharge time to outpatient management
-
4.
Total number of patients whose treatment required operation—wound closure by skin autografts
Total healing time was defined as the time necessary to reach 95% of reepithelialization by each patient. Hospital discharge was enabled to all patients, an outpatient management of whom could have been done safely and comfortably.
3 Results
One hundred nine patients were included in the study. From these patients 59 were males (54%) and 50 females (46%). The male/female ratio was by adults 1.16:1 and 1.2:1 by children. The mean age of the patients was 7.6 years (min., 6 months; max., 80 years). The majority of 109 patients were children (96 cases). The mean BSAB was 13% (min., 3%; max, 43%). By all patients the burns were caused by hot liquids (water, tea, coffee, soup). The numbers of patients, sex, mean age, mean BSAB, minimal and maximal values (min.-max.), and standard deviations (S.D.) are shown in Table 1.
According to BSAB the patients were divided into three groups:
-
1.
Within 10% (48 patients with mean extent 7.6%)
-
2.
11–20% (45 patients with mean extent 13.4%)
-
3.
More than 20% (16 patients with mean extent 29.4%)
The results are displayed in Table 2.
Healing was within 14 days. Among 109 patients 78 (71%) patients healed within 14 days with a mean time 9.6 days (S.D.: 3.2). One sample t-test which compared mean healing times achieved within 14 days with the value of 14 days proved the significant difference (p = 0.0001 < 0.05) by the level of significance 0.05. In the group with extents larger than 20%, the lowest amount of patients healed within 14 days.
This fact shows the correlation between the larger extents of burns and increased healing times in this group. Healing within 14 days serves for expression of the healing dynamics and as a parameter for comparison with another studies focused on the alternative treatment options.
3.1 Total Healing Time
Also in this category, the values in groups with extents up to 10% and from 10 to 20% were almost identical and within 14 days. In the group with extents more than 20%, the mean value of total healing time was 24.6 days which is in accordance to the increased value of mean healing time within 14 days in this group. For all the 109 cases, the mean total healing time was 15.1 days (S.D.: 11.6) with no significant difference between mean total healing times and the value of 14 days (p = 0.3 > 0.05).
3.2 Discharge Time
As the treatment of residual defects could have been done in many cases on the outpatient basis, the mean values of discharge time in each group were lower than mean values of total healing time. Among 109 patients altogether, the mean discharge time was up to 10 days.
3.3 Operations
In four cases, it was necessary to perform tangential excision of the deep parts of the wounds and their coverage with split-thickness skin autografts or the coverage of residual defects of larger extent, the spontaneous healing of which would require much more time. In the group with extents up to 10%, there were no operations. In the group with extents from 10 to 20%, one case of a 7-year-old girl with BSAB 13.5% was treated by operation. Because of the wound deepening of BSAB 3.5%, the tangential excision and temporary coverage with porcine skin xenografts had been performed on day 10 postburn. Skin xenografts had been replaced by split-thickness skin autografts on the second stage. The wound cultures at the day of admission were sterile, on day 4 postburn sterile—after multiplication Staphylococcus aureus. From 16 patients in the group with BSAB above 20%, three cases were operated, which represented 18.7% from this group. In two cases (one girl and one boy with the same age of 2 years and with BSAB 22% and 23%, respectively), the tangential excision and coverage with split-thickness skin autografts of BSAB 6 and 6.5%, respectively, had been performed. In one case (girl 2 years old, BSAB:21.5%), it was necessary to cover the residual defects, which represented BSAB 2% with split-thickness autografts.
Among 109 patients, 4 patients underwent surgery, which represented 3.6%. The mean surface operated was 4.5% TBSA (total body surface area).
4 Discussion
The authors compare the results of the retrospective study with cryopreserved skin xenografts with the results obtained at other workplaces, where another types of skin substitutes in this indication have been used.
A retrospective study of the treatment of scalds by glycerolized skin allografts had been performed by Brans [7]. In the period of 4 years, 45 patients with mean age of 23 months and mean BSAB 10.2% (from 3% to 23%) had been treated during the first 24 h postburn. From all patients 21 patients (47%) healed within 14 days. Better outcome of patients from the study of the treatment of scalds by skin xenografts (71%) could be only partially explained by the lower mean age of patients treated by skin allografts. The decisive could be the difference in the viability of these skin substitutes.
A prospective randomized study had been performed by Leicht [8], based on the comparison of the treatment of scalds by lyophilized allografts (LA) with the treatment by open technique. From 50 patients with scalds, 25 with the mean age 1.4 years and mean BSAB 8.35% received LA within the first 24 h postburn. From these 25 patients, 15 of them (60%) healed within 14 days. Better results achieved in the study with xenografts (71% healed within 14 days) could be explained by the higher mean age (7.6 years) but also by the similar reason mentioned previously, for as much as lyophilized skin allografts do not retain the viability in comparison with cryopreserved skin xenografts.
The results of another studies showed the necessity of the presence of bioactive surface to accelerate the reepithelialization through the contact orientation and stimulation of cell proliferation [9, 10]. From this point of view and with regard to the results achieved, the partially retained bioactivity of skin xenografts seems to be more advantageous. Partially retained bioactivity of cryopreserved skin xenografts (CXE) was observed also by Liangpeng Ge [11], where CXE has been shown to maintain a level of skin metabolism equal to 77% of the fresh sample when measured immediately after thawing (the viability value of fresh skin samples before cryopreservation was defined as 100%). The average viabilities of thawed skin samples were 77.01% (quick freezing) and 66.37% (slow freezing), respectively, measured by 3-(4,5)-dimethylthiazol-2,5-diphenyl tetrazolium bromide (MTT) salt assay immediately after thawing.
It is also possible to preserve skin allografts by deep freezing which partially retains their viability. However, utilization of them is recommended in such cases, where integration of the dermal component as a part of permanent wound closure is desired [12], because the glycerol-preserved or lyophilized allograft provides no viable coverage material and lacks the beneficial effect of integration and vascularization of viable allogeneic grafts [13].
Transmissions of human diseases via allografts, particularly infections with viruses such as HIV, CMV, and hepatitis, are a risk [14,15,16]. Although the disease transmissions through allografts are shown to be low [17], utilization of them should be reserved for wound closure in excised full-thickness burns of large extent with limited donor sites, where the benefit of wound closure (when combined with skin autografts) of large full-thickness burns would balance the possible (although very low) risk of disease transmission.
In cryopreserved skin xenografts, zoonotic infections above all porcine endogenous retroviruses (PERV) are a potential threat, but, to date, no evidence of pig-human PERV transfer has been observed [18]. Moreover, the actual risk for PERV infection, replication, and pathogenic outcome in human recipients of xenotransplantation products is still undefined [19]. Also in authors’ clinical practice, for almost 30 years of using of cryopreserved skin xenografts, such complication has never been observed.
A prospective, randomized trial of 32 patients with partial skin thickness burns, mostly scalds (25 from 32), is reported by Healy [20] comparing E-Z Derm® (silver-impregnated porcine xenograft) with Jelonet® (petroleum jelly-impregnated widely meshed gauze) as a burn dressing. The bacterial colonization rate, need for surgical treatment, time for spontaneous healing, analgesic requirements, and frequency of dressing changes were assessed in each group. No statistically significant differences were found between the two groups, for any of these factors. Surgical treatment was required in 7 of the 16 E-Z Derm-treated patients, compared with 8 of the Jelonet group (mean body surface area burned 2.3% and 1.8%, respectively). Of the burns which healed spontaneously, the mean time to healing was 12.9 days for the E-Z Derm-treated group and 12.5 days for the Jelonet-treated group. Although the number of patients included in our study and the mean body surface area were higher (109 patients, 13 ± 8%), the mean healing time was comparable (15.1 days). Moreover, the number of patients who required surgical treatment in our study was significantly lower (4 patients from 109 vs. 7 patients from 16 in E-Z Derm group). In E-Z Derm, the collagen is chemically cross-linked with an aldehyde group, which makes it more resistant to bacterial collagenous degradation and reduces antigenicity, one of the early concerns about xenografts [21] but adherence is reduced [22], which could explain the differences between study results.
In prospective study performed by Zajicek and colleagues [23], 43 pediatric patients with superficial scald burns treated with Xe-Derma® (dry sterile biological cover derived from a cellular pig dermis) were compared with 43patients treated with Askina THINSite® (hydrocolloid dressing). No significant difference in the epithelialization time, percentage of conversion from superficial to deep dermal burns, and percentage of infectious complications was detected between the two groups. Xe-Derma® showed better adherence to the wound with no need to change in comparison to Askina THINSite®, where in 40% of patients (17 out of 43) had to be changed. In the Xe-Derma group and also in the Askina THINSite group, a part of the area covered with them converted in 16 and 18 patients, respectively. In converted areas, Flammazine® or autografting (the number of patients with autograft procedure not specified) was used for further treatment. According to the results of authors’ retrospective study with cryopreserved skin xenografts, only 4 (3 of them with BSAB >20%) from 109 patients needed autograft procedure (mean surface operated 4.5% TBSA). Lower count of wound conversions can be explained by partially retained bioactivity of cryopreserved skin xenografts.
For the coverage of partial-thickness burns also, amnion obtained from the placentas of selected donors is being used. In 2007 Singh [24] compared the burn wound healing rate between the radiation-sterilized amniotic membranes and glycerol-preserved amniotic membranes. Fifty patients with partial-thickness burns (41 of them with scalds, the rest caused by flame) up to the 70% of BSAB were selected in the study. The wounds of each patient had been divided on halves, first half treated by glycerol-preserved and the second one by irradiated membrane, respectively. There were no significant differences in the rate of healing between the gamma-irradiated amniotic membranes and glycerol-preserved membranes indicating no adverse effect of the gamma irradiation on the efficacy of the membranes. According to the authors, in all the patients, membranes are desiccated and separated in 10–14 days time leaving behind epithelialized surface. Exact results of the healing were not stated.
The usage of allogeneic cultivated keratinocytes as a temporary wound coverage is advantageous mainly by IIb-degree burns, where the capability of keratinocytes to promote epithelialization by releasing the growth factors and mediators of the wound healing is combined with the ability of epithelialization from the adnexal structures of the viable parts of the dermis. On this approach “the Viennese concept” is based, published by Rab [25] in 2005:
-
1.
Early tangential excision of partial- and full-thickness scalds (days 4–7 after trauma).
-
2.
Coverage of the partial-thickness burns by cryopreserved allogeneic cultivated keratinocytes.
-
3.
In scalded areas which have to be excised to the subcutaneous tissue, the coverage of autologous split skin grafts is still the method of choice.
The authors compared 22 scalded children with the wounds tangentially excised and covered with cultivated allogeneic keratinocytes on days 4–7 after trauma with 14 children who underwent the same procedure on days 4–7 after trauma covered with split skin autografts. They observed significantly lower volume of the blood transfusion and significantly better long-term results pertaining the hypertrophic scar formation in the group of patients covered by cultivated allogeneic keratinocytes. In compliance with the results achieved, according to the authors, the higher costs resulting from the usage of keratinocytes are justified. “The Viennese concept” is promising mainly by the management of scalds in the period after first 24 h after trauma, when the usage of temporary skin substitutes is more risky because of possible infectious complications which can in many cases disable their application.
Lower incidence of hypertrophic scars in the group of patients covered with cultivated allogeneic keratinocytes as a long-term result corresponds with the finding of Ghaffari [26], who observed significantly lower amount of collagen produced by dermal fibroblasts during their cultivation with keratinocytes. This fact he explains by the presence of keratinocyte-derived collagen-inhibitory factors (KD-CIFs) with the molecular weight 30–50 kDa, which are regulating type I collagen expression and synthesis in dermal fibroblasts.
There is a reason to suppose that this regulation acts also by other types of biological skin substitutes with preserved viability of keratinocytes when used.
During cutaneous wound healing, keratinocyte proliferation and migration are critical for reepithelialization. In addition the epidermis secretes growth factors, cytokines, proteases, and matricellular proteins into the wound microenvironment that modify the extracellular matrix and stimulate other wound cells that control the inflammatory response, promote angiogenesis, and facilitate tissue contraction and remodeling. Wound keratinocytes express at least seven different integrins—the major cell adhesion receptors for the extracellular matrix—that collectively control essential cell-autonomous functions to ensure proper reepithelialization, including migration, proliferation, survival, and basement membrane assembly. Moreover, it has become evident in recent years that some integrins can regulate paracrine signals from wound epidermis that stimulate other wound cells involved in angiogenesis, contraction, and inflammation. Importantly, it is likely that abnormal integrin expression or function in the epidermis contributes to wound pathologies such as over-exuberant healing (e.g., hypertrophic scar formation) or diminished healing (e.g., chronic wounds). Many challenges arise from the complex roles that multiple integrins play in wound epidermis, which may be regulated through extracellular matrix remodeling that determines ligand availability [27]. Future research should be focused on understanding of different integrin functions coordination in wound epidermis to determine how best to target them clinically to achieve maximum therapeutic benefit.
Besides biological skin substitutes also another types of skin substitutes of different origin and constitution are used for coverage of partial-thickness burns. In 1998 Ou [28] published a retrospective study of the treatment of scalds by Biobrane. In the period of 2.5 years, 106 patients with scalds with an average BSAB 12.5% had been treated within 24 h postburn. By 24 from these patients, the coverage was aborted because of the low adherence, 14 of them healed spontaneously. By 10 remaining patients, it was necessary to perform the coverage with split skin autografts. The cases of low adherence and accumulation of exudate below Biobrane were by the authors explained by the presence of devitalized upper dermis. Therefore they emphasized accurate initial diagnosis of the depth of burns because, according to them, best results can be obtained only on superficial partial-thickness burns. Patients operated represented 9.4% of all the patients from the study, in the study with cryopreserved skin xenografts 3.6%. Neither the healing within 14 days nor total healing time was stated. The mean time of separation of the skin substitute was 11.1 days (from 3 to 18 days).
In the period immediately after an injury, during initial treatment, it is sometimes difficult to make an exact differentiation of IIa- and IIb-degree burns. Therefore, as more suitable appears the usage of such types of skin substitutes, the adherence of which should not be influenced by this depth range.
On partial-thickness face burns, a biosynthetic skin substitute Transcyte has been used to comply with the changing irregular surfaces of the face. In a comparative study [29], the usage of Transcyte was compared with open technique (bacitracin ointment). The authors observed significantly shorter healing time, shorter time for the wound care, and lower pain in the group treated with biosynthetic skin substitute.
At present, there is still research for the permanent skin substitute the quality of which would be the closest to the ideal skin substitute [30]:
-
1.
Inexpensive
-
2.
Long shelf life
-
3.
Used off the shelf
-
4.
Nonantigenic
-
5.
Durable
-
6.
Flexible
-
7.
Prevents water loss
-
8.
Bacterial barrier
-
9.
Drapes well
-
10.
Easy to secure
-
11.
Grows with child
-
12.
Applied in one operation
-
13.
Does not become hypertrophic
-
14.
Does not yet exist
Also from these ideal properties, the concepts of the characteristics of a good wound dressing by which all burns should be covered in a primary care are more or less coming out [31]:
Considered Essential
-
1.
Maintain moist wound environment
-
2.
Contours easily
-
3.
Non-adherent but retains close contact with the wound
-
4.
Easy to apply and remove
-
5.
Painless on application and removal
-
6.
Cost-effective
-
7.
Protects against infection
Considered Desirable
-
1.
Lasts for 10 days (one application)
-
2.
Minimal dressing changes
-
3.
Waterproof to allow for washing and bathing
Conclusions
From the bioactive properties of cryopreserved skin xenografts, it follows:
-
1.
Their adherence to the fresh wound surface decreases the leakage of electrolytes and salts (little or no exudate under the xenografts); good adherence also reduces the risk of secondary wound contamination.
-
2.
Increased speed of epithelialization by growth factors, which they contain.
-
3.
Faster wound healing is reducing the wound fibroplasia by which the final quality of scars is improved.
-
4.
Unless the total healing time exceeds 14 days, the risk of scar hypertrophy is minimal.
Early application of cryopreserved skin xenografts on all IIa- and IIb-degree scalds can overwhelm the problem with their exact differentiation. Moreover, it decreases the risk of secondary wound deepening which would demand excision and coverage with split skin grafts. From clinical knowledge and experiences obtained from the usage of cryopreserved skin xenografts in given indication, the following results:
-
1.
The treatment algorithm for indication and application of cryopreserved skin xenografts is at authors’ clinical workplace on highly professional level.
-
2.
The best effect of the application of skin xenografts is achieved when applied within the first 24 h after trauma, which reduces the risk of secondary infection.
-
3.
According to authors’ experiences, the wound surfaces covered with xenografts are during the dressing changes less painful; the consumption of analgetics is also lower.
-
4.
The costs associated with the processing and storage of cryopreserved skin xenografts are 0.2€ per cm2, which is significantly less amount than that needed for the purchase of synthetic or biosynthetic skin substitutes.
-
5.
According to the results of the author’s study, treatment of superficial scald burns with cryopreserved skin xenografts particularly in children proved to be an effective, safe, and reliable method.
The retrospective study proved the clinical efficiency of utilization of cryopreserved skin xenograft for the treatment of partial-thickness scald burns. This method with its treatment effect is fully comparable with the other ones, which are using biological or another type of skin substitutes in given indication, realized at other clinical workplaces.
Appropriate choice of skin substitute should always depend on careful consideration of the characteristics, indications for use, clinical experiences, results of clinical research, and also price of each available skin substitute. Only by this approach, it is possible to achieve the main goal—early wound closure of the best quality.
References
Ahuja RB, Bhattacharya S (2002) An analysis of 11,196 burn admissions and evaluation of conservative management techniques. Burns 28:555–561
Pruitt BA, Wolf SE, Mason AD (2007) Epidemiological, demographic, and outcome characteristics of burn injury. In: Herndon DN (ed) Total burn care. Elsevier, Philadelphia, pp 14–32
Forjuoh SN (2006) Burns in low- and middle-income countries: a review of available literature on descriptive epidemiology, risk factors, treatment, and prevention. Burns 32:529–537
Koller J, Orság M, Ondriašová E, Gräffinger I, Bukovcan P (1994) Analysis of 1119 Burn injuries treated at the bratislava burn department during a five-year period. Acta Chir Plast 36(3):67–70
Bukovcan P, Koller J (2010) Treatment of partial-thickness scalds by skin xenografts – retrospective study of 109 cases in the three- years period. Acta Chir Plast 52(1):7–12
Moserová J, Bĕhounková E, Vrabec R, Svátek V (1974) Methods of collection and storage of porcine dermoepidermal grafts. Rozhl Chir 53(3):190–194
Brans TA, Hoekstra MJ, Vloemans AFPM, Kreis RW (1994) Long-term results of treatment of scalds in children with glycerol-preserved allografts. Burns 20:10–13
Leicht P, Muchardt O, Jensen M, Alsbjörn BA, Sørensen B (1989) Allograft vs. exposure in the treatment of scalds – a randomized controlled clinical study. Burns 15:1–3
Brown R, Smith KD, Angus McGrouther D (1997) Strategies for cell engineering in tissue repair. Wound Rep Reg 5:212–221
Git M, Toda K, Grinell F (1990) Activation of human keratinocyte migration on type 1 collagen and fibronectin. J Cell Sci 96:197–205
Ge L, Sun L, Chen J, Mao X, Kong Y, Xiong F, Wuc J, Wei H (2010) The viability change of pigskin in vitro. Burns 36:533–553
Brož L, Vogtová D, Königová R (1999) Experience with banked skin in the Prague burn centre. Acta Chir Plast 41:54–58
Atiyech BS, Hayek SN, Gunn SW (2005) New technologies for burn wound closure and healing – Review of the literature. Burns 31:94456
Pirnay JP, Vandenvelde C, Duinslaeger L, Reper P, Vanderkelen A (1997) HIV transmission by transplantation of allograft skin: a review of the literature. Burns 23(1):1–5
Kealey GP (1997) Disease transmissions by means of allograft. J Burn Care Rehabil 18:S10–S11
Hermans MHE (2014) Porcine xenografts vs. (cryopreserved) allografts in the management of partial thickness burns: Is there a clinical difference? Burns 40:408–415
Csonge L, Pellet S, Szenes A, Istvan J (1995) Antibiotics in the preservation of allograft and xenograft skin. Burns 21(2):102–105
Boneva RS, Folks TM (2004) Xenotransplantation and risks of zoonotic infections. Ann Med 36(7):50
Wilson CA (2008) Porcine endogenous retroviruses and xenotransplantation. Cell Mol Life Sci 65(21):3399–3412
Healy CMJ, Boorman JG (1989) Comparison of E-Z Derm and Jelonet dressings for partial thickness burns. Burns 15(1):52–54
Ersek RA, Gadaria U, Surak G, Denton DR (1986) A new wound dressing that solves the retention and storage problems: a clinical history. Contemp Surg 28(3):72–79
Chiu T, Pang P, Ying SY, Burd A (2004) Porcine skin: friend or foe? Burns 30:739–741
Zajicek R, Matouskova E, Broz L, Kubok R, Waldauf P, Königova R (2011) New biological temporary skin cover Xe-Derma® in the treatment of superficial scald burns in children. Burns 37:333–337
Singh R, Purohit S, Chacharkar MP, Bhandari PS, Bath AS (2007) Microbiological safety and clinical efficacy of radiation sterilized amniotic membranes for treatment of second-degree burns. Burns 33:505–510
Rab M, Koller R, Ruzicka M, Burda G, Kamolz LP, Bierochs B, Meissl G, Frey M (2005) Should dermal scald burns in children be covered with autologous skin grafts or with allogeneic cultivated keratinocytes?—“The Viennese concept”. Burns 31:578–586
Ghaffari A, Kilani RT, Ghahary A (2009) Keratinocyte-conditioned media regulate collagen expression in dermal fibroblasts. J Invest Dermatol 129(2):340–347
DiPersio CM, Zheng R, Kenney J, Van De Water L (2016) Integrin-mediated regulation of epidermal wound functions. Cell Tissue Res 365:467–482
Ou LF, Lee SY, ChenYC YRS, Tang YW (1998) Use of biobrane in pediatric scald burns – experience in 106 children. Burns 24:49–53
Demling RH, DeSanti L (1999) Management of partial thickness facial burns (comparison of topical antibiotics and bio-engineered skin substitutes). Burns 25:256–261
Tompkins RG, Burke JF (1992) Burn wound closure using permanent skin replacement materials. World J Surg 16:47–52
Alsbjörn B, Gilbert P, Hartmann B, Kaźmiersky M, Monstrey S, Palao R, Roberto MA (2007) Van TrierA, VoinchetV. Guidelines for the management of partial-thickness burns in a general hospital or community setting – Recommendations of a European working party. Burns 33:155–160
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Bukovčan, P., Koller, J. (2017). Treatment of Partial-Thickness Scalds by Skin Xenografts. In: Shiffman, M., Low, M. (eds) Burns, Infections and Wound Management. Recent Clinical Techniques, Results, and Research in Wounds, vol 2. Springer, Cham. https://doi.org/10.1007/15695_2017_28
Download citation
DOI: https://doi.org/10.1007/15695_2017_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-10685-0
Online ISBN: 978-3-030-10686-7
eBook Packages: MedicineMedicine (R0)