Abstract
Burns are common injuries. Advances in treatment during the last decades resulted in ever-decreasing mortality rates. Severe burns still pose a challenge to burn care specialists: large areas of tissue loss require coverage with split-thickness skin grafts, while there is a limited availability of donor sites. The higher the initial graft take rate, the quicker a patent skin barrier is restored and the higher is the chance of survival. Topical negative pressure (TNP) dressings have been seen to beneficially influence wound healing by various means: perfusion is enhanced, granulation tissue forms rapidly, and wound infection can be prevented. Furthermore, fixation of skin grafts with TNP seems to be superior to conventional bolster dressings. In the severely burned, skin graft take rates above 90% can reliably be achieved with TNP resulting in higher survival rates and improvement of the final functional and aesthetic outcome.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Burns are common and devastating injuries, often accompanied by severe physical and psychological impairment. Advances in burn care have effected a steady decline of burn mortality rates during the past decades [1]. While until the mid-twentieth century, the burn eschar was left to be digested by bodily and bacterial enzymes, the 1970s brought the early excision of the burns. This paradigm change in the approach to burn wounds resulted in a dramatic increase of survival rates after burn injuries [2].
Nowadays, extensive burns remain a challenge for burn surgeons for two main reasons: on the one hand, large areas of tissue loss require rapid coverage with autologous skin transplants, while on the other hand, there is a limited availability of donor sites [3]. The chance of complete graft take decreases with increasing percentage of burned total body surface area (TBSA). While in burns smaller than 35% TBSA take rates average 95%, the mean take rate in burns exceeding 35% TBSA is much lower [4].The chance of survival is closely related to the success of early skin grafting. Not only does a restored skin barrier prevent from further fluid and temperature loss and life-threatening hypothermia. Untreated burn wounds represent the ideal habitat for microorganisms and consequently act as origin for bloodstream infections. Today, septic complications are the most common cause of death after burns [2]. Prolonged and incomplete graft healing furthermore facilitates the development of hypertrophic scars and contractures which further impair the functional and aesthetic outcome of the injury [5].
High skin graft take rates are of utmost importance and determine the course of the recovery process to significant extent. Topical negative pressure (TNP) has found various applications in wound management since its introduction in the 1990s [6]. Due to its healing-promoting properties, it was soon proposed as method for skin graft fixation [7]. In acute burns, TNP dressings have been found superior to gauze bandages with regard to burn wound progression [8]. Skin graft fixation in burns using TNP helped to achieve good wound healing results [9].
2 General Effects of Topical Negative Pressure (TNP)
TNP wound therapy was first described by Morykwas and Argenta in 1997 [10, 11]. The TNP dressing consists of a reticulated open-cell polyurethane foam that is fit into the wound and sealed toward the surrounding with an adhesive occlusive drape. Negative pressure is then applied through a specialized tubing system and a suction device. Subsequently wound exudate is removed, and the wound tissue is exposed to mechanical forces exerted by the subatmospheric pressure [6]. Since its upcoming the technique has been used for wounds of various etiologies, and different mechanisms and effects on wound healing have been discovered. Firmly established are the effects of TNP on local blood flow, formation of granulation tissue, and its regulatory influence on the microbiome of the wound [12]. Table 1 provides an overview over the known effects of TNP on wound healing.
3 Tissue Perfusion and Edema Reduction
The effects of TNP on microcirculation have been investigated in various models. Many of the wound-healing-promoting properties of TNP are being deduced on its perfusion-altering properties.
In an early animal trial with pigs, random-pattern flap survival was significantly increased (by 20% on average) when −125 mmHg TNP was applied. This effect was mainly interpreted as the result of improved microcirculation. The effect has been seen to be pressure dependent. At −125 mmHg TNP, blood flow increased, whereas it was decreased at −400 mmHg [10]. This increase in blood flow seems to be accompanied by morphologic changes in the tissue, including increased vascular diameter, increased angiogenesis, and enhanced endothelial proliferation [13]. The changes in blood flow are, however, unevenly distributed across the wound and also depend on the type of tissue. There is a zone of relative hypoperfusion close to the wound margins, while approximately two centimeters from the wound perfusion increases to its maximum before returning to baseline levels again in the periphery. In musculature the zone of relative hypoperfusion is comparably small, while there is a strong increase in perfusion. In subcutaneous tissue, the increase in blood flow is less pronounced, and the zone of relative hypoperfusion is larger. Blood flow at the wound edges increases multifold when TNP is discontinued—an effect that is most likely the result of reactive hyperemia [14, 15]. There is evidence that negative pressures between −80 mmHg and the clinical standard of −125 mmHg can be considered as equivalent regarding wound perfusion [16]. Recent research explored novel and potentially promising indications for TNP treatment with regard to tissue perfusion. TNP doubled microvascular blood flow in normal, ischemic, and reperfused myocardium in pigs [17].
Although it is not yet fully understood how TNP causes improved microcirculation, it seems feasible that the applied subatmospheric pressure creates an interstitial fluid gradient and reduces edema. Thus the extrinsic cause for impaired vascular perfusion is removed [10]. So far this hypothesis however lacks profound scientific foundation—also because tissue edema is notoriously difficult to quantify. Nonetheless, a reduction of edema can be observed clinically [6, 11, 18], and recent findings also suggest that TNP-treated tissue is “dryer” than untreated and has greater fluid storage potential [19].
4 Tissue Proliferation and Cellular Response
According to Wolff’s law established in the late 1800s, all living tissues react to mechanical stresses in dynamic fashion. While pressure results in tissue atrophy with only few exceptions (e.g., epiphyseal cartilage), tensile forces promote tissue proliferation. Similarly also TNP exerts tractive effort on the wound surface. For example, full-thickness skin defects in pigs treated with −125 mmHg TNP were completely filled with granulation tissue after 8 days. During that time, wounds treated with −25 mmHg were filled up to 20% with granulation tissue, and wounds treated with −500 mmHg were filled up to just 6% with granulation tissue. At this pressure also a significant thickening of the wound walls occurred, resulting in artificial deepening of the defect [20]. On the other hand, also improper handling of TNP dressings can have deleterious consequences: when an air leak was simulated in −125 mmHg TNP dressings, experimental wounds in pigs increased almost twofold in size [20]. On the molecular level, TNP seemed to reduce concentrations of pro-matrix metalloproteinase-9 (pro-mmp-9) as well as the active enzyme mmp-9 in chronic and acute wounds. Mmp-9 is a type IV collagenase, and high levels are associated with prolonged wound healing. TNP also helped to maintain a low ratio of mmp-9 to the tissue inhibitor of metalloproteinases 1 (TIMP-1) in the wound. A low ratio of mmp-9/timp-1 is associated with improved wound healing [21].
5 Effects on Wound Microbiome
TNP is often referred to as a mean for maintaining a clean wound surface by removing exudates. However, also regulatory effects on the wound microbiome seem to exist. A significant reduction of bacteria counts in experimental wounds in pigs occurred after application of −125 mmHg TNP for 4 days [10]. It seemed as if the application of TNP had the ability to prevent systemic spread of local infections. In a murine burn wound sepsis model, full-thickness scalds contaminated with Pseudomonas aeruginosa inocula received either wet-to-dry dressings or TNP therapy. After 2 weeks of treatment, 33% of TNP-treated mice had survived compared to 10% in the wet-to-dry dressings group [22].
Observations from in vitro models support the aforementioned findings: reduced growth rates and loss of the ability to create biofilms were observed when Staphylococcus aureus was cultured under −125 mmHg negative pressure conditions [23]. TNP application to biofilm forming P. aeruginosa for 2 weeks resulted in a small but statistically significant reduction of bacterial counts compared to untreated controls. Silver-impregnated TNP foam speeded this process up, and the difference reached significance after only 24 h [24]. The synergistic effects of silver-impregnated foam and TNP application however depended on the bacterial strains used. Application of silver foam alone already led to a significant reduction of S. aureus and MRSA counts, whereas the effects on P. aeruginosa and Staphylococcus epidermidis were negligible until TNP is added [25].
6 Treatment of Acute Burns with TNP
According to Jackson’s [26] original definition of tissue damage in burn wounds, a burn consists of three concentric zones: the innermost coagulation zone is characterized by irreversible tissue damage. Next to it there is the hypoperfused zone of stasis, where both viable and necrotic cells are present, which is followed by the zone of hyperemia that is characterized by active edema formation. The zone of stasis and the zone of hyperemia are at risk for necrosis but potentially salvageable if perfusion can be kept upright. When progressive edema and local inflammation further impair vascular flow in these two zones, thrombotic obliteration of the affected vessels results in necrosis of formerly viable tissue [27].
The process of burn wound progression usually occurs during the first 3–5 days after trauma. During this time frame, a burn that has initially been diagnosed to be superficial may progress in size and depth to a deep dermal or full-thickness skin defect requiring surgery and skin grafting. Consequently burn wound progression significantly adds to the initial morbidity of the injury [28].
Due to its perfusion-altering effects, TNP has been ascribed the potential of counteracting burn wound progression [8, 29]. The effects of TNP on acute burns have been studied both in animals [30] and humans [8, 29, 31]. The main influence of TNP on burn wounds seems to lie in the reduction of burn wound edema which in turn increases blood flow (Fig. 1) [8, 29].
7 Observations from Animal Trials
Application of −125 mmHg TNP on burn wounds in pigs for 6 h resulted in a significantly decreased maximum depth of cellular death below the wound surface when compared to controls treated with sterile dressings. Thereby it seemed as if TNP had greatest efficacy when applied early after the injury [30]. The tissue-preserving effect of TNP is probably based in part on its ability to restore the integrity of damaged collagen fibers. Burns are accompanied by denaturation of collagen strands within the damaged tissue. It is likely that the surface tension exerted by unfolded collagen fibers causes a fluid gradient from the vasculature to the extracellular space [32, 33]. Explants of burned skin from pigs treated with −125 mmHg TNP in situ presented with a fluid storage capacity similar to that of unsevered tissue when put into a water bath. This finding was attributed to the mechanical compression of dermal structures that prevents fluid influx in situ. This in turn probably reduced swelling and further unfolding of damaged collagen [19].
8 Clinical Observations
Clinical studies investigated the use of TNP to prevent burn wound progression in hand burns [8, 29]. Burn wound perfusion was the primary endpoint and monitored using indocyanine green angiography (ICGA). The technique allowed for objective qualitative and quantitative measurement of vascular perfusion. Indocyanine green dye was injected intravenously, and vascular dye uptake in the region of interest was recorded with a specialized video camera using dynamic laser-fluorescence angiography. The maximum recorded pixel intensity was assessed to quantify wound perfusion. ICGA accurately predicts survival in the zone of ischemia [34] and can be used to identify areas requiring surgical intervention [35, 36].
In the studies published [8, 29], patients with bilateral second-degree hand burns were assigned to the study protocol within 6 h after trauma. The more severely injured hand received −125 mmHg TNP therapy, while the other was treated with sulfadiazine cream and conventional gauze dressings. For sufficient and easy coverage of the treated hand, a special TNP glove was used (Fig. 2). ICGA measurements were carried out upon admission and daily thereafter. If burn wound progression necessitated surgery, conservative treatment was discontinued. Surgical therapy consisted of a combination of tangential excision and split-thickness skin grafting or dermabrasion and autologous keratinocyte application.
In conventionally treated hands, vascular perfusion as assessed with ICGA was significantly decreased on days 1–3 after trauma relative to baseline levels. In TNP-treated hands, vascular perfusion could be maintained, i.e., did not decrease compared to baseline levels [8, 29]. Also dye uptake, meaning the time from intravenous injection of indocyanine green dye to maximum pixel intensity recorded, was significantly slower in controls than in treated hands [8, 29]. In addition, skin grafting was required less often in TNP-treated hands. Clinically an impressive reduction of edema was observed [8].
According to case studies, TNP may also have beneficial effects on frostbites. Poulakidas et al. [37] treated an adult male with frostbites to one foot. Although the wounds were in a detrimental state upon admission and TNP therapy was initiated 72 h after trauma, all wounds had healed after 4 weeks. In children with frostbites to the hands, TNP therapy helped to prevent the loss of digits. Follow-up X-rays revealed that full integrity of epiphyseal cartilages could be preserved. Therefore, TNP therapy may be a method to prevent long-term complications in pediatric frostbites [38].
9 Skin Engraftment and TNP to Enhance Skin Graft Take Rates
During the first days after grafting, the skin transplant is nourished by diffusion of nutrients from the wound bed. In this phase of treatment, the parts of the transplant that are not in contact with the wound bed will not take, i.e., die off. After 48–72 h, the vessels from the wound bed connect to existing graft vasculature, and reperfusion sets in [39, 40]. During the following days, the autochthonous graft vasculature is successively replaced by sprouting vessels from the wound bed, and the skin graft is incorporated [41, 42]. Seroma or hematoma formation underneath the graft may hinder successful engraftment, and shearing forces may disrupt the early vascular connections between transplant and wound bed. Local infection often has detrimental consequences and may result in complete graft loss. Desiccation must be avoided by all means [43].
TNP combines all features required for ideal skin graft treatment in one application: immobilization of the graft and ensuring close contact to the wound bed, maintenance of a moist wound milieu, and reduction of bacterial load [6]. Possibly TNP alters diffusion of nutrients from the wound bed to the skin graft [44]. First clinical experiences with TNP for skin graft fixation have been made by Schneider et al. [7]. The authors found that TNP was especially useful in difficult recipient areas and is applicable to a wide range of wounds. Results from later clinical studies support this rationale.
In an early case study, the use of TNP dressings for split-thickness skin graft fixation in chronic leg ulcers was described. After only 5 days of treatment, take rates ranged from 95 to 100% [45]. Because there was no representative control group, no conclusions could be drawn concerning the superiority of TNP over conventional dressings. A later report compared conventional dressings and TNP for skin graft fixation in wounds of different etiology in 61 consecutive patients. Although there were no differences concerning take rates, patients in the TNP arm of the study required re-grafting significantly less often [43]. A retrospective analysis compared the outcome of meshed split-thickness skin grafts in 74 leg ulcers before and after implementation of TNP dressings. The mean take rate in the TNP-treated group (93%) was significantly higher than that of the group managed with conventional dressings (67%). No severe side effects of TNP were reported; however, occasional discomfort occurred in TNP-treated areas when suction was applied, and some patients reported sleeping problems because of the noises of the electric pump [46].
Currently, few randomized controlled trials exist that compare TNP and conventional skin graft bolster dressings. Moisidis et al. [47] reported increased or equal take rates in 75% of TNP-treated grafts compared to conventional dressings. In a subjective evaluation, TNP-treated grafts were also considered qualitatively equal or better in 85% of the cases [47]. A randomized, double-masked, controlled trial reported significantly better take rates, significantly shorter hospitalization times, and significantly fewer reoperations in skin-grafted patients treated with TNP [48]. In a recent analysis of patients receiving skin grafts for burn wound coverage or secondary reconstruction, equally significant higher take rates were seen when TNP was used. TNP-treated grafts also healed faster, so they required dressings for a significantly shorter period of time [49].
TNP can also be used to prepare wound beds for skin grafting. While full-thickness skin grafts have advantages over split-thickness skin grafts—including better skin pliability, lower shrinking tendency, and better color match—high take rates cannot be achieved as reliably. Preconditioning of wound beds with TNP prior to full-thickness skin grafting resulted in average take rates of 95% even in large wounds [50].
10 Technique
Various methods to enhance graft take in burns have been proposed, including fibrin sealant [51], medical honey [52], or amniotic membrane [53]. Large burns requiring skin grafting represent a prime indication for TNP wound therapy in a way for two reasons: (1) especially second-degree burns go along with significant water evaporation, and (2) local and generalized edema compromise vascular perfusion of the wound bed [8, 54]. With the knowledge that TNP dressings allow for wound drainage, enhance perfusion of burn wounds through edema reduction, and improve skin graft take rates, TNP has been applied for skin graft fixation in burns on several occasions [3, 9, 55,56,57,58,59].
11 Experiences with TNP for Skin Graft Fixation in Burn Patients
Table 2 gives an overview over the results of clinical studies and case reports published on the topic. In general, good experiences have been made with the technique, and in all studies, the reported take rates were above 90% even in the severely burned [9, 55,56,57,58,59].
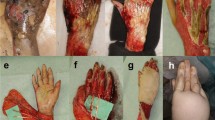
Schintler et al. [55] were the first to describe the use of TNP for skin graft fixation in extensive burns. A 6-year-old boy with 40% TBSA deep flame burns on the neck, the trunk, and the right arm was treated with fascial-level excision and split-thickness skin grafts meshed 1:2. All grafted areas were treated with TNP for 5 days; 7 days after surgery take rate was 100% (Figs. 3 and 4). Throughout the whole course of the therapy, the patient was alert and in an unexpectedly good general condition [55].
Take rate is almost 100% in the same patient (Fig. 3) 5 days postsurgery
Roka et al. [56] as well as Kamolz et al. [9] further explored the use of TNP for skin graft fixation in larger cohorts. Roka described a cohort of 29 patients with second- to third-degree burns and a mean Baux score of 78.6%, while Baux scores exceeded 100 in six patients. The mean grafted TNP-treated TBSA was 20.2%, and the median time of TNP treatment was 4 days. The survival rate was 97%—one male with a Baux score of 121 succumbed to his injuries, while one female with a Baux score of 127 however survived. There was no data available on take rates, but it was noted that all survivors could be discharged with completely healed wounds after a mean hospitalization time of 46.6 days [56]. The cohort of 37 patients described by Kamolz [9] received TNP dressings for at least 25% grafted TBSA. Although Baux scores in this cohort were higher (mean 88.6, above 100 in eight patients) and treatment modalities did not differ significantly from those of Roka et al., hospitalization time (46.6 days) and survival rate (97%) remained the same. Of 37 patients, 36 could be discharged, and the skin graft take rate was 95% and above in all cases (Fig. 5) [9]. Notably, in neither study any TNP-related complications such as severe wound site infections were described [9, 56].
Hoeller et al. [57] reviewed 60 pediatric burn patients who had received TNP for skin graft fixation. The mean burned TBSA was 4.5%, and children of all age groups—infants (<24 months), toddlers/preschoolers (2–6 years), school-age children (6–13 years), and adolescents (>13 years)—were analyzed. With 3.5% the mean TNP-treated TBSA was comparably small. After a median TNP application time of 5 days, the mean take rate was 96%. It was found that smaller grafted TBSA resulted in better take rates. In contrast the amount of TNP-treated TBSA had no influence on take rates. Three major complications were noted. In two cases major bleeding occurred hours after TNP installation which led to termination of therapy. Take rate was 95% in both cases though. In one case a bigger part of the skin graft became necrotic leading to the worst take rate in the cohort of 70%. Minor alterations were observed in 17 cases, of which one was contamination of a TNP system with stool after installation in the anogenital region. Nevertheless the mean take rate in this subgroup was 80%.
Psoinos et al. [59] reported a case of an 8-month-old female who sustained scalds averaging 6% TBSA to her lower back and the buttocks. Four percent of the wound area, mainly located in the gluteal region, required excision and skin grafting. After 5 days of TNP treatment, take rate was 100%. In this case TNP application was found especially useful to avoid fecal contamination of the grafted areas on the buttocks. Instead of the conventional foam dressing, gauze was used as wound filler material.
In another study with 12 patients, skin graft recipient sites as well as donor sites were covered with TNP dressings resulting in an average TNP-treated TBSA of 35.1%. Average graft take rate was 97%, and all patients survived. Although this data was available only for four patients, the reported median epithelialization time of donor sites after TNP treatment was 11 days. No major side effects were described. However, one patient developed acute kidney injury during the course of TNP treatment [58].
12 Method of Application
After depth-adapted burn wound debridement, split-thickness skin grafting is performed. Mesh grafts are fixed to the wound surface with staples. When Meek grafts are used, the silken carrier sheets are tacked down accordingly [3]. The grafts are then covered with sterile fatty gauze or other non-adherent dressings such as soft silicone wound contact layer [55]. This is done to prevent adherence of the TNP foam to the skin graft. The sterile polyurethane TNP foam dressing is clipped in shape to fit the wound dimensions and placed atop the non-adherent dressing. Most manufacturers provide foam dressings of various sizes [58], and for coverage of the entire hand, also special TNP gloves are available (Fig. 2) [8]. The entire area is covered with the occlusive transparent drape that is usually provided with the foam. Alternatively any available occlusive drape can be used, like Opsite [49]. An opening is cut into the film for the suction tubing, and a continuous pressure between −75 and −125 mmHg is applied (Fig. 6).
When large areas have to be covered or when the wound lies in a region of complex contour that is subject to repeated motion—such as the axilla, the groins, or the circumference of the arm—the foam is stapled to the wound surface (Fig. 7). If more than one foam piece is required to cover the wound, the individual foam pieces are connected to one another with staples.
Large TNP dressings or multiple wound surfaces may necessitate the use of more than one negative pressure pump [9, 56]. In some cases it may be feasible though to connect TNP dressings on wound sites that are separated by viable skin to one another with thin polyurethane foam strips. To prevent direct contact with the skin and consequent maceration, the strips are wrapped up in occlusive drape. With this technique negative pressure can be distributed evenly to all wound sites, and resources can be saved [60].
When a tight seal cannot be achieved right away, it has proven useful to apply negative pressure temporarily with surgical suction units before switching to the portable TNP device. This proceeding allows for investigation of the dressing for leakages and also effectively removes the first portion of wound exudate from the dressing [9]. Some favor the technique of stapling the occlusive drape to the skin in the wound periphery to guarantee its adherence in mobile body parts [58]. In most cases a sufficient seal can be achieved without this approach though [9].
Application of TNP dressings to the fingers, hands, toes, and feet can
be difficult. Even with the usually very pliable occlusive drape, a tight seal may be hard to achieve in web spaces. When special TNP gloves are not readily available, a good method to overcome those difficulties is the “sterile glove technique.” Here a conventional sterile surgical glove is used instead of the occlusive drape. After the foam dressing has been applied, the hand or foot is dressed with a sterile glove. In small defects a single tight-fitting glove often suffices to seal the dressing. In larger defects, the glove dressing may require completion with a piece of occlusive drape proximally. An opening for the TNP suction tubing is created as usual. When using this technique, the proximal rubber band of the glove should always be removed because it has been seen to cause pressure marks. The fingertips of the glove can be cut off to monitor perfusion of the digits. When applied with care, this technique is very efficient, and strangulation of digits is unlikely. The method is, however, not recommended in patients with preexisting vascular diseases [61].
13 Additional Benefits of TNP for Skin Graft Fixation in Burns
The TNP foam becomes rigid when negative pressure is applied. In certain body parts, this makes an additional splint unnecessary because enough suspension is provided by the dressing. Burned hands can effectively be immobilized in the intrinsic plus position solely by using TNP gloves (Fig. 2) [8, 29].
Despite their splint-like effect, TNP dressings retain part of their flexibility also after negative pressure activation. This allows for active mobilization of joints also during the early stages of therapy [3]. Successful early mobilization with TNP dressings in place is possible in adults as well as in children [9, 55,56,57]. Especially in babies and toddlers, where compliance cannot be expected and there is a high level of activity, mobilization can be allowed unscrupulous with TNP dressings holding the skin grafts firmly in place. Portable TNP devices furthermore give children who would normally be bed bound opportunity to play outside the bed [57]. In some cases it may be necessary to spare particular body regions from TNP application to facilitate mobilization. In circular burns to the lower limbs, sparing the popliteal fossa may increase the range of motion. To further soften the dressings, negative pressure can be reduced to −50 mmHg or turned off temporarily [58].
Because the TNP system removes blood and exudates from the wound surface and stores it in a container, a relatively clean dressing can be guaranteed for a longer period of time. As a result dressing changes are required less often. The dressing can be left in place from 4 up to 7 days [50, 56]. The fluid captured in the container also allows for indirect evaluation of the wound. A significant amount of blood in the container may indicate hemorrhage, and purulent fluid could be an early sign for wound infection [56].
Additionally, the fluid output of the TNP system can be used as an orientation for fluid requirements. When wound healing progresses and the skin barrier is restored by reepithelialization emerging from the skin grafts, the amount of drainage fluid gradually decreases [55]. To estimate total evaporative fluid loss, skin grafts as well as donor sites may be covered with TNP dressings. In general, the fluid output of grafted areas is smaller than that of conservatively managed burn wounds or that of donor sites [8, 58].
TNP dressings are comparably expensive. In the long run, TNP may however have financial advantages over conventional wound dressings. Since TNP dressings have to be changed less often, less analgesics, sedatives, and also qualified health-care personnel are needed [56]. Higher initial take rates also decrease the risk for complications as well as the need for re-grafting. Complications and frequent reoperations go along with longer hospitalization times which in turn result in higher treatment costs [43, 47]. Consequently the higher costs of TNP therapy are likely to be compensated by better outcome [9, 56].
14 Discussion
With the use of TNP dressings as bolster, high skin graft take rates above 90% can be achieved in adults [9, 56, 58] as well as in children with severe burns [55, 57, 59]. Even in patients with Baux scores exceeding 100, high take rates can be achieved reliably [9, 56]. TNP dressings are especially useful for fixation of large skin grafts in body regions of inferior take rate, such as the gluteal region, the lower back, or the posterior aspect of the thorax [3]. There is no information available on the ideal duration of TNP application. However, most authors reported application times between 4 and 6 days until the first dressing change with satisfactory results [9, 55,56,57].
Despite the vast evidence that TNP interferes actively with wound healing processes, no significant side effects are known. In the treatment of burns, hardly any TNP-related complications have been described, and in all of them, a direct association to the wound dressings was questionable. By and large the reported events did not affect the final outcome, and all complications could be salvaged [57, 58].
The studies on TNP application for skin graft fixation in burns lack a representative control group in all cases. Still, randomized controlled trials on TNP for skin graft fixation in wounds of different etiology showed the beneficial effects of TNP on take rates, even when the dressing had to be improvised [49]. Concerning the quality of data, objective methods for wound assessment were not used in most of the cases.
Two studies [9, 56] used specialized burn size assessment software (BurnCase 3D, RISC Software GmbH, Linz, Hagenberg, Austria [62]) for estimation of grafted TBSA as well as take rates and thus provided objective data.
Not only the healing of skin grafts but also take rates of dermal substitutes can be improved by using TNP. The use of TNP has been seen to allow for earlier skin grafting in substitutes requiring a two-step procedure, such as Integra®. This in turn reduced the length of hospital stay and should on the long run decrease the risk for complications due to faster wound healing [63].
Currently TNP gauze dressings experience a revival with wound healing results similar to those of the usual foam dressings [59, 64]. Gauze dressings use smaller negative pressures of about −80 mmHg. In surgical wounds of different etiology, TNP gauze dressings produce an overall volume reduction of 15.1% per week—a value comparable to that of the foam alternative [64]. There is also evidence that gauze dressings produce less pain and discomfort around the wound site than foam dressings [65]. This makes TNP gauze a user-friendly alternative to foam dressings worthwhile considering. With regard to burns, TNP gauze dressings have been applied for skin graft fixation with good success in a pediatric burn patient [59]. It remains to be seen how TNP gauze will impact future burn wound management. First results are encouraging though.
Conclusions
TNP dressings can be used for skin graft fixation to reliably achieve take rates above 90% in severe burns. The application rarely leads to complications, and reported adverse events are manageable. Additional benefits of TNP comprise a splinting effect in extremity burns, a reduced need for dressing changes, the possibility of early mobilization without the risk of graft shear, and a good acceptance by patients.
References
Smolle C, Cambiaso-Daniel J, Forbes AA, Wurzer P, Hundeshagen G, Branski LK et al (2017) Recent trends in burn epidemiology worldwide: a systematic review. Burns 43:249–257
Kamolz LP (2010) Burns: learning from the past in order to be fit for the future. Crit Care 14:106
Lumenta DB, Kamolz LP, Frey M (2009) Adult burn patients with more than 60% TBSA involved-meek and other techniques to overcome restricted skin harvest availability—the Viennese concept. J Burn Care Res 30:231–242
Thourani VH, Ingram WL, Feliciano DV (2003) Factors affecting success of split-thickness skin grafts in the modern burn unit. J Trauma 54:562–568
Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, Herndon DN (2016) Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet 388:1427–1436
Schintler MV (2012) Negative pressure therapy: theory and practice. Diabetes Metab Res Rev 28(Suppl 1):72–77
Schneider AM, Morykwas MJ, Argenta LC (1998) A new and reliable method of securing skin grafts to the difficult recipient bed. Plast Reconstr Surg 102:1195–1198
Kamolz LP, Andel H, Haslik W, Winter W, Meissl G, Frey M (2004) Use of subatmospheric pressure therapy to prevent burn wound progression in human: first experiences. Burns 30:253–258
Kamolz LP, Lumenta DB, Parvizi D, Wiedner M, Justich I, Keck M, Pfurtscheller K, Schintler M (2014) Skin graft fixation in severe burns: use of topical negative pressure. Ann Burns Fire Disasters 27:141–145
Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W (1997) Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 38:553–562
Argenta LC, Morykwas MJ (1997) Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 38:563–576
Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J (2006) Vacuum-assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg 117:121s–126s
Chen SZ, Li J, Li XY, Xu LS (2005) effects of vacuum-assisted closure on wound microcirculation: an experimental study. Asian J Surg 28:211–217
Wackenfors A, Sjögren J, Gustafsson R, Algotsson L, Ingemansson R, Malmsjö M (2004) Effects of vacuum-assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen 12(6):600
Wackenfors A, Gustafsson R, Sjögren J, Algotsson L, Ingemansson R, Malmsjö M (2005) Blood flow responses in the peristernal thoracic wall during vacuum-assisted closure therapy. Ann Thorac Surg 79:1724–1730
Borgquist O, Ingemansson R, Malmsjö M (2010) Wound edge microvascular blood flow during negative-pressure wound therapy: examining the effects of pressures from -10 to -175 mmHg. Plast Reconstr Surg 125:502–509
Lindstedt S, Malmsjö M, Ingemansson R (2007) Blood flow changes in normal and ischemic myocardium during topically applied negative pressure. Ann Thorac Surg 84:568–573
Banwell PE, Musgrave M (2004) Topical negative pressure therapy: mechanisms and indications. Int Wound J 1:95–106
McGee MP, Morykwas M, Campbell D, Hoge K, Argenta L (2014) Interstitial-matrix edema in burns: mechanistic insights from subatmospheric pressure treatment in vivo. Wound Repair Regen 22:96–102
Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC (2001) Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg 47:547–551
Mouës CM, van Toorenenbergen AW, Heule F, Hop WC, Hovius SER (2008) The role of topical negative pressure in wound repair: expression of biochemical markers in wound fluid during wound healing. Wound Repair Regen 16:488–494
Liu Y, Zhou Q, Wang Y, Liu Z, Dong M, Wang Y, Li X, Hu D (2014) Negative pressure wound therapy decreases mortality in a murine model of burn-wound sepsis involving Pseudomonas aeruginosa infection. PLos One 9:e90494
Li T, Wang G, Yin P, Li Z, Zhang L, Liu J, Li M, Zhang L, Han L, Tang P (2015) Effect of negative pressure on growth, secretion and biofilm formation of Staphylococcus aureus. Antonie Van Leeuwenhoek 108:907–917
Ngo QD, Vickery K, Deva AK (2012) The effect of topical negative pressure on wound biofilms using an in vitro wound model. Wound Repair Regen 20:83–90
Valente PMD, Deva A, Ngo Q, Vickery K (2016) The increased killing of biofilms in vitro by combining topical silver dressings with topical negative pressure in chronic wounds. Int Wound J 13:130–136
Jackson DM (1953) The diagnosis of the depth of burning. Br J Surg 40:588–596
Singh V, Devgan L, Bhat S, Milner SM (2007) The pathogenesis of burn wound conversion. Ann Plast Surg 59:109–115
Johnson RM, Richard R (2003) Partial-thickness burns: identification and management. Adv Skin Wound Care 16:178–187
Haslik W, Kamolz LP, Andel H, Meissl G, Frey M (2004) The use of subatmospheric pressure to prevent burn wound progression: first experiences in burn wound treatment. Zentralbl Chir 129(Suppl 1):s62–s63
Morykwas MJ, David LR, Schneider AM, Whang C, Jennings DA, Canty C, Parker D, White WL, Argenta LC (1999) Use of subatmospheric pressure to prevent progression of partial-thickness burns in a swine model. J Burn Care Rehabil 20:15–21
Molnar JA, Simpson JL, Voignier DM, Morykwas MJ, Argenta LC (2005) Management of an acute thermal injury with subatmospheric pressure. J Burns Wounds 4:e5
McGee MP, Morykwas M, Shelton J, Argenta L (2012) Collagen unfolding accelerates water influx, determining hydration in the interstitial matrix. Biophys J 103:2157–2166
McGee MP, Morykwas MJ, Argenta LC (2011) The local pathology of interstitial edema: surface tension increases hydration potential in heat-damaged skin. Wound Repair Regen 19:358–367
Fourman MS, Phillips BT, Crawford L, McClain SA, Lin F, Thode HC, Dagum AB, Singer AJ, Clark RA (2014) Indocyanine green dye angiography accurately predicts survival in the zone of ischemia in a burn comb model. Burns 40:940–946
Kamolz LP, Andel H, Auer T, Meissl G, Frey M (2006) Evaluation of skin perfusion by use of indocyanine green video angiography: rational design and planning of trauma surgery. J Trauma 61:635–641
Kamolz LP, Andel H, Haslik W, Donner A, Winter W, Meissl G, Frey M (2003) Indocyanine green video angiographies help to identify burns requiring operation. Burns 29:785–791
Poulakidas S, Cologne K, Kowal-Vern A (2008) Treatment of frostbite with subatmospheric pressure therapy. J Burn Care Res 29:1012–1014
Poulakidas SJ, Kowal-Vern A, Atty C (2016) Pediatric frostbite treated by negative pressure wound therapy. J Burn Care Res 37(5):e489–e492
Lindenblatt N, Calcagni M, Contaldo C, Menger MD, Giovanoli P, Vollmar B (2008) A new model for studying the revascularization of skin grafts in vivo: the role of angiogenesis. Plast Reconstr Surg 122:1669–1680
Lindenblatt N, Platz U, Althaus M, Hegland N, Schmidt CA, Contaldo C, Vollmar B, Giovanoli P, Calcagni M (2010) Temporary angiogenic transformation of the skin graft vasculature after reperfusion. Plast Reconstr Surg 126:61–70
Calcagni M, Althaus MK, Knapik AD, Hegland N, Contaldo C, Giovanoli P, Lindenblatt N (2011) In vivo visualization of the origination of skin graft vasculature in a wild-type/gfp crossover model. Microvasc Res 82:237–245
O’Ceallaigh S, Herrick SE, Bennett WR, Bluff JE, Ferguson MWJ, McGrouther DA (2007) Perivascular cells in a skin graft are rapidly repopulated by host cells. J Plast Reconstr Aesthet Surg 60:864–875
Scherer LA, Shiver S, Chang M, Meredith JW, Owings JT (2002) The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg 137:930–933
Wang X, Zhang Y, Han C (2014) Topical negative pressure improves autograft take by altering nutrient diffusion: a hypothesis. Med Sci Monit 20:61–63
Sposato G, Molea G, Di Caprio G, Scioli M, La Rusca I, Ziccardi P (2001) Ambulant vacuum-assisted closure of skin-graft dressing in the lower limbs using a portable mini-VAC device. Br J Plast Surg 54:235–237
Körber A, Franckson T, Grabbe S, Dissemond J (2008) Vacuum assisted closure device improves the take of mesh grafts in chronic leg ulcer patients. Dermatology 216:250–256
Moisidis E, Heath T, Boorer C, Ho K, Deva AK (2004) A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg 114:917–922
Llanos S, Danilla S, Barraza C, Armijo E, Piñeros JL, Quintas M, Searle S, Calderon W (2006) Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double-masked, controlled trial. Ann Surg 244(5):700
Petkar KS, Dhanraj P, Kingsly PM, Sreekar H, Lakshmanarao A, Lamba S, Shetty R, Zachariah JR (2011) A prospective randomized controlled trial comparing negative pressure dressing and conventional dressing methods on split-thickness skin grafts in burned patients. Burns 37:925–929
Landau AG, Hudson DA, Adams K, Geldenhuys S, Pienaar C (2008) Full-thickness skin grafts: maximizing graft take using negative pressure dressings to prepare the graft bed. Ann Plast Surg 60(6):661
Branski LK, Mittermayr R, Herndon DN, Jeschke MG, Hofmann M, Masters OE, Norbury WB, Traber DL, Tangl S, Redl H (2011) fibrin sealant improves graft adherence in a porcine full-thickness burn wound model. Burns 37:1360–1366
Emsen IM (2007) A different and safe method of split thickness skin graft fixation: medical honey application. Burns 33:782–787
Mohammadi AA, Johari HG, Eskandari S (2013) Effect of amniotic membrane on graft take in extremity burns. Burns 39:1137–1141
Lamke LO, Liljedahl SO (1971) Evaporative water loss from burns, grafts and donor sites. Scand J Plast Reconstr Surg 5:17–22
Schintler M, Marschitz I, Trop M (2005) The use of topical negative pressure in a paediatric patient with extensive burns. Burns 31:1050–1053
Roka J, Karle B, Andel H, Kamolz L, Frey M (2007) Use of V.A.C. therapy in the surgical treatment of severe burns: the Viennese concept. Handchir Mikrochir Plast Chir 39:322–327
Hoeller M, Schintler MV, Pfurtscheller K, Kamolz LP, Tripolt N, Trop M (2014) A retrospective analysis of securing autologous split-thickness skin grafts with negative pressure wound therapy in paediatric burn patients. Burns 40:1116–1120
Fischer S, Wall J, Pomahac B, Riviello R, Halvorson EG (2016) Extra-large negative pressure wound therapy dressings for burns - initial experience with technique, fluid management, and outcomes. Burns 42:457–465
Psoinos CM, Ignotz RA, Lalikos JF, Fudem G, Savoie P, Dunn RM (2009) Use of gauze-based negative pressure wound therapy in a pediatric burn patient. J Pediatr Surg 44:e23–e26
Low OW, Chong SJ, Tan BK (2013) The enhanced total body wrap—the new frontier in dressing care for burns. Burns 39:1420–1422
Kamolz L-P, Lumenta DB (2013) Topical negative pressure therapy for skin graft fixation in hand and feet defects: a method for quick and easy dressing application—the ‘sterile glove technique’. Burns 39:814–815
Parvizi D, Giretzlehner M, Wurzer P, Klein LD, Shoham Y, Bohanon FJ, Haller HL, Tuca A, Branski LK, Lumenta DB, Herndon DN, Kamolz LP (2016) BurnCase 3D software validation study: burn size measurement accuracy and inter-rater reliability. Burns 42:329–335
Jeschke MG, Rose C, Angele P, Füchtmeier B, Nerlich MN, Bolder U (2004) Development of new reconstructive techniques: use of Integra in combination with fibrin glue and negative-pressure therapy for reconstruction of acute and chronic wounds. Plast Reconstr Surg 113:525–530
Campbell PE, Smith GS, Smith JM (2008) Retrospective clinical evaluation of gauze-based negative pressure wound therapy. Int Wound J 5:280–286
Fraccalvieri M, Ruka E, Bocchiotti MA, Zingarelli E, Bruschi S (2011) Patient’s pain feedback using negative pressure wound therapy with foam and gauze. Int Wound J 8:492–499
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Smolle, C., Brinskelle, P., Steiner, A., Schintler, M., Kamolz, LP. (2017). Skin Graft Fixation in Severe Burns: Use of Topical Negative Pressure. In: Shiffman, M., Low, M. (eds) Burns, Infections and Wound Management. Recent Clinical Techniques, Results, and Research in Wounds, vol 2. Springer, Cham. https://doi.org/10.1007/15695_2017_24
Download citation
DOI: https://doi.org/10.1007/15695_2017_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-10685-0
Online ISBN: 978-3-030-10686-7
eBook Packages: MedicineMedicine (R0)