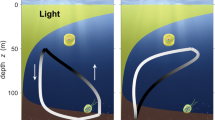
Abstract
Many phytoplankton organisms are denser than their aquatic medium, and so sink relative to the surrounding water. Decreasing cell density relative to the medium allows upward movement, using gas vesicles in cyanobacteria and decreasing the density of the vacuole in large-celled eukaryotes. Upward movement can also occur in organisms with flagella. These three mechanisms can maintain the organisms in the photic zone with a small estimated minimum energy cost relative to those of cell growth (<0.2% in eukaryotic algae and cyanobacteria). When there is limited circulatory water movement in the upper mixed layer the capacity for upward movement relative to surrounding water allows the possibility of periodic vertical migration, making use of opposing gradients of the resources of photon flux density (high near the surface) and nitrogen, phosphorus, and iron (high near the chemocline). For non-flagellate organisms, increased density is needed for the downward portion of the migration. Periodic vertical migration also occurs for flagellates near the surface of muddy or sandy substrates, avoiding excessive light or the possibility of removal in overlying water in tidal habitants. Such movements are also possible in these habitats, and microbial mats on rocky substrata, for cyanobacteria and pennate diatoms with gliding motility. Estimates of the minimum energy cost of periodic vertical migration in these benthic habitats are again a small fraction (10−4%) of the estimated minimum energy cost of growth. High frequency variations in sinking rate of a planktonic marine diatom appear to require a larger fraction of the energy used in cell growth (probably at least 16%) than other mechanisms considered here. The limited data available suggests that the lack of flagella on male gametes of algae (rhodophytes), have little effect on outbreeding and algae diversification. Other dispersal mechanisms (seagrass pollen, wave-driven macrophyte movement) affecting algae outbreeding are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Background
Miyata et al. (2019) propose a phylogeny of motility of organisms, with distinction between active and passive movement. Active movement involves metabolic supply of energy to mechanochemical motors (e.g. flagella: see Appendix) or, in aqueous habitats, regulation of density (Miyata et al. 2019). Passive movement involves prior metabolic inputs to a mechanism that functions after there is no further energy input. Examples are ejection of seeds from the fruits of some legumes upon release of tension produced by desiccation, and wings and parachutes on fruits and seeds (Denny 1993, 2016; Vogel 2020; Miyata et al. 2019; Mann and Lozier 2006). Motion depending on mechanochemical motors potentially, when combined with sensory inputs and signaling pathways, allows directional movement relative to the substratum for movement on surfaces, or for unattached organisms, the moving fluid environments. Motion by buoyancy changes in water only allows movement relative to the force of gravity. Passive movement through fluids does not permit directed movement.
In the case of planktonic photosynthetic organisms, motion relative to the surrounding water using mechanochemical motors (flagella in eukaryotes) or changes in density (ballast; gas vesicles in cyanobacteria, modulation of vacuolar density in eukaryotes) has roles in resource acquisition including movement through the opposing vertical gradient of photosynthetically active radiation and dissolved nutrients such as phosphate and combined nitrogen. For low concentrations of nutrients, motion relative to the surrounding water can increase availability by decreasing the thickness of the diffusion boundary layer. Vertical movement can help to avoid abiotic factors that can limit productivity, e.g. ultraviolet radiation, and biotic factors such as grazers and viruses that remove phytoplankton organisms. Flagellar motility has an important role in sexual reproduction in phytoplankton species with flagellate gametes. Flagellar and gliding motility in benthic microalgae can change position of cells in relation to, for example, the much steeper photosynthetically active radiation gradient in microbial mats and in sediments. Changes in cell density are less significant in benthic microalgae than in phytoplankton. The phenomena discussed in this paragraph are examined in more detail in the body of this paper.
For attached macroscopic organisms, in addition to dispersal by gametes (or structures that carry gametes, e.g. pollen) or products of sexual reproduction, there is also the possibility of lateral vegetative (asexual) spread by elongation growth or detached parts (Collado-Vides 2001). Again, these topics are considered in detail in the rest of the paper.
2 Classification of Aquatic Oxygenic Photosynthetic Organisms in Relation to Vegetative Motility
Luther (1949) and Den Hartog and Segal (1964; see also Raven 1981) proposed a classification of aquatic photosynthetic organisms. Planophytes are unattached to the solid substrate, and include macroscopic benthopleustophytes resting on the substrate, mesopleustophytes between the substrate and the air–water interface, and acropleustophytes floating at the water surface, as well as phytoplankton that are microscopic acropleustophytes (Luther 1949; Den Hartog and Segal 1964; Raven 1981). Organisms attached to substrate are divided into rhizophytes (macroscopic organisms on substrata of small size relative to the organism, and with roots or rhizoids penetrating into the substrata) and haptophytes (microscopic to macroscopic organisms attached to substratum structures that are large relative to the size of the organism) (Luther 1949; Den Hartog and Segal 1964; Raven 1981).
3 Phytoplankton and Benthic Microalgae
3.1 Density and Sinking of Phytoplankton
Phytoplankton are generally denser than their aquatic environment, and so sink under the influence of gravity relative to the surrounding water (Boyd and Gradmann 2002). Turbulence has been shown to increase, rather than, as some have suggested, decrease, sedimentation (Ruiz et al. 2004; see also Ross 2006 and Arrieta et al. 2020). The density differential is larger, other things being equal, in mineralized cells, e.g. diatoms and coccolithophores (Boyd and Gradmann 2002; Raven and Waite 2004). Waite and Harrison (1992) and Waite et al. (1992) showed that metabolic energy is needed for low sinking rates of the centric diatom Ditylum brightwellii: illuminated controls sank less rapidly than illuminated cells in the presence of metabolic inhibitors or cells in the dark. Following from this, Waite et al. (1997) show that the predicted (from Stokes’ Law, assuming size-independent density) increase in sinking rate of diatoms over a range of cell sizes applies to dead or metabolically inactivated diatoms, but metabolically active cells show lower sinking rates for large-cell organisms than predicted by Stokes’ Law. This observation shows that diatoms can use energy to modulate sinking rate through specific physiological mechanisms that decrease cell density, as described in Sect. 3.2. Furthermore, some larger phytoplankton cells can have positive buoyancy, e.g. centric diatoms such as some species of Coscinodiscus and Rhizosolenia, and also Ethmodiscus rex, the non-flagellate vegetative phase of the dinoflagellate Dinocystis lunaria and the cyst (phycoma) phase of the prasinophycean chlorophyte alga Halosphaera (Boyd and Gradmann 2002; Beardall et al. 2008; Acuña et al. 2010). Changes in cell density of these large cells can result in periodic vertical migration as discussed in the next section.
3.2 Periodic Vertical Migration of Phytoplankton
One important effect of motility of phytoplankton is periodic vertical migration related to the inverse gradients of photosynthetically active radiation (highest at the surface of the water body) and of nutrients (highest at the chemocline near the base of the photic zone) and also any periodic vertical migration of grazing organisms. The mechanism of vertical migration is the vertical component of flagellar motility, and the necessary vertical vector of changes in density of the phytoplankton organism encompassing both higher and lower organism densities than the aquatic medium.
Motility of eukaryotic phytoplankton expressing flagella in the vegetative stage occurs in both marine and fresh water habitats with, among other functions, periodic vertical migration (e.g. Raven and Richardson 1984; Ralston et al. 2007; Hall and Paerl 2011; Bollens et al. 2012; Inoue and Iseri 2012; Schuh and Menden-Deuer 2014). These periodic vertical migrations act to move nutrients to the water surface (Inoue and Iseri 2012).
Occurrence of periodic vertical migration can also occur in cyanobacteria with upward movement when gas vesicles are inflated and the organism density is less than that of the medium, and downward movement when accumulated ballast materials (e.g. polysaccharides) and/or increased turgor deflating gas vesicles causes the density to exceed that of the medium (Walsby 1994). These movements can occur for either marine or freshwater habitats (Walsby 1994). Fogg and Walsby (1971) suggested that periodic vertical migration of cyanobacteria functions in exploiting separation of light and nutrients in the environment of phytoplankton, although Bormans et al. (1999) found little evidence of this in the publications then available. However, Brockes and Ganf (2001; see also Hunter et al. 2008) found density responses of the colonial Microcystis aeruginosa to variations in nitrogen, phosphorus, and photon flux density availability consistent with periodic vertical migration. Evidence is consistent with periodic (diel) vertical migration of the diazotrophic colonial cyanobacterium in the central Atlantic (Villareal and Carpenter 1990, 2003), where P (sometime with Fe colimitation) limits N2 fixation (Sañudo-Wilhelmy et al. 2001; Mills et al. 2004; see also Schlosser et al. 2014; Garcia et al. 2015).
Villareal (1986), Villareal et al. (1999, 2014) showed that nitrate was moved upward in the surface ocean in mats of the diatom Rhizosolenia. Wirtz and Smith (2020) show that, in the oligotrophic ocean, long-period (many days) periodic migrations can significantly increase primary productivity, additional to the known effects of diel periodicity migrations, so that vertical pumping of nutrients can be responsible for half of total marine primary productivity, which in turn is almost half of total primary productivity on Earth (Field et al. 1998).
Since fresh water is less dense than sea water, decreasing the density of cells below that of fresh water by means of vacuolar accumulation of solutes yielding very low-density solutions is not possible (Boyd and Gradmann 2002), even with active water transport (Raven and Doblin 2014). Periodic vertical migration in fresh water is, as mentioned above, possible for cyanobacteria with gas vesicles such as the colonial Microcystis (Hunter et al. 2008), and for flagellates such as Volvox (Sommer and Gliwicz 1986).
3.3 Energetics of Periodic Vertical Migration in Flagellates
Raven and Richardson (1984) calculated the energetics of movement as a fraction of energy used in growth for a marine dinoflagellate; the outcomes are summarized in Table 1. Using on the minimum energy cost of motility based on the work done against friction in flagella operation plus the cost of flagella synthesis, a first-order estimate of the energy cost of motility is 10−3 that of cell synthesis (Table 1). Substituting the mechanistic (ATP use) energy cost of motility for the work done against friction, the minimum energy cost of motility (operation plus synthesis of flagella) is 2 × 10−3 that of cell synthesis (Tables 1 and 2).
For freshwater flagellate algae there is also the question of volume regulation. Metabolism requires a higher intracellular osmolarity than the osmolarity of freshwater, so there is a driving force for water entry (Raven 1982, 2000, 2018). The presence of flagella requires that there is an area of plasmalemma not protected from osmotic expansion by the cell wall (if present) (Raven 1982, 2000, 2018). Volume maintenance under these conditions requires active water efflux, typically involving contractile vacuoles (Raven 1982, 2000, 2018). Hence, the energy cost of vertical periodic migration in freshwater flagellates is expected to be higher than that for marine flagellates.
3.4 Energetics of Buoyancy and Periodic Vertical Migration in Cyanobacteria
Walsby (1994) has computed the energy cost per unit time for an Escherichia coli-sized cyanobacterial cell with a generation time of 1 day, i.e. a specific growth rate of 0.69 per day, and computed the cost of gas vesicle synthesis during growth as 2.3 × 10−15 W cell−1. Walsby (1994) did not compute the energy cost of cell growth; the calculations that follow are based on the cell properties that Walsby used, i.e. fresh mass of 9.5 × 10−16 kg per cell, and a generation time of 1 day (specific growth of 0.69 per day). Assuming a fresh/dry weight ratio of 3.33, the cell dry weight is 2.9 × 10−16 kg so, with 0.4 g C per g of dry matter, there is 9.7 × 10−15 mol C per cell. From Kliphuis et al. (2012), the absorbed photon cost per cell C during growth is 23 mol photons (400–700 nm); for photochemistry using 680 nm excitation (175 kJ per mol photon) the energy used is 8.89 × 10−8 J per cell. If only 0.3 of this energy is stored in the growing cell, with a generation time of 1 day, this corresponds to 8.89 × 10−8 × 0.4/24 × 3,600 = 2.3 × 10−15 W cell−1 (Table 1). The cost of gas vesicle synthesis is then 1.3 × 10−3 of the cost of cell synthesis. (Tables 1 and 2).
Individual cyanobacterial cells with the characteristics modeled only move upwards at ≤2 μm s−1 (Walsby 1994; Tables 1 and 2). However, association of cells in colonies can increase the movement speed to ≥1 mm s−1 based on calculations using Stokes’ Law (Walsby 1994). While faster movement relative to surrounding water decreases the boundary layer thickness of organisms of a given size and thus increases solute diffusion to the organism surface and increase nutrient influx, this is partly offset in the case of colony formation by the intrinsically greater diffusion boundary layer around larger organisms (Beardall et al. 2008). Furthermore, colonies have further constraints imposed by the needs for diffusion of nutrients through the matrix to non-surface cells in the colony, as well as increased package (self-shading) restriction on light availability to the average colony cell in a given radiation field (Beardall et al. 2008), as well as diversion of some photosynthate from cell multiplication to producing the extracellular matrix of the colony. How motility at ~0.12 μm s−1, by an unknown mechanism, of cells within colonies of the freshwater cyanobacterium Microcystis wesenbergii (Mulling et al. 2014) might address some of these negative aspects of enhanced motility by coloniality is not clear.
3.5 The Swimming Cyanobacterium
Are there alternatives to gas vesicles in allowing upward movement of planktonic cyanobacteria relative to the surrounding water? While no cyanobacteria have bacterial flagella, about a third of open ocean isolates of Synechococcus can swim through open water at 5–25 μm s−1 (Waterbury et al. 1985; Ehlers and Koiller 2011; Ehlers and Oster 2012). Possible mechanisms of this motility are presented by Ehlers and Koiller (2011) as acoustic streaming based on waves in the (outer?) cell membrane and Ehlers and Oster (2012) as helical waves at the cell surface (outer membrane), driven by mechanochemical motors attached to peptidoglycan in the periplasm. Energization of the outer membrane of cyanobacteria is incompletely understood (Raven and Sánchez-Baracaldo 2021; Rees and Raven 2021).
3.6 Regulation of Sinking Rate of Planktonic Diatoms in Relation to Virus Infection
Raven and Waite (2004) suggested that the interference of virus attack with the ability to decrease cell density of diatoms could cause infected cells to sink faster than uninfected cells, thus limiting spread of the infection. When Raven and Waite (2004) wrote there was very little knowledge of viral infection of diatoms; since then several viruses of marine diatoms (Yuji et al. 2015) have been characterized. While virus infection can convert planktonic particulate organic carbon into dissolved organic carbon (Fuhrman 1999; Wilhelm and Suttle 1999), and thus decrease the extent of the biological pump per unit of primary productivity, Yamada et al. (2018) found that the presence of a virus of Chaetoceros tenuissima (3–10 μm equivalent spherical diameter) caused a 5–59-fold increase in the number of cell aggregates 50–400 μm equivalent spherical diameter relative to virus-free control cultures. This aggregation increases the rate of particulate organic matter sedimentation of the cells in the clump, although viral infections may overall increase the rate of conversion of diatom particulate organic into dissolved organic matter (Fuhrman 1999; Suttle 2007).
The influence of virus infection on diatom sinking can be further affected by Si limitation, Kranzler et al. (2019) showed that Si limitation of diatom growth increased viral infection and the resulting cell mortality. Virus infection can also promote the loss of diatoms from the water column via induction of spore production decreasing the possibility of infection of remaining vegetative cells in marine diatoms (Pelusi et al. 2021).
3.7 Energetics of Intermittent Sinking in Planktonic Diatoms
Gemmell et al. (2016) showed that the centric diatom Coscinodiscus wailesii showed unsteady sinking. In the light, with periods of rapid sinking alternating with periods of slow sinking with a periodicity of the order of 10 s, when exposed to high nutrient conditions for hours after a period of growth at low-nutrient concentrations. This alternation was shown not to enhance nutrient flux to the cell surface, important under low-nutrient conditions, relative to steady sinking at the same mean rate (Gemmell et al. 2016). Du Clos et al. (2019) showed that the unsteady cell movement was muted when the sinking occurred in the dark. Du Clos et al. (2021) found that there was increasing unsteadiness of sinking of nutrient-limited C. wailesii after addition of NO3− and Si(OH)4, but not HPO42−. They suggested that this unsteadiness may be a response to patchy nutrients, helping to maximize light exposure and minimize energy costs of unsteady sinking. The energy cost of oscillatory sinking was calculated by Lavoie and Raven (2020). These authors showed that the least energy expensive mechanism of unsteady sinking, i.e. episodic cell volume increases with active water influx on the time scale of the periodicity of sinking rate variation, still had a minimum energy cost of 0.16 of the baseline energy cost of growth (Table 2). This energy cost of unsteady sinking exceeds that of generation of buoyancy by gas vesicles or active water influx or upward swimming using flagella (Tables 1 and 2), in periodic vertical migration by over an order of magnitude. To what extent oscillatory sinking is beneficial for diatoms in nutrient patchy environments as well as the physiological mechanisms involved require further investigation.
3.8 Energetics of Buoyancy and Periodic Vertical Migration of Marine Planktonic Diatoms
Some species of Rhizosolenia and Coscinodiscus, and Ethmodiscus rex, are capable of positive buoyancy (Moore and Villareal 1996; Villareal et al. 1999; Boyd and Gradmann 2002; Raven and Doblin 2014; Lavoie et al. 2016). The mechanism for positive buoyancy with the lowest energy cost, i.e. 4.84 × 10−8 W cell−1, is density reduction by active water influx for E. rex (Raven and Doblin 2014). With 0.478 μmol C per cell of E. rex (Villareal et al. 1999) and 23 mol absorbed photons of photosynthetically active radiation per mol C incorporated into cell material (Kliphuis et al. 2012), cell doubling requires 11 μmol absorbed photons per cell or, for the photons used in photochemistry at 680 nm, 4 J per cell. Assuming 33% of absorbed photons are converted to energy stored in cell material, i.e. 1.32 J, and a specific growth rate of 0.069 per day (Villareal et al. 1999), i.e. cell doubling time of 10 days, the power used in growth is 1.32/10 × 24 × 3,600 or 1.52 × 10−6 W. The energy used in active water transport is only used in the ascending leg of the vertical cycling, assumed to take 5 days, active water transport costs a mean 2.42 × 10−8 W cell−1. Buoyancy generation costs then uses 0.016 of the energy used in growth (Tables 1 and 2).
3.9 Periodic Vertical Migration of Benthic Cyanobacteria and Microalgae
Such vertical migration can occur in epilithic or hypolithic microbial mats, including stromatolites and thrombolites (Bebout and Garcia-Pichel 1995; Nadeau et al. 1999; Consalvey et al. 2004; Lichtenberg et al. 2020), and where the photosynthetic micro-organisms occur at the interface of bulk waters and small inorganic plus organic particles of muddy or sandy materials (Round and Palmer 1966; Moss 1977; Paulíčova et al. 2008).
Avoidance of UV radiation appears to be a major function of vertical migration of cyanobacteria in microbial mats in the hypersaline Solar Lake in Egypt (Bebout and Garcia-Pichel 1995) and Antarctica (Nadeau et al. 1999). Consalvey et al. (2004) found the dominant diatom vertical migration in estuarine microbial mats involved downward movement in anticipation of the incoming tide, and upward migration as the tide recedes. Epipelic/epipsamic diatoms and Euglena spp. in an estuary move to the surface in the light, constrained by downward movement as the tide comes in (Round and Palmer 1966).
3.10 Energetics of Periodic Vertical Migration by Benthic Cyanobacteria and Microalgae
Raven (1983) calculated the minimum energy cost of gliding motility in such filamentous cyanobacteria as Oscillatoria and Phormidium at 2.7 × 10−6 m s−1 based on work done against friction, suggesting that the energy used was 0.01 of that available from respiration, or perhaps 0.005 of that from photosynthesis, assuming constant movement (Table 2). Marques da Silva et al. (2020) computed the energy cost of vertical migration at 10−6 m s−1 of raphid pennate diatoms such as Nitzschia spp. in a 400 μm photic zone; the small depth of the photic zone is a result of light attenuation by particles in the sediment. Marques da Silva et al. (2020) calculated the minimum energy cost of movement based on work done to overcome gravity and friction, and compared it to the energy stored from photosynthesis. The minimum energy cost of movement over 24 h is only 10−6 of the energy stored in photosynthesis over 24 h, granted one vertical migration cycle each 24 h (Marques da Silva et al. 2020) (Table 2).
4 Sexual and Asexual Reproduction in the Absence of Flagella
4.1 Ancestral Sexual Reproduction in Eukaryotes
Genes for meiosis occur in many cultured algae in which sexual reproduction has not been reported, consistent with the occurrence of haploid-diploid alternation typical of eukaryote sexual reproduction (Grimsley et al. 2010; Fučiková et al. 2015). It is possible that the Last Eucaryotic Common Ancestor had sexual reproduction involving flagellate cells, with chemotaxis allowing the gametes to find one another (Venuleo et al. 2017). As shown in the Appendix, there have been several independent losses of flagellar motility among photosynthetic eukaryotes.
4.2 Spores of Multicellular Red Algae
Pickett-Heaps et al. (2001) measured the gliding motility on surfaces at 0.66 μm s−1 of the unicellular Porphyridium, and the Chantransia stage spores (10 μm diameter) of the multicellular Batrachospermum glide at ~2.2 μm s−1; the latter speed is of the same order of magnitude of speed of cyanobacterial movement (see Table 2). The motility mechanism of algal spores has been subsequently studied by Ackland et al. (2007), who showed that pseudopodia of archaespores of the multicellular alga Porphyra pulchella operate using, as expected, actin and myosin.
4.3 Zygnematophyceae
The Zygnematophyceae (=Conjugatophyceae) is the most speciose of the classes of the algal Streptophyta, and has benthic and planktonic representatives (Van den Hoek et al. 1995; Algaebase). Sexual reproduction of these aflagellate (see Appendix) algae involves cells (desmids) or filaments (Zygnematales) of compatible genotypes coming into contact, dissolution of the parts of the cell walls that are in contact, rounding up of cell contents with loss of the aqueous vacuole, and amoeboid movement of the contents of one cell into the other followed by fusion of the protoplasts and formation of a zygospore (van den Hoek et al. 1995). The other classes of algal Streptophyta are the Klebsormidiophyceae (benthic and planktonic), Coleochaetophyceae (benthic), and Charophyceae (benthic) that have flagellate male gametes in oogamous sexual reproduction (van den Hoek et al. 1995).
4.4 Pennate Diatoms (Bacillariophyceae Sensu Stricto)
Although the basal diatoms are the oogamous planktonic centric (Mediophyceae, according to Algaebase) organisms with flagellate male gametes (van den Hoek et al. 1995; Nakov et al. 2018), flagella have been lost at least twice in diatom evolution (Nakov et al. 2018).
One loss was in the ancestor of the pennate diatoms, with basal paraphyletic araphid taxa and derived monophyletic raphid species, i.e. diatoms with a raphe, which is a slit in the cell wall (frustule) (Round et al. 1990; Cox 2012). Most araphid diatoms are benthic, e.g. the araphid Ardissonea crystallina that is motile, leaving a mucus trail (Pickett-Heaps et al. 1991). Gametes move using ‘pseudopodia’ and are functionally dimorphic (anisogamous) (Sato et al. 2011; Nakov et al. 2018). Sato et al. (2011) describe mobility of male gametes of the araphid Pseudostaurosira trainorii as based on extrusion and retrieval of microtubule-based threads, possibly with kinesin or tubulin forming a mechanochemical motor, with sex pheromones that guide the initially ‘random walk’ motility? Davidovich et al. (2012) describe related motility of male gametes of the araphid Tabellaria spp.
The other loss was in Ardissonea crystallina, a member of the benthic marine toxariid clade of centric diatoms (Davidovich et al. 2017). The gametes are functionally dimorphic and have amoeboid movement (Davidovich et al. 2017). There must be close approach of sexually compatible strains not just for the pennate diatoms where the partners become enveloped in a common mucilage sheath before the amoeboid gametes fuse, but also to a lesser extent for centric diatoms with flagellate male and non-motile female gametes (van den Hoek et al. 1995; Nakov et al. 2018).
Notwithstanding the apparent role, from ultrastructural evidence, of microtubules in gliding locomotion of the araphid Pseudostaurosira trainorii (Sato et al. 2011), there is substantial evidence (Edgar and Zavortink 1983; Poulsen et al. 1999; Bertrand 2008; Yamoaka et al. 2016) showing that actomyosin is involved in gliding of both raphid and araphid pennate diatoms.
Montresor et al. (2016) review evidence for sex in planktonic marine diatoms. Almost all of the data on the occurrence of sex in marine planktonic comes from work on cultures, with very limited direct data (e.g., observation of empty gametangia) from nature (Montresor et al. 2016). However, there is also evidence of the occurrence of sexual reproduction in nature from molecular genetic data (Montresor et al. 2016). Botte et al. (2013) and Montresor et al. (2016) point out the need for calm water for diatom aggregation and sexual reproduction in planktonic diatoms. Collective sinking of aggregated algae has indeed been shown to promote pairing and reproduction in planktonic pennate diatoms (Font-Muňoz et al. 2019). Diversification rates in diatoms have been related to their locomotion and life history, as are diversification and the extent of outbreeding in the Zygnematophyceae, Rhodophyta, and seagrasses (Nakov et al. 2018; Table 2 of Collins et al. 2013).
5 Vegetative Reproduction and Dispersal of Aquatic Macrophytes
Collado-Vides (2001) reviews the spatially limited lateral dispersal of marine macroalgae by separation of ramets, i.e. genetically identical but physically separate organisms as a mean of vegetative reproduction with little lateral spread. This can occur by heterotrichy (Fritsch 1942), i.e. growth of filaments along the substrate with erect branches, with the possibility of separation of ramets by scission (e.g. by herbivores or abiotic forces) of the horizontal filaments. Heterotrichy occurs in the Rhodophyta: Floridiophyceae (e.g. Bostrychia, Gelidium), Chlorophyta: Chlorophyceae (e.g. Stigeoclonium), and Ulvophyceae (e.g. the acellular Caulerpa, Penicillus, Udotea, and the multicellular Trentepohlia), and Ochrophyta: Phaeophyceae (e.g. Ectocarpales) (Fritsch 1942; Collado-Vides 2001). Horizontal growth rate of the stolons of five Caulerpa spp. in situ is up to 10 mm per day (Williams et al. 1985), and that of Caulerpa sertularioides in the laboratory is 4 mm per day (Mosquera-Murillo and Peñasalanera 2016). As for heterotrichy, scission of the horizontal axis by herbivory or abiotic damage can separate ramets.
Frond growth from a common holdfast for haptophytes (Raven 1981) gives less lateral spread, e.g. in the Rhodophyta: Floridiophyceae (e.g. Corallina, Mazaella), Chlorophyta: Ulvophyceae (e.g. Blidingia), and Ochrophyta: Phaeophyceae (e.g. Ascophyllum) (Collado-Vides 2001). Another possibility for ramet separation is branch bending and re-attachment to the rocky substratum followed by separation of the newly attached structure, e.g. Laurencia (Ochrophyta: Phaeophyceae) (Collado-Vides 2001). Finally, there is crustose growth with lateral spread and, again, the possibility of separation of ramets, e.g. Mesophyllum (Rhodophyta: Floridiophyceae) and Ralfsia (Ochrophyta: Phaeophyceae) (Collado-Vides 2001). Matsuda (1989) examined three crustose species of the Floridiophyceae (Rhodophyceae), and found the maximum horizontal growth rate of 0.13 mm per day.
Seagrasses have rhizomes allowing horizontal growth as a mean of vegetative reproduction; the highest value reported by Marba and Duarte (1998) is for Halophila ovalis growing at 9.7 mm per day. For comparison with terrestrial vascular plants, Marrs and Watt (2006) report elongation rates of 3.5 mm per day for the fern Pteridium aquilinum.
Apart from ramets formed by breaking of horizontal structures discussed in the three preceding paragraphs, macroalgal detachment also contributes to vegetative reproduction and dispersal. Long distance dispersal by detached, viable portions of benthic macrophytes that are exposed directly to the water body is in principle enhanced if the detached portions are buoyant. This has been examined for Durvillaea (Fucales: Pheophyceae) which has species with gas spaces in their thallus (e.g. D. antarctica) and are buoyant, and species lacking gas spaces (e.g. D. pomatorum) (Fraser et al. 2020). Fraser et al. (2020) point out that the occurrence of gas spaces limits the depth at which algae can occur, and also that there have been multiple gains and losses of gas spaces in the genus. Li et al. (2020) examined genetic connectivity among attached populations, and a free-floating population, of the gas vacuolate Sargassum horneri (Fucales: Phaeophyceae) in Chinese marginal seas, and found few shared haplotypes between the attached and nearby rafted populations: the rafted population had an unknown origin. Burnett and Koehl (2017) showed that pneumatocysts of Egregia densa (Laminariales: Phaeophyceae) provide buoyancy with minimal effect on drag in wave-driven flow. Other things being equal, the occurrence of pneumatocysts provides buoyancy with minimal increase in the possibility of wave-driven detachment. As expected, wounding increased breaking, but rapid growth rate also increased the chance of breaking, of Egregia densa (Burnett and Koehl 2019).
De Bettignies et al. (2020) examined survival in situ of fragments of Laminaria hyperborea (Laminariales: Phaeophyceae) sporophytes, a kelp that lacks gas spaces. They found that some fragments maintained photosystem activity after 25 weeks, when only 16% of the original biomass remained. Importantly, some reproductive activity was retained after 20 weeks. It thus appears that despite the absence of gas spaces, reproductive capacity could be important for dispersal granted adequate wave or current activity.
6 Elongate Male Gametes and Pollen Grains in Aquatic Macrophytes
6.1 Rhodophyta
Non-flagellate spermatia of red algae are produced by haploid male gametophytes. Spermatia are surrounded by an extracellular covering and also have two elongate extracellular appendages at 180° (Fetter and Neushul 1981; Brawley and Johnson 1992; van den Hoek et al. 1995; Kaczmarska and Dowe 1997. Engel et al. 1999; Engel 2002; Santelices 2002; Mine et al. 2003; Engel et al. 2004; Maggs et al. 2011). The appendages are believed to increase the chance of encounter of a spermatium in moving water with a trichogyne on a female gametophyte. Destombe et al. (1990) show that spermatia of Gracilaria verrucosa remain fertile for 5 h after release, and can fertilize cystocarps 80 m from the spermatia source. Measurements of the fraction of eggs of red algae that are fertilized in the natural environment gave higher values than had been assumed. Santelices (2002) cites 30–80% of eggs fertilized in the brooding-type red algae, which tend to be lower than the 70–100% of broadcast-type fucoid brown algae, but higher than had been assumed. This relatively large fraction of eggs that are fertilized without using flagellar apparatus invites reconsideration of the hypothesis that the carposporophyte phase of the life cycle, producing many diploid carpospores from each zygote is an evolutionary response to a low fraction of eggs that are fertilized.
6.2 Seagrasses
Seagrasses, submerged marine flowering plants, are less phylogenetically diverse than submerged freshwater flowering plants: they are all members of the monocotyledonous order Alismatales. Most seagrasses are rooted in fine-grained substrates, although some (e.g. Phyllospadix) grow on rocky shores (Raven 1981; Williams 1995). Movement processes in seagrasses include vegetative spread of clones through rhizomatous lateral growth through the fine-grained substrate or, for Phyllospadix, over rocky substrates, drifting of vegetative fragments, and dispersal of sexually produced propagules, and pollen dispersal (McMahon et al. 2014).
The seagrasses are the only flowering plants with submerged pollen release, transfer and pollination (Pettitt 1980, 1981; Pettitt et al. 1980; McConchie and Knox 1989; Cox and Humphries 1992; Reusch 2003; Vermaat et al. 2004; Ackerman 2006; Kendrick et al. 2012; Sinclair et al. 2014). Cox and Humphries (1992) show that 67% of seagrass genera are dioecious, and the remaining 33% are monoecious, although at least 40% of species are monecious, i.e. the monoecious genera are more speciose (Sinclair et al. 2014). Pollen grains of seagrasses are filiform, or functionally filiform by release of spherical grains attached in a filament (Ackerman 2006). Vermaat et al. (2004) used the dioecious Enhalus acoroides and showed that there was a large increase in fruit production from carpels (= pollination success) when seagrass cover was over 50%. Moreover, there was no effect of the apparent sex ratio on pollination success indicating that pollen dispersal and pollination success is effective (Vermaat et al. 2004). Sinclair et al. (2014) used the monoecious clonal Posidonia australis, and found very high (0.93–0.97 for two sites) genetic diversity in embryos. The pollen dispersal distances inferred from paternity assignment for the two sites are 30.8 and 26.8 m, greater than the mean clonal patch sizes, 12.8 and 13.8 m. Sinclair et al. (2014) tabulate (their Table 4) outbreeding multilocus outcrossing rate reported for monoecious seagrasses; the range is generally 0.61–1.0, with one report of a very wide range of 0.1–0.89. Table 4 of Sinclair et al. (2014) shows a multilocus outcrossing rate of 0.03–0.97 for wind-pollinated terrestrial monoecious Cyperaceae and Poaceae. The data show that the mean multilocus outcrossing rate for the terrestrial wind-pollinated herbaceous plants is less than that for the seagrasses. The quantitative significance of pollination by invertebrate fauna, particularly at night (van Tussenbroek et al. 2016), relative to water movements is not clear. It is certain that the pollen dispersal mechanism in seagrass (without relying on flagella) can lead to significant outbreeding.
7 Conclusions
Movement of organisms or parts of organisms, either active by mechanochemical motors or passively using water movements, are important in the life of aquatic photolithotrophs. Most of these organisms are denser than their aquatic medium, so they, or detached parts, sink relative to the surrounding water in the absence of appropriate mechanochemical motors. Phytoplankton generally sink through their aquatic medium, and retention in the photic zone is not aided by turbulence. Upward movement of non-flagellate organisms is allowed, by decreased density of the aqueous vacuole in large-celled eukaryotes, and gas vesicles in cyanobacteria. Such movement can also occur in organisms with flagella. The small minimum energy cost of these three mechanisms of upward movements (Tables 1 and 2) can maintain the organisms in the photic zone using a small fraction of the energy used in cell growth. When there is limited circulatory water movement in the upper mixed layer there is the capacity for upward movement relative to surrounding water using one of the three upward movement processes. Such movement allows periodic vertical migration relative to the water surface, thus increasing resource gain rate from the opposing gradients of the resources of photon flux density (high near the surface) and nitrogen, phosphorus, and iron (high near the chemocline). Increased density is needed for the downward portion of the migration of non-flagellate organisms, and the periodic vertical migration can only function when there is limited water movement near the surface.
Periodic vertical migration is not limited to phytoplankton; it, and other migrations, can occur in benthic habitats, e.g. for photosynthetic flagellates near the surface of muddy or sandy substrates, moving deeper in avoiding excessive light or in avoiding removal in overlying water in tidal habitats at high tide. Such movements can also occur for cyanobacteria and pennate diatoms with gliding motility in microbial mats on rocky substrata. As for the mechanochemical motility in phytoplankton, the estimates of the minimum energy cost of migration based on work done against friction in these benthic habitats are again a small fraction of the energy cost of growth, although more work is needed to better constrain this energy cost (Table 2). A higher fraction of the energy used in cell growth appears to be needed for high frequency variations in sinking rate of a planktonic marine diatom, but these estimates rely on putative cellular mechanisms that have not been fully resolved yet. More experimental work is thus required to determine the cellular mechanisms and hence refine bioenergetic understanding (Table 2). The limited data available suggests that lacking flagella on male gametes of algae (rhodophytes), have little effect on the extent of fertilization, or of outbreeding, relative to organisms with flagellated male gametes. Other dispersal mechanisms (seagrass pollen, detached portion of benthic macrophytes) are also effective to enhance algae fertilization.
References
Ackerman JD (2006) Sexual reproduction of seagrasses: pollination in the marine context. In: Larkum AWD, Orth RJ, Duarte C (eds) Seagrasses: biology, ecology and conservation. Springer, Dordrecht, pp 89–109
Ackland JC, West JA, Pickett-Heaps J (2007) Actin and myosin regulate pseudopodia of Porphyra pulchella (Rhodophyta) archaespores. J Phycol 43:129–138
Acuña JL, López-Alvarez M, Nogueira E, Conzález-Taboada F (2010) Diatom flotation at the onset of the spring bloom: an in situ experiment. J Exp Mar Biol Ecol 400:115–125
Arrieta J, Jeanneret R, Roig P, Tuval I (2020) On the rate of sinking diatoms: the transport of active buoyancy-regulating cells in the ocean. Phil Trans R Soc A 378:20190529
Bailey JC, Bidigare RR, Chistensen SJ, Andersen RA (1998) Phaeothamniophyceae classis nova: a new lineage of chromophytes based upon photosynthetic pigments, rbcL sequence analysis and ultrastructure. Protist 149:245–263
Beardall J, Allen D, Bragg J, Finkel ZV, Flynn KJ, Quigg A, Rees TAV, Richardson A, Raven JA (2008) Allometry and stoichiometry of unicellular, colonial and multicellular phytoplankton. New Phytol 181:95–309
Bebout BM, Garcia-Pichel F (1995) UV B-induced vertical migrations of cyanobacteria in a microbial mat. Appl Environ Microbiol 61:4215–4222
Bertrand J (2008) Movements des diatomées VIII. Synthese et hypothése. Diatom Res 23:19–29
Blanc G et al (2010) The Chlorella vulgaris NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 22:2943–2955
Bollens SM, Quenetta JA, Rollwagen-Bollers G (2012) Predator-enhanced diel vertical migration in a planktonic dinoflagellate. Mar Ecol Prog Ser 447:49–54
Bormans M, Sherman BS, Webster IT (1999) Is buoyancy regulation in cyanobacteria an adaptation to exploit separation of light and nutrients. Mar Freshw Res 50:897–906
Botte V, Ribero D’Alcala M, Montresor M (2013) Hydrodynamic interactions at low Reynolds numbers: an overlooked mechanism favouring diatom encounters. J Plankton Res 35:914–918
Bowler C et al (2008) The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456:239–244
Boyd CM, Gradmann AED (2002) Impact of osmolytes on buoyancy of marine phytoplankton. Mar Biol 50:445–452
Brawley SH, Johnson LE (1992) Gametogenesis, gametes and zygotes: an ecological perspective on sexual reproduction in algae. Br Phycol J 27:233–252
Brockes JD, Ganf GG (2001) Variations in buoyancy responses of Microcystis aeruginosa to nitrogen, phosphorus and light. J Plankton Res 23:1299–1411
Brooks E, Rindi F, Suo Y, Ohatani S, Green M (2015) The Trentepohliales (Ulvophyceae, Chlorophyta): an unusual algal order and its novel plant pathogen, Cephaleuros. Plant Dis 9:740–753
Burnett NP, Koehl MAR (2017) Pneumatocysts provide buoyancy with minimal effect on drag in wave-driven flow. J Exp Mar Biol Ecol 497:1–10
Burnett NP, Koehl MAR (2019) Mechanical properties of the wave-swept kelp Egregia menziesii change with season, growth rate and herbivore wounds. J Exp Biol 222:jeb190595
Carvalho-Santos Z, Azimzadeh J, Pereira-Leal JB, Betencourt-Dias M (2011) Tracing the origins of centrioles, cilia, and flagella. J Cell Biol 194:165–175
Collado-Vides L (2001) Clonal architecture in marine macroalgae: ecological and evolutionary perspectives. Evol Ecol 15:531–545
Collins S, Rost B, Rynearson TA (2013) Evolutionary potential of marine phytoplankton under ocean acidification. Evol Appl 7:140–155
Colp MJ, Archibald JM (2019) Evolution: new protest predators under the Sun. Curr Biol 29:R936–R938
Consalvey M, Paterson DM, Underwood GJC (2004) The ups and downs of life in a benthic biofilm: migration of benthic diatoms. Diatom Res 10:181–192
Cox EJ (2012) Ontogeny, homology, and terminology – wall morphogenesis as an aid to character recognition and character state definition for pennate diatom systematics. J Phycol 48:1–21
Cox PA, Humphries CJ (1992) Hydrophilous pollination and breeding system evolution in seagrasses: a phylogenetic approach to the evolutionary ecology of the Cymodoceaceae. Bot J Linn Soc 113:217–226
Davidovich NA, Kaczmarska I, Karpov SA, Davidovich OI, MacGillivary L, Mather L (2012) Mechanism of male gamete motility in araphid pennate diatoms from the genus Tabularia (Bacillariophyta). Protist 163:480–494
Davidovich NA, Davidovich OI, Podunay YA, Gastineau R, Kaczmarska I, Poulíčlová A, Witkowski A (2017) Ardissonea crystallina has a type of sexual reproduction that is unusual for centric diatoms. Sci Rep 7:14670
De Bettignies F, Dauby P, Thomas F, Grobet A, Delage L, Bohner O, Loisei S, Devault D (2020) Degradation dynamics and processes associated with the accumulation of Laminaria hyperborean (Phaeophyceae) kelp fragments: an in situ experimental approach. J Phycol 56:481–1492
Den Hartog C, Segal C (1964) A new classification of waterplant communities. Acta Botanica Neerlandica 13:67–393
Denny M (1993) Air and water. The biology and physics of life’s media. Princeton University Press, Princeton, NJ, p 360
Denny M (2016) Ecological mechanics. Principles of life’s physical intractions. Princeton University Press, Princeton, NJ, p 503
Destombe C, Godin J, Remy J-M (1990) Viability and dissemination of spermatia of Gracilaria verrucosa (Gracilariales, Rhodophyta). In: Lindstrom SL, Gabrielson PW (eds) Thirteenth international seaweed symposium. Developments in hydrobiology, vol 58. Springer, Dordrecht, pp 219–223
Du Clos KT, Karp-Boss TA, Villareal TA, Gemmell BJ (2019) Coscinodiscus wailesii mutes unsteady sinking in dark conditions. Biol Lett 15:2010816
Du Clos KT, Karp-Boss L, Gemmell BJ (2021) Diatoms rapidly alter sinking behaviour in response to changing nutrient concentrations. Limnol Oceanogr 66:892–900
Edgar LA, Zavortink M (1983) The mechanism of diatom locomotion. II. Identification of actin. Proc R Soc B 218:345–358
Ehlers KM, Koiller J (2011) Could cell membrane produce acoustic streaming. Making the case for Synechococcus self-propulsion. Math Comput Model 53:1489–1504
Ehlers K, Oster G (2012) On the mysterious propulsion of Synechococcus. PLoS One 7:e36081
Engel CR (2002) Reproductive ecology of an intertidal red seaweed, Gracilaria gracilis: influence of high and low tides on fertilization success. J Mar Biol Assoc UK 82:189–192
Engel CR, Watier R, Destombe C, Valero M (1999) Performance of non-motile male gametes in the sea: analysis of paternity and fertilization success in a natural population of a red seaweed, Gracilaria gracilis. Proc R Soc B 266:1879–1886
Engel CR, Destombe C, Valero M (2004) Mating system and gene flow in the red seaweed Gracilaria gracilis: effect of haploid-diploid life history and intertidal rocky shore landscape on fine-scale genetic structure. Heredity 92:289–298
Fetter R, Neushul M (1981) Studies on developing and released spermatia in the red alga Tiffaniella snyderae Rhodophyta. J Phycol 17:141–159
Field CB, Behreenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281:237–240
Fogg GE, Walsby AE (1971) Buoyancy regulation and the growth of growth of planktonic blue-green algae. Mitt Int Verein Theor Angew Limnol 19:182–188
Font-Muňoz JS, Jeanneret R, Arrieta J, Anglès D, Jordi A, Tuval I, Basterretxea G (2019) Collective sinking selective cell pairing in planktonic pennate diatoms. Proc Natl Acad Sci U S A 116:15997–16002
Fraser CI, Velásquez M, Nelson WA, Macaya EC, Hay CH (2020) The biogeographic importance of buoyancy in macroalgae:a case study of the southern bull-kelp genus Durvillaea (Phaeophyceae) including descriptions of two new species. J Phycol 56:23–36
Fritsch FE (1942) Studies of the comparative morphology of the algae. 1. Heterotrichy and juvenile stages. Ann Bot 6:397–412
Fučiková K, Pažoutová M, Rindi F (2015) Meiotic genes and sexual reproduction in the green algal class Trebouxiophyceae (Chlorophyta). J Phycol 51:429–430
Fuhrman JA (1999) Marine viruses and their biogeochemical and ecological effects. Nature 399:541–548
Garcia NS, Fu F, Sedwick PN, Hutchins PA (2015) Iron deficiency increases growth and nitrogen fixation rates of phosphorus-limited marine cyanobacteria. ISME J 9:238–245
Gawryluk RMR, Tikhonenkov DV, Hehenberger E, Husnik F, Mylnikov AP, Keeling PJ (2019) Non-photosynthetic predators are sister to red algae. Nature 572:240–243
Gemmell BJ, Buskey EJ, Villareal TA (2016) Dynamic sinking behaviour in marine phytoplankton: rapid changes in buoyancy may aid nutrient uptake. Proc Roy Soc B Biol Sci 283:20160816
Grimsley N, Péquin B, Bachy C, Moreau H, Piganeau G (2010) Cryptic sex in the smallest eukaryotic marine green alga. Mol Biol Evol 27:47–54
Guiry MD, Guiry GM (2021) Algaebase. World-wide electronic publication, National University of Ireland, Galway. https://www.algaebase.org; searched on 03 July 2021
Guo JT, Weatherby K, Canter D, Šlapeta J (2010) Effect of nutrient concentration and salinity on immotile-motile transformation of Chromera velia. Eukar Microbiol 57:444–446
Hall NS, Paerl H (2011) Vertical migration patterns of phytoflagellates in relation to light and nutrient availability in a shallow microtidal estuary. Mar Ecol Prog Ser 425:1–19
Hunter PD, Tyler AN, Willby NJ, Gilvear DJ (2008) The spatial dynamics of vertical migration by Microcystis aeruginosa in a eutrophic shallow lake: a case study using high spatial resolution time series airborne remote sensing. Limnol Oceanogr 53:2391–2406
Inoue T, Iseri Y (2012) Diel vertical migration and nutrient transport of the dinoflagellate Peridinium bipes f. occumatum in a thermally stratified reservoir. Water Sci Technol 66:1212–1219
Jackson C, Clayden S, Reyes-Prieto A (2015) The Glaucophyta: the blue-green plants in a nutshell. Acta Soc Bot Polon 84:149–165
Kaczmarska I, Dowe LL (1997) Reproductive biology of the red alga Polysiphonia lanosa (Ceramiales) in the Bay of Fundy, Canada. Mar Biol 128:695–703
Kai A, Yoshij Y, Nakayama T, Inuye I (2008) Aurearenophyceae classis nova, a new class of Heterokontophyta based on a new marine unicellular alga Aurearena cruciate gen. et sp. nov. inhabiting sandy beaches. Protist 159:435–457
Kawachi M, Inouye I, Honda D, O’Kelly CJ, Bailey JC, Bidigare RR, Anderson RA (2002) The Piguiophyceophyceae classis nova, a new class of photosynthetic stramenopiles whose members produce large amounts of omega-3 fatty acids. Phycol Res 50:31–47
Kawai Mn Maeba S, Sasaki M, Okuda K, Henry EC (2003) Schizocladia schioensis, a new filamentous marine chromophyte, belonging to a new class, Schizocladiophyceae. Protist 154:211–228
Kellmar M (2016) Fine-tuning motile cilia and flagella: evolution of the dynein motor proteins from plants to humans at high resolution. Mol Biol Evol 33:3249–3267
Kendrick GA, Waycott M, Carruthers TJB, Cambridge ML, Hovey R, Krauss SL, Lavery PS, Les DH, Lowe RJ, MascaróI Vidal C, Ooi JLS, Orth R, Rivers DO, Ruiz-Montoya L, Sinclair EA, Statton J, Kornelis van Dijk J, Verduin JJ (2012) The central role of dispersal in the maintenance and persistence of seagrass populations. Bioscience 62:56–65
Khan S, Scholey JM (2018) Assembly, functions and evolution of Archaella, Flagella, and Cilia. Curr Biol 28:R278–R292
Kliphuis AMJ, Klok AJ, Martens DE, Lamers PP, Janssen M, Wijfels RH (2012) Metabolic modelling of Chlamydomonas reinhardtii: energy requirements for autotrophic growth and maintenance. J Appl Phycol 24:253–266
Kranzler DF, Krause JW, Brzezisnki MA, Edwards BR, Biggs WP, Maniscola M, McGrow JP, Van Mooy BAS, Bidle KD, Allen AE, Thamatrakoln K (2019) Silicon limitation facilitates virus infection and mortality of marine diatoms. Nat Microbiol 4:1790–1797
Lavoie M, Raven JA (2020) How can large-celled diatoms rapidly modulate episodic sinking episodically. J Exp Bot 71:3386–3389
Lavoie M, Raven JA, Levasseur M (2016) Energy cost and putative benefits of cellular mechanisms modulating buoyancy in aflagellate marine phytoplankton. J Phycol 52:239–251
Leadbeater DSC, McCready SMM (2000) The flagellates: historical perspectives. In: Leadbeater BSC, Green JC (ed) Flagellates: unity, diversity and evolution. The systematics association special issues volume 59. Taylor and Francis, London, pp 1–26
Leliaert F, Smith DR, Moreau H, Herron MD, Vergruggen H, Delwiche CF, De Clerck O (2012) Phylogeny and molecular evolution of the green algae. Crit Rev Plant Sci 31:1–46
Leliaert F, Tronholm A, Lemièux C, Turmel M, De Priest MS, Bhattacharya D, Karol KG, Fredericq S, Zechmen ZW, Lopez-Bautista JM (2016) Chloroplast phylogenomic analyses reveal the deepest-branching lineage of the Chlorophyta, Palmophylophyceae class. nov. Sci Rep 16:25367
Li J-J, Liu Z-Y, Zhong X-H, Zhuand L-C, Bi Y-X, Qin S (2020) Limited genetic connectivity among Sargassum horneri (Phaeophyceae) populations in the Chinese marginal seas despite their high dispersal capacity. J Phycol 56:994–1005
Lichtenberg M, Cartaxana P, Kühl M (2020) Vertical migration optimizes photosynthetic efficiency of motile cyanobacteria in coastal mat. Front Mar Sci 7:359
Liu YJ, Hodson MC, Hall BD (2006) Loss of the flagellum happened only once in the fungal lineage: phylogenetic structure of Kingdom Fungi inferred from RNA polymerase II subunit genes. BMC Evol Biol 6:74
Luther H (1949) Vorschlan zu einer okologischen Grunenleiting von Hydrophyten. Acta Botan Fenn 44:1–15
Maggs CA, Fletcher HL, Fewer D, Loade L, Mineur J, Johnson MP (2011) Speciation in red algae: members of the Ceramiales as model organisms. Integr Comp Biol 51:492–504
Mann KH, Lazier JR (2006) Dynamics of marine ecosystems: biological-physical interactions in the oceans. Blackwell Science Publishing, Oxford
Marba N, Duarte CM (1998) Rhizome elongation and seagrass clonal growth. Mar Ecol Prog Ser 174:269–280
Marques da Silva J, Duarte B, Borissovitch AB (2020) Travelling expenses: the energy cost of diel vertical migration of epipelic microphytobenthos. Front Mar Sci 7:433
Marrs RH, Watt AS (2006) Biological Flora of the British Isles: Pteridium aquilinum (L) Kühn. J Ecol 94:1272–1321
Matsuda S (1989) Succession and growth rates of incrusting crustose corraline algae (Rhodophyta, Cryptonemiales) in the upper fore-reef environment of Ishigaki Island, Ryuki Islands. Coral Reefs 7:185–195
McConchie CA, Knox RB (1989) Pollen-stigma interaction in the seagrass Posidinia australis. Ann Bot 63:235–248
McMahon K, van Dijk K-J, Ruiz-Montoya L, Kendrick GA, Krauss DL, Waycott M, Verduin J, Lowe R, Statton J, Brown E, Duarte C (2014) The movement ecology of seagrasses. Proc Roy Soc B Biol Sci 281:20140878
Merchant SS et al (2007) The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318:245–250
Mills MM, Ridame C, Davey JM, La Roche J, Geider RJ (2004) Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature 429:282–294
Mine I, Kobouchi Y, Okuda K (2003) Fine structure of spermatial surface in the red alga Antithamnion nipponicum (Rhodophyta). Phycol Res 51:109–117
Mitchell DR (2017) Evolution of cilia. Cold Spring Harbour Persp Biol 9:a028290
Miyata M, Robinson RC, Uyeda TQP, Fukumori Y, Fukushima S-I, Haruba S, Homma M, Inaba K, Ito M, Kaito C, Kato K, Kenri T, Kinesita Y, Kojima D, Minamino T, Mori H, Nakamura S, Shibata S, Nakame S, Shimabukaro K, Tamakoshi M, Taoka A, Tashiro Y, Tulum I, Wada M, Wayayashi K-i (2019) Tree of motility – a proposed history of motility systems in the tree of life. Genes Cells 25:6–21
Moestrup Ø, Hoffman LR (1975) A study of spermatozoids of Dichotomosiphon tuborosum (Chlorophyceae). J Phycol 11:225–235
Montresor M, Vitale L, D’Alello D, Ferante MI (2016) Sex in planktonic diatoms: insights and challenges. Mar Ecol 30:1–15
Moore TK, Villareal TA (1996) Buoyancy and growth characteristics of three positively buoyant marine diatoms. Mar Ecol Prog Ser 132:2513–2525
Mosquera-Murillo Z, Peñasalanera EJ (2016) Effect of salinity on growth of the green alga Caulerpa sertularioides (Bryopsidales, Chlorophyta) under laboratory conditions. Hydrobiologia 26:277–282
Moss B (1977) Adaptations of epipelic and epipsammic freshwater algae. Oecologia 28:103–108
Mulling BTM, Wood SA, Hamilton DP (2014) Intra-colony motility of Microcystis wesenbergii cells. N Z J Bot 52:152–159
Nadeau T-L, Howard-Williams C, Castenholz RW (1999) Effects of solar UB and visible irradiance of photosynthesis and vertical migration of Oscillatoria (Cyanobacteria) in an Antarctic microbial mat. Aquat Environ Ecol 7:231–243
Nakov T, Beaulieu JM, Alverson AJ (2018) Accelerated diversification is related to life history and locomotion in a hyperdiverse lineage of microbial eukaryotes (Diatoms, Bacillariophyta). New Phytol 219:462–473
Paulíčova A, Hašler P, Lysákova M, Spears B (2008) The ecology of freshwater epipelic algae: an update. Phycologia 47:437–450
Pelusi A, De Luca P, Manfellotto F, Thamatrakoln K, Bidle KD, Montresor M (2021) Virus-induced spore formation as a defense mechanism in marine diatoms. New Phytol 229:2251–2259
Pettitt JM (1980) Reproduction of seagrasses: nature of the pollen and receptive surface of the stigma in the Hydrocharitaceae. Ann Bot 45:257–271
Pettitt JM (1981) Reproduction of seagrasses: pollen development in Thalassia hemprichii, Halophila stipulacea and Thalassodendron ciliatum. Ann Bot 498:609–622
Pettitt JM, McConchie CA, Ducker SC, Knox RB (1980) Unique adaptations for submarine pollination in seagrasses. Nature 266:487–489
Pickett-Heaps J, Hill DRA, Blaze KL (1991) Active gliding motility in an araphid marine diatom Ardissonea (formerly Synedra) crystallina. J Phycol 27:718–725
Pickett-Heaps J, West JA, Wilson SM, McBride DL (2001) Time-lapse videomicroscopy of cell (spore) movement in red algae. Eur J Phycol 36:9–11
Poulsen NC, Spector I, Spurk TP, Schulz TF, Wetherbee R (1999) Diatom gliding is the result of an actin-myosin motility system. Cytoskeleton 44:23–33
Ralston DK, Mcgillikuddy DJ Jr, Townsend DW (2007) Asynchronous vertical migration and bimodal distribution of motile phytoplankton. J Plankton Res 29:803–821
Raven JA (1981) Nutritional strategies of benthic plants; the acquisition of C, N and P by rhizophytes and haptophytes. New Phytol 88:1–30
Raven JA (1982) The energetics of freshwater algae: energy requirements algae: energy requirements for biosynthesis and volume regulation. New Phytol 92:1–20
Raven JA (1983) Cyanobacterial motility as a test of the quantitative significance of proticity transmission along membranes. New Phytol 94:511–519
Raven JA (2000) The flagellate condition. In: Leadbeater BSC, Green JC (eds) Flagellates: unity, diversity and evolution. The systematics association special issues volume 59. Taylor and Francis, London, pp 27–48
Raven JA (2018) The potential effect of low osmolarity of cell function through decreased concentration of enzyme substrates. J Exp Bot 69:4667–4673
Raven JA, Doblin MA (2014) Active water transport in algae: where, why and how. J Exp Bot 65:6279–6292
Raven JA, Richardson K (1984) Dinophyte flagella: a cost-benefit analysis. New Phytol 98:259–276
Raven JA, Sánchez-Baracaldo P (2021) Gloeobacter and the implications of a freshwater origin of cyanobacteria. Phycologia. https://doi.org/10.10180/00318884.2021.18811729
Raven JA, Waite AM (2004) The evolution of silicification in diatoms: inescapable sinking and sinking as escape? New Phytol 164:45–61
Raven JA, Suggett DJ, Giordano M (2020) Inorganic carbon concentrating mechanisms in free living and symbiotic dinoflagellates and chromerids. J Phycol 65:6279–6292
Rees TAV, Raven JA (2021) The maximum growth rate hypothothesis is correct for eukaryotic photosynthetic organisms, but not cyanobacteria. New Phytol 230:601–611
Reusch TBH (2003) Floral neighbourhoods in the sea: how floral density, opportunity for outcrossing and population fragmentation affect seed set in Zostera marina. J Ecol 91:610–615
Ross ON (2006) Particles in motion: how turbulence affects plankton sedmentation from an oceanic mixed layer. Geophys Res Lett 33:L10609
Round FE, Palmer JD (1966) Persistent, vertical-migration rhythms in benthic microflora: field and laboratory studies in diatoms from the banks of the river Avon. J Mar Biol Assoc UK 46:191–214
Round FE, Crawford RM, Mann DG (1990) The diatoms. Biology and morphology of the Genera. Cambridge University Press, Cambridge. p 747
Ruiz J, Macios D, Peters F (2004) Turbulence increases average settling velocity of phytoplankton. Proc Natl Acad Sci U S A 101:17720–17724
Santelices B (2002) Recent advances in fertilization ecology of macroalgae. J Phycol 38:4–10
Sañudo-Wilhelmy SA, Kustka AB, Gobler CJ, Hutchins DA, Yang M, Lwiza K, Burns J, Capone DG, Raven JA, Carpenter EA (2001) Phosphorus limitation of nitrogen fixation by Trichodesmium in the central Atlantic Ocean. Nature 411:66–69
Sato S, Beakes G, Idei M, Nagumo T, Mann DG (2011) Novel sex cells and evidence for sex pheromones in diatoms. PLoS One 6:e26923
Schlosser C, Klav JK, Wake BD, Snow JP, Honet DT, Woodward EMS, Lohan MC, Honey DT, Woodward EMS, Achterberg FP, Moore CM (2014) Seasonal ITCZ migration dynamically controls the location of the (sub)tropical Atlantic biogeochemical divide. Proc Natl Acad Sci U S A 111:1438–1442
Schmidt M, Horn S, Ehlers K, Wilhelm C, Schnetter R (2015) Guanchochroma wildpretti gen. et sp. nov. (Ochrophyta) provides new insight into the diversification and evolution of the algal class Synchromophyceae. PLoS One 10:e013821
Schuh R, Menden-Deuer S (2014) Going ballistic in the plankton: anisotropic swimming behavior of the marine protists. Limnol Oceanogr Fluids Environments 4:1–16
Sinclair EA, Gecan I, Krauss SL, Kendrick GA (2014) Against the odds: complete outbreeding in a monoecious clonal seagrass Posidonia australis (Posidoniaceae). Ann Bot 113:1185–1196
Sommer U, Gliwicz ZM (1986) Long range vertical migration of Volvox in tropical Lake Cahona Bassa (Mozambique). Limnol Oceanogr 31:650–653
Suttle CA (2007) Marine viruses –major players in global ecosystem. Nat Rev Microbiol 5:801–812
Van den Hoek C, Mann DG, Jahns HM (1995) Algae. An introduction to phycology. Cambridge University Press, Cambridge, pp xiv + 623
Van Tussenbroek BI, Villamill N, Márquez-Guzmán J, Wong R, Monroy-Vealáquez LV, Solis-Weis V (2016) Experimental evidence of pollination in marine flowers by invertebrate fauna. Nat Commun 7:129880
Venuleo M, Raven JA, Giordano M (2017) Intraspecific chemical communication in microalgae. New Phytol 215:516–530
Vermaat JE, Rollon RV, Lacap CDA, Billot C, Alberte F, Nacorda NME, Wiegman F, Terrados JT (2004) Meadow fragments and reproductive output of the SE Asian seagrass Enhalus acoroides. J Sea Res 52:321–328
Villareal TA (1986) Positive buoyancy in the oceanic diatom Rhizosolenia debayana H. Peragallo. Deep Sea Res A Oceanographic Res Papers 35:1037–1043
Villareal TA, Carpenter EJ (1990) Diel buoyancy regulation in the marine diazotrophic cyanobacterium Trichodesmium thiebaultii. Limnol Oceanogr 35:1832–1837
Villareal TA, Carpenter EJ (2003) Buoyancy regulation and the potential for vertical migration in the oceanic cyanobacterium Trichodesmium. Microb Ecol 45:1–10
Villareal TA, Pilskain C, Brzezinski M, Lipschultz F, Dennett M, Gardner GB (1999) Upward transport of oceanic nitrate by migrating diatom mats. Nature 397:423–425
Villareal TA, Pilskain C, Montoya JP, Dennett M (2014) Upward nitrate transport by phytoplankton in oceanic waters: balancing nutrient budgets in oligotrophic seas. Peer J 2:e302
Vogel S (2020) Life in moving fluids. The physical biology of flow, 2nd edn, Revised and Expanded. Princeton University Press, Princeton, NJ, p 503
Waite A, Harrison PJ (1992) Role of sinking and ascent in sexual reproduction in the marine diatom Ditylum brightwellii. Mar Ecol Prog Ser 87:113–122
Waite A, Thompson PA, Harrison PJ (1992) Does energy control the sinking rates of marine diatoms? Limnol Oceanogr 37:468–477
Waite A, Fisher A, Thompson PA, Harrison PJ (1997) Sinking rate versus cell volume relationship illuminates sinking rate control in marine diatoms. Mar Ecol Prog Ser 157:97–108
Walsby AE (1994) Gas vesicles. Microbiol Rev 58:94–144
Waterbury JB, Willey JM, Franks DG, Valois FW, Watson SW (1985) A cyanobacterium capable of swimming motility. Science 169:3429–3434
Wickstead B, Gull K (2007) Dyneins across eukaryotes: a comparative genomic analysis. Traffic 8:1708–1721
Wilhelm SW, Suttle CA (1999) Viruses and nutrient cycles in the sea: viruses play critical roles in the structure and function of aquatic food webs. Bioscience 49:781–788
Williams SL (1995) Surfgrass (Phyllospadix torreyi) reproduction: reproductive phenology, resource allocation, and male rarity. Ecology 76:1953–1970
Williams SL, Breda VA, Anderson TW, Nyden BB (1985) Growth and sediment disturbance of Caulerpa spp. (Chlorophyta) in a submarine canyon. Mar Ecol Prog Ser 21:275–281
Wirtz K, Smith SL (2020) Vertical migration by bulk phytoplankton sustains biodiversity and nutrient input to the surface ocean. Sci Rep 10:1142
Woo YH et al (2015) Chromerid genomes reveal the evolutionary path from photosynthetic algae to obligate intracellular parasites. Elife 4:e069774
Yamada T, Tomura Y, Fukuda H, Nogata T (2018) Aggregate formation during the viral lysis of a diatom. Front Mar Sci 5:article 167
Yamoaka N, Suetome Y, Yoshida T, Sonube S (2016) Motion analysis and unltrastructural study of a colonial diatom, Bacillaria paxillifer. Microscopy 65:211–221
Yuji T, Yoyoda T, Kei K (2015) Marine diatom viruses and their hosts: resistance mechanisms and population dynamics. Persp Phycol 2:65–81
Zhu H, Hu Z, Kiu G (2017) Morphology and molecular evolution of Trentepohliales (Chlorophyta) from China. Eur J Phycol 52:330–341
Acknowledgements
Discussion with John Beardall, Susan Brawley, Juliet Brodie, Martina Doblin, Zoe Finkel, Mario Giordano, Gary Kendrick, David Mann, Antonietta Quigg, Katherine Richardson, Anya Waite, Diana Walker, Tony Walsby, Michelle Waycott and Mark Westoby have been very helpful, Comments on the manuscript by Ulrich Lüttge and Glen Wheeler have greatly improved it.
The University of Dundee is a registered Scottish charity, No SC 015096.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Communicated by Ulrich Lüttge
Appendix. Flagella: What they Are and Where They Occur Among Photosynthetic Organisms
Appendix. Flagella: What they Are and Where They Occur Among Photosynthetic Organisms
1.1 Definition of Flagella
The term ‘flagella’ (singular flagellum) is used for both the bacterial and eukaryotic waggling aqueous motility device. Khan and Scholey (2018) use the terms ‘flagella’ for the bacterial structure, ‘cilia’ for the eukaryote structure, and ‘archaella’ for the analogous structure in the Archaea. However, here the eukaryote organelle is termed, as is it commonly called in plant science, ‘flagella’ (Leadbeater and McCready 2000). It has been suggested that flagella in this sense occurred in the Last Eukaryotic Common Ancestor (Mitchell 2017).
1.2 Centrioles/Basal Bodies Are Ancestral
Carvalho-Santos et al. (2011) provide phylogenetic evidence on the ancestral nature of Centrioles/Basal Bodies, and their losses and those of flagella and cilia.
1.3 Flagellar and Cytoplasmic Dynein Phylogeny
Wickstead and Gull (2007) showed that cytoplasmic dyneins were lost from Rhodophyta, Viridiplantae, and Entamoeba; flagella dyneins are absent from Rhodophyta and flowering plants.
1.4 Fungi: Only Photosynthetic as Lichen Symbioses
Liu et al. (2006) showed that there was only a single loss of the flagellum in fungi: excluding the microsporidia as a sister group to the fungi, the only flagellate fungi are the basal Chytridiomycota. Using a broad definition of symbiosis, some chytrids are parasitic symbionts of algae. Using the narrow definition of symbiosis as mutualistic, Glomeromycotina are mutualistic with embryophytes or (in one case) a cyanobacterium, and Ascomycota and Basidiomycota are mutualistic with vascular plants as mycorrhizas and cyanobacteria and trebouxiophycean and ulvophycean (Trentepohliales) green algae as lichens.
1.5 Number of Species With and Without Flagella in the Glaucophyta, Rhodophyta, and Algal Streptophyta
Flagella are common in the Glaucophyta (Jackson et al. 2015) with 25 species (Guiry and Guiry 2021) (15-2-2018). Flagella are lacking in the Rhodophyta with 7,034 species (Guiry and Guiry 2021). The sister group to the Rhodophyta, the non-photosynthetic phagotrophic Rhodelphidia, have flagella (Gawryluk et al. 2019; Colp and Archibald 2019). The algal Streptophyta (=Charophyta) have 5,068 species, of which the flagella-less Zygnematphyceae have 4,150 species, and almost all of the remaining 918 species have flagella (Guiry and Guiry 2021).
1.6 Chlorophyta
1.6.1 Palmophyllophyceae, Prasinophyceae, Chlorophyceae, Trebouxiophyceae, and Ulvophyceae
The Palmophyllophyceae and Prasinophyceae are basal Chlorophyta (Leliaert et al. 2012; Leliaert et al. 2016). The known Palmophyllophyceae are benthic palmellmoid organisms; flagella are unknown (Leliaert et al. 2016). The Prasinophyceae are planktonic and have members with (Micromonas, Mantoniella, Pyramimonas) and without (Ostreococcus, Pycnococcus) expressed flagella (Leliaert et al. 2012).
Chlorella variabilis NC64A (Trebouxiophyceae) is not known to have flagella, but has 103 out of the 360 (29%) flagella-specific proteins in the chlorophycean Chlamydomonas (Blanc et al. 2010). Figure 4 of Blanc et al. (2010) compares flagella proteins from Chlamydomonas reinhardtii with those of C. variabilis NC64A and with Ostreococcus tauri, O. lucimaris, Micromonas CCMP, and Micromonas RCC (Prasinophyceae) and Thalassiosira pseudonana (Bacillariophyceae). Of the organisms with flagella, the two Micromonas strains had all but one of the 50 Chlamydomonas proteins, while T. pseudonana only has 16 of the 50 Chlamydomonas proteins. The two Ostreococcus species each have same 4 of the 50 Chlamydomonas proteins. The trebouxiophycean Asterochloris has flagellate spores, and has dynein genes (Kellmar 2016).
Merchant et al. (2007) used ‘cilia cut’ to seek Chlamydomonas flagellar proteins in organisms known to have flagella (Homo sapiens, Phytophthora sp.) and those that lack flagella: Arabidopsis (angiosperms), Cyanidioschyzon (Rhodophyta), Neurospora, Dictyostelium, and Archaea and Bacteria). Phaeodactylum (Bacillariophyceae) lacks flagella genes (Bowler et al. 2008).
The Ulvophyceae are benthic multicellular or coenocytic macroalgae; the reproductive unicells are generally flagellate (Leliaert et al. 2012). This is the case not only for the marine ulvophyceans, and freshwater ulvophycean Dichotomosiphon (Moestrup and Hoffman 1975), but also for the subaerial Trentepohliales (Brooks et al. 2015; Zhu et al. 2017). The Chlorophyta have 6,799 species (Guiry and Guiry 2021)
1.7 Euglenophyta (Euglenozoa)
These secondary endosymbionts of a green algal endosymbiont in an excavate endosymbiont are universally flagellate. 1,521 species (Guiry and Guiry 2021).
1.8 Chlorarachniophyceae (Cercozoa/Rhizaria)
These secondary endosymbionts of a green algal endosymbiont in a rhizarian endosymbiont were first known as amoebae, but some are flagellate. 15 species (Guiry and Guiry 2021).
1.9 Alveolata
1.9.1 Dinophyta
Dinoflagellates are apparently ancestrally photosynthetic involving secondary endosymbiosis and, subsequently, tertiary endosymbiosis, but some have lost the capacity to photosynthesize (Raven et al. 2020). Many dinoflagellates are flagellate phytoplankton; others are non-flagellate most of the time, but have a flagellate reproductive phase. 3,490 species (Guiry and Guiry 2021).
1.10 Chromerida
Chromerids are photosynthetic symbionts of corals (Raven et al. 2020); the flagellate stage of Chromera velia is found more frequently in low salinity cultures (Guo et al. 2010). The chromerid flagella apparatus was modified as part of the host invasion apparatus in their evolutionary descendants, the non-photosynthetic apicomplexan parasites (Woo et al. 2015). 2 species (Chromera and Vitrella) (Guiry and Guiry 2021).
1.11 Ochrophyta
1.11.1 Bacillariophyceae (as Bacillariophyta in Guiry and Guiry 2021)
Vegetative diatom cells are non-flagellate. (Ancestral) centric diatoms have flagellate male gametes; (derived) pennate diatoms have non-flagellate gametes. 16,803 species (Guiry and Guiry 2021). Nakov et al. (2018) relate accelerated diversification in diatoms to their life history and locomotion.
1.11.2 Bolidophyceae
Flagellate planktonic motile cells and non-flagellate cyst (Palmales) phases. 18 species (Guiry and Guiry 2021).
1.11.3 Chrysomerophyceae
Filamentous, with flagellate spores (Kai et al. 2008). 7 species (Algaebase).
1.11.4 Chrysophyceae
Flagellate planktonic unicells or colonies; some filaments or macroscopic (Hydrurus) non-motile benthic organisms with flagellate reproductive phase. 764 species (Guiry and Guiry 2021).
1.11.5 Dictyochophyceae
Marine flagellate planktonic cells. 161 species (Guiry and Guiry 2021).
1.11.6 Eustigmatophyceae
Coccoid freshwater (a few marine) cells; reproductive cells flagellate. 102 species (Guiry and Guiry 2021).
1.11.7 Pelagophyceae
Marine plankton, coccoid or flagellate; some benthic palmelloid or filamentous. 25 species (Guiry and Guiry 2021).
1.11.8 Phaeophyceae
Alternation of phases, except in the Fucales. Motile spores, except in Dictyotales. Both gametes flagellate in isogamous/anisogamous species, male gamete flagellate in oogamous species. 2,061 species (Guiry and Guiry 2021).
1.11.9 Phaeothamniophyceae
Filamentous with flagellate spores (Bailey et al. 1998). 35 species (Guiry and Guiry 2021).
1.11.10 Pinguiophyceae
Planktonic, with flagellate stages (Kawachi et al. 2002). 5 species (Algaebase).
1.11.11 Raphidophyceae
Plankonic or psammophilic flagellates in fresh, brackish or marine habitats. 40 species (Guiry and Guiry 2021).
1.11.12 Schizocladiophyceae
Marine, branching filaments: flagellate zoospores. Kawai Mn Maeba et al. (2003). 1 species (Guiry and Guiry 2021).
1.11.13 Synchromophyceae
There are no reports of flagella in the Synchromophyceae (Schmidt et al. 2015). 7 species (Guiry and Guiry 2021).
1.11.14 Synurophyceae
Planktonic flagellate unicells or colonies. 417 species (Guiry and Guiry 2021).
1.11.15 Tribophyceae (Xanthophyceae)
Unicellular, multicellular or (Vaucheria) coenocytic. Some vegetative cells are planktonic flagellates. Reproductive cells flagellate; compound zoospores in Vaucheria. 695 species (Guiry and Guiry 2021).
1.11.16 Haptophyta
Marine (e.g., coccolithophores, and the colony-forming Phaeocystis), and a few freshwater, plankton, many with flagella. 936 species (Guiry and Guiry 2021).
1.11.17 Cryptophyta
Freshwater and marine planktonic flagellates. 218 species (Guiry and Guiry 2021).
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Raven, J.A., Lavoie, M. (2021). Movement of Aquatic Oxygenic Photosynthetic Organisms. In: Lüttge, U., Cánovas, F.M., Risueño, MC., Leuschner, C., Pretzsch, H. (eds) Progress in Botany Vol. 83. Progress in Botany, vol 83. Springer, Cham. https://doi.org/10.1007/124_2021_55
Download citation
DOI: https://doi.org/10.1007/124_2021_55
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-12781-6
Online ISBN: 978-3-031-12782-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)