Abstract
Phi thickenings, lignified bands of secondary cell wall encircling root cortical cells, align between adjacent cells to form a complex network that frames the endodermis and central stele. Since their description in numerous angiosperms and gymnosperms in the two decades from the 1860s, there has been little research into the functions of these enigmatic structures. Their cage-like organisation led to speculation that phi thickenings mechanically strengthen the root, but more recent ideas include that they regulate biotic interactions or control ion movements in a manner similar to the Casparian strip. There is, however, sparse direct evidence supporting these suggestions. In this review, we focus on the roles that phi thickenings might play within roots, and although we conclude that the primary function of phi thickenings is to mechanically stiffen the root apex, we emphasise that phi thickenings need not have a single role and that they can be “re-tooled” to perform other functions. We describe several experimental systems for studying phi thickening functions. Geranium and Pelargonium roots, in which phi thickenings form in cortical cells immediately under the epidermis, are well suited for cell biology investigations, whereas the large aerial roots of epiphytic orchids are ideal for investigating root biomechanics. For genetic analysis, however, the differential induction of phi thickenings in Brassica roots in response to water stress or hormones provides a powerful experimental platform to identify regulatory mechanisms and directly test our models of phi thickening functionality. Since phi thickenings typically form in the root apex, we propose that these structures function primarily to strengthen or stiffen the plant root. We reject the concept that phi thickenings function to block ion flows in a manner analogous to the Casparian strip, but instead see the potential for limiting ion flows within the root cortex as drawback to the presence of thickenings. This negative outcome due to the presence of phi thickenings may explain why phi thickenings are only induced in response to specific biotic or abiotic challenges in some species and are not formed constitutively. In some instances, however, phi thickenings may have been “re-tooled” to perform other roles including blocking uptake of ions, notably in the case of Brassicaceae species growing under extreme conditions.
Communicated by Ulrich Lüttge
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction and a History of Phi Thickenings
Phi thickenings are typically thin bands of lignified, secondary cell wall that form around the radial cell walls in the root cortex, a cellular location where only a thin, primary cell wall would normally be found. This narrow band of thickenings encircles the entire cortical cell, and as the thickenings are typically coordinated between adjacent cells, an entire network of lignified thickenings surrounding the central stele is produced. However, unlike the endodermis, phi thickenings do not become suberised, and unlike secondary cell wall development in xylem, the cells that form phi thickenings (phi cells) do not undergo programmed cell death. This review will discuss the possible functions of these phi thickenings within the root.
1.1 Van Tieghem and His Discovery of Phi Thickenings
In 1871, the French botanist Van Tieghem published a long monograph in Annales des Sciences Naturelles Botanique on the structural symmetry of vascular plants (Van Tieghem 1871). In a section of gymnosperms (p. 187), he provided the first generally acknowledged description of structures that are now known as phi thickenings, although this term was not one that Van Tieghem himself used:
L’avant-dernière assise corticale possède un caractère spécial. Ses cellules, à section carrée ou hexagonale, ont au milieu de leurs faces latérales et transverses une bande d'épaississement saillante en dedans et arrondie, et les bandes des cellules voisines se correspondent exactement. Cette bande d'abord blanche, et devenant plus tard jaune clair, forme un cadre rectangulaire qui donne beaucoup de solidité à la cellule.
This statement translates as:
The penultimate cortical layer [that is, the inner cortical layer] has a special character. Its cells are square or hexagonal in cross section, and have in the middle of their lateral and transverse walls a thickened strip that is rounded and projects into the cell. The strips of the neighbouring cells correspond exactly. This band, initially white, and later becoming light yellow, forms a rectangular frame which gives a lot of solidity to the cell.
Van Tieghem reported these thickenings in a range of different gymnosperms, including Taxus baccata (European yew) which he illustrated in cross section, and other species from the Cupressaceae (cypresses) and Taxaceae (yews). In a subsequent series of papers in 1887 and 1888 (Van Tieghem 1887a, b, c, 1888a, b; Van Tieghem and Monal 1888), he also described thickenings in a range of different angiosperm families including the Brassicaceae (the crucifers or cabbage family), the Rosaceae (rose family), the Caprifoliaceae (honeysuckles), the Fabaceae (the legumes), and the Geraniaceae (geraniums). Further, by using clearing and the cell wall stain basic fuchsin, he demonstrated that these cell wall thickenings were lignified. In many cases, Van Tieghem’s work on phi thickenings in a particular species, genus, or family remains the only published description of these structures in those taxa.
1.2 Nicolai and the Discovery of Phi Thickenings
Contrary to statements in all publications concerning phi thickenings since the 1920s, Van Tieghem was not the first to describe these enigmatic structures. In a general study of root development, Nicolai (1865) described cortical cell wall thickenings in gymnosperm roots including Taxus, writing on page 62 that:
die innerste Rindenschicht in eine Schutzscheide verwandelt. Bald nachdem dieses geschehen ist, sieht man in den anderen Zellreihen der Rinde, und zwar zunächst in der Zellreihe, die die Schutzscheide umgiebt, eine eigenthümliche Verdickung, die durch einen verholzten und sich allmälich sehr stark verdickenden, senkrechten Streifen der radialen Wand hervorgerufen wird.....
Ausser bei Coniferen sah ich eine ähnliche Verdickung nur bei Pyrus malus, hier aber auf den Zellkreis beschränkt, der unmittelbar die Schutzscheide umgiebt.
In translation:
the innermost bark [cortical] layer is transformed into a protective sheath [the endodermis]. Shortly after this has happened, one sees a peculiar thickening in the other cell rows of the cortex, first in the cell layer that surrounds the endodermis, which is caused by a lignified and gradually thickening, vertical strip of the radial wall.
Except for conifers, I saw a similar thickening only in Pyrus malus [apple], but there it is limited to the ring of cells that immediately surround the endodermis.
Later in the same article, he also described similar, albeit more complex thickenings in the aerial roots of the orchid Cattleya. Although cited by Kroemer (1903) and Scott and Whitworth (1928), this initial description of cell wall thickenings seems to have been forgotten. However, while illustrations were not provided, the written descriptions are consistent with the development of phi thickenings in gymnosperm roots, with their initial development in the inner cortex immediately outside the endodermis and subsequent formation of smaller and slightly more random thickenings in layers further out. Moreover, as suggested by Nicolai (1865), phi thickenings also occur in Pyrus malus in a single ring in the inner cortex (Riedhart and Guard 1957; Mackenzie 1979; Peterson et al. 1981), while large and complex phi thickenings are present in the aerial roots of the orchid Cattleya (Brundrett et al. 1988) and in the related genera Laelia (Idris et al., manuscript in preparation) and Rhyncholaelia (Collings, unpublished data). Thus, it would seem that credit for the initial descriptions of phi thickenings should belong to Nicolai (1865) who not only provided the first description of these structure in gymnosperms, but also gave the first description of similar structures in angiosperms.
1.3 The Origin of the Name “Phi Thickenings”
The term “phi thickenings” was coined neither by Van Tieghem nor Nicolai. In a monograph on the vascular system of plants, Russow (1875) wrote:
Hierher gehört die in den Wurzeln der Cupressineen und Taxineen vorkommende, zuerst von van Tieghem beschriebene Scheide, welche dadurch ausgezeichnet ist, dass die zur Oberfliche des Centralcylinders rechtwinklig stehenden Wände der sie zusammensetzenden Zellen mit einem planconvexen Verdickungsband versehen sind. Um diese merkwürdig gebaute Scheide, zum Unterschiede von anderen Aussenscheiden, kurz zu bezeichnen, wollen wir sie Φ-Scheide nennen, weil der Querdurchschnitt der verdickten Wand dem griechischen Buchstaben Φ gleicht.
This statement translates as:
This description includes the protective layer of cells found in the roots of the Cupressaceae and Taxaceae, first described by van Tieghem, which are distinguished by the semi-circular cell wall thickenings in the cell walls that run at right angles to the surface of the central cylinder [stele]. To briefly describe this strange structure, in contrast to other thickened cell walls, we might call them Φ thickenings because the cross-section of the thickened wall resembles the Greek letter Φ.
Russow went on to describe similar phi thickenings in a range of angiosperms from the Rosaceae, Caprifoliaceae, Fabaceae, and Berberidaceae (barberries), but as with Van Tieghem’s catalogue of species that contain phi thickenings, the phi thickenings in many of the species that Russow identified have not subsequently been studied.
1.4 The Late Nineteenth Century: A Golden Age for Phi Thickening Discovery
Considering the relative paucity of phi thickening research in the years since 1900, there was a surprising variety of research into these structures in the two decades years immediately after Nicolai, Van Tieghem, and Russow’s initial observations. These included similar observations of phi thickenings in a range of gymnosperm species by Klein (1872a, b, c), Reinke (1873) and de Bary (1877), observations of phi thickenings in Brassica oleracea roots (Woronin 1878) and observations made by Schwendener (1874) in his book Das mechanische Princip im anatomischen Bau der Monocotylen (The Mechanistic Principle on the Anatomical Structure of Monocots). In a section entitled “Mechanically-effective parenchyma cells” (page 159), he wrote:
Als mechanisch wirksame Elemente und zwar als Einrichitungen gegen radiale Druckkräfte betrachte ich endlich auch die Zellen der Schutzscheide in Wurzeln und Rhizomen, jedoch nur soweit sie verdickte Wandungen besitzen; ebenso jene eigenthümlichen Membranverdickungen, welche zuweilen im Parenchym ausserhalb der Schutzscheide auftreten und um diese letztere ein zusammenhängendes Netzwerk mit longitudinal gestreckten Maschen herstellen. Wo solche Verdickungsleisten unter der Epidermis oder sonst im Parenchym vorkommen, mögen, sie einem analogen Zwecke dienen. Aber auch hier ist es bloss eine hypothetische Ansicht, die ich ausspreche; die wissenschaftliche Prüfung und Durchführung derselben würde eingehendere Untersuchungen voraussetzen, als ich sie bis dahin angestellt habe.
This translates as:
Finally, I consider the cells of the endodermis in roots and rhizomes as mechanically effective elements, namely as devices against radial pressure forces, but only as far as they have thickened walls. Likewise, the peculiar cell wall thickenings, which sometimes occur in the parenchyma outside the endodermis, and around which they form a coherent network with a longitudinally elongated meshwork. Wherever such thickenings occur under the epidermis or in the parenchyma, they may serve an analogous purpose. But here, too, this is just a hypothesis view that I am expressing; the scientific examination of this concept would require more detailed investigations than I have so far completed.
Schwendener contributed the first and most direct early statement about a possible mechanical role for the phi thickenings but more than a century later, we are still waiting for “more detailed investigations” to be conducted into the mechanical effects played by phi thickenings in plant roots.
1.5 The Scope of This Review
As we near the 150th anniversary of Van Tieghem’s initial observations of phi thickenings and of the observations by Nicolai, Reinke, de Bary, Russow, Klein, Schwendener, and Woronin, it is worth considering how far we have progressed in understanding these enigmatic structures. While the list of species that exhibit thickenings has increased substantially, having been described in four and fourteen different orders of the gymnosperms and angiosperms, respectively (Aleamotuʻa et al. 2019), we remain no closer to understanding the functions that these structures play within the root. Thus, the intent of this review is to focus primarily on the functions of phi thickenings, and as such, discussion of the distribution, structures, and chemical composition of phi thickenings will be limited. Readers interested in these topics are directed to several relevant reviews (de Melo 2011; Fernández-García et al. 2014; Aleamotuʻa et al. 2019). Furthermore, our discussion of the development of these structures, at both a molecular and cellular level, is also limited. Although there is little primary research on development to review, our thinking on this topic is discussed elsewhere (Aleamotuʻa et al. 2019).
1.6 Suggested Roles for Phi Thickenings
Phi thickenings can be induced in roots by a range of abiotic and biotic stresses (Sect. 3). Because of this induction, and because basic structure of phi thickenings has been conserved across diverse taxa from gymnosperms to angiosperms, it is normally assumed that there must be a functional reason for the plant to make these structures. Various functions of phi thickenings in plant roots have been proposed, summarised into three broad themes. Thickenings might:
-
provide mechanical strength or support to the root,
-
regulate apoplastic transport in a manner analogous to the Casparian strip, and,
-
regulate interactions of the root with fungi and other microorganisms.
In this review (Sects. 4–6), we consider the evidence for and against each of these functions. We conclude that phi thickenings are likely to function differently in roots of different species, and that in certain circumstance, all three proposed functions are likely to be valid explanations of phi thickening function. However, we suggest that the default function for phi thickenings is to mechanically strengthen the plant root, and that only in certain cases have phi thickenings been re-tooled to perform other roles.
2 Four Experimental Systems for Testing Phi Thickening Functions
A fundamental problem in considering the function of phi thickenings is the paucity of experimental studies that have been conducted to assess their function. Almost exclusively, data concerning phi thickenings is limited to a description of their localisation in different species or, in some cases, to descriptions of factors that induce their formation. Specific tests of function have, however, been rare. In this section, we define four experimental systems that we have recently used and explain why they provide excellent experimental approaches in which to investigate the functionality of phi thickenings. These model systems differ, however, in the locations in which the phi thickenings develop within the root. The suggested models are:
-
1.
roots of the Geraniaceae, notably in species of Pelargonium and Geranium,
-
2.
aerial roots of epiphytic orchids, including species from the genera Laelia, Rhyncholaelia, and Cattleya,
-
3.
roots of the Brassicaceae, notably Brassica oleracea and B. napus, and.
-
4.
reticulate networks present in the inner face of the inner cortex of roots from the Brassicaceae.
These four systems show significant positional differences in their phi thickenings. In his initial review, Van Tieghem (1888b) noted that there were three different localisations of thickenings within roots. Type I thickenings, as exemplified by the Brassica root and the reticulate network, are found in the cell layer immediately outside the endodermis. Type II thickenings are found in roots of species from the Geraniaceae and are localised to the cortical cell layer immediately beneath the epidermis. Type III thickenings are found in the intervening cells of the root cortex, in between the peri-epidermal layer (to use Van Tieghem’s terminology) and the sub-epidermal layer, with the Laelia root providing an excellent example of this localisation. Thus, these four systems can be thought of as complementary. We include information about these systems in the hope that others might be inspired to use these as models in their own research into the functions of phi thickenings.
2.1 Roots from Geranium and Pelargonium
Phi thickenings in the Geraniaceae occur in the cell layer immediately below the epidermis, as observed by Bergendal (1883) in Geranium molle and G. sylvaticum, by Van Tieghem and Monal (1888) in a wide range of species from the genera Geranium, Pelargonium, and Erodium, and by Kroemer (1903) in G. rotundifolium. More detailed histochemical and structural investigations were provided in numerous papers between the 1920s and 1980s in which G. rotundifolium (Scott and Whitworth 1928), P. hortum and P. peltatum (Haas et al. 1976; Peterson et al. 1981; Perumalla et al. 1990; Peterson and Cholewa 1998; Meyer and Peterson 2011) were studied. These studies confirmed the network of lignified phi thickenings in the sub-epidermal layer that develop near the root tip and showed that the thickenings were typically semi-circular in shape in individual cells so that the paired structure did indeed appear like the Greek letter phi. The studies also demonstrated that in older parts of the root, smaller thickenings could develop in cell corners in multiple layers of cells deeper into the cortex.
The most significant advantage of Geranium and Pelargonium roots for testing phi thickenings is their constitutive formation immediately below the epidermis, making for more practical cell biological experimentation than in species where the thickenings are located deeper within the root. This localisation allowed the permeability of phi thickenings to be tested with fluorescent apoplastic tracers, with experiments demonstrating dye diffusion through the root as far as the endodermis, thus questioning the possible role of phi thickenings as an apoplastic barrier (Peterson et al. 1981; Perumalla et al. 1990; Meyer and Peterson 2011). The interpretation of this data will be discussed further (Sects. 5.2 and 5.3). The Geraniaceae system does, however, have one major weakness for studying the function of phi thickenings: with thickening formation being constitutive, and with no known cultivars in which thickenings do not form, and no known inhibitors of the thickening formation pathway, control experiments that lack phi thickenings are not yet possible.
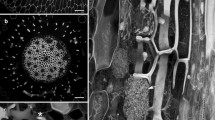
We recently commenced a reinvestigation of the Geranium and Pelargonium roots. Rather than growing roots from stem cuttings, or taking them from plants growing in soil, we used surface-sterilised seeds of two species, Geranium robertianum and Pelargonium australe, grown aseptically on agar plates as this provides an experimental system more suitable for the application of drugs and other compounds. Using lignin and cellulose stains (basic fuchsin and calcofluor white) and confocal imaging, we confirmed that phi thickenings form a complex network of lignified walls in the sub-epidermal cells (Fig. 1) beginning only several millimetres from the root tip. Reconstructed root cross sections, generated in ImageJ from high resolution optical stacks, showed that the highly elongate thickenings occupied much of the cells’ radial walls (Fig. 1b). Moreover, while the outer face of these sub-epidermal cells was also weakly lignified (Fig. 1b, arrow), extra phi thickenings present in other layers of the cortex were not present. These three observations were made in both species, but run counter to previously published reports. One possible explanation for these differences is that the narrow primary roots that form following seed germination on agar plates produce slightly different phi thickenings and anatomy to roots induced from cuttings when grown in soil. While the structures that we observed in Geranium robertianum and Pelargonium australe have some similarities to a hypodermis, the sub-epidermal structure present in the roots of many plant species (Perumalla et al. 1990; Peterson and Perumalla 1990), the strong secondary cell wall indicates that they are phi thickenings.
Confocal imaging of roots of Pelargonium australe. Whole roots were fixed, cleared, and then double-labelled with basic fuchsin that stained lignin (left column) and calcofluor white for cellulose (right column). (a) Maximum projection of a longitudinal section collected near the root tip showing the sub-epidermal phi thickenings. (b) Cross sections generated using the reslice function in ImageJ. These (and subsequent computer-generated cross sections) come from optical stacks collected with the confocal pinhole minimised in order to optimise Z resolution. The weakly lignified Casparian strip (CS) is a broad band within the radial wall of the endodermis. The sub-epidermal cells contain phi thickenings (ϕ) that extend along most of the radial wall, and there is some lignification of the outer wall of this cell layer (arrow). Scale bar = 50 μm for both images. Ep epidermis, ϕ phi thickenings, En endodermis, CS Casparian strip, X xylem
2.2 Roots of Epiphytic Orchids
Our second model system for research into phi thickenings is the epiphytic orchid root and, in particular, the aerial roots from species in the Cattleya group including those from the genera Cattleya, Laelia and Rhyncholaelia. While phi thickenings are rare in monocots (de Melo 2011; Fernández-García et al. 2014; Aleamotuʻa et al. 2019), they are common in orchids, being more frequent in epiphytic and lithophytic species where roots grow in little or no soil, than in species growing in soil (Olatunji and Nengim 1980; Burr and Barthlott 1991). Our initial characterisation of orchid phi thickenings investigated the structure and development of thickenings in the South American epiphyte Miltoniopsis sp. (Idris and Collings 2015), and we subsequently investigate the function and induction of these structures showing that their presence was unrelated to the development of orchid mycorrhizae, that they did not block the uptake of fluorescent tracer dyes, and that the thickenings were induced by water stress (Idris and Collings 2019). The Miltoniopsis root system is not, however, experimentally ideal as the development of thickenings is slow and variable. Instead, we have investigated Rhyncholaelia digbyana, a species that has dramatic aerial roots which contain impressive, multiple-ring networks of phi thickenings (Fig. 2). These structures, which in longitudinal sections resemble the reinforcing girders of a building (Fig. 2b), might be a more suitable system for studying the mechanics of phi thickenings. Similar rings of phi thickenings were observed in Cattleya roots by Nicolai (1865), and more recently in Cattleya aurantiaca (Brundrett et al. 1988), Cattleya skinneri (Joca et al. 2017), Encyclia sp. and Prosthechea caetensis (de Oliviera Pires et al. 2003), and Laelia anceps (Idris et al., manuscript in preparation).
Confocal imaging of aerial roots of the epiphytic orchid Rhyncholaelia digbyana. Images are maximum projections of berberine-stained lignin in sections near the root tip. (a) Cross section showing extensive cortical phi thickenings (ϕ). (b) Longitudinal section showing the complex organisation of the phi thickening network. The velamen layer (V) that surrounds the root is another complex, lignified network. Scale bar = 200 μm for both images. V velamen, Ex exodermis, ϕ phi thickenings, En endodermis, X xylem, P phloem
2.3 Brassica Roots
Our third suggested model system for studying phi thickenings is the roots of Brassica oleracea and B. napus. The initial report of phi thickenings in Brassica roots came from an investigation of the cabbage disease clubroot and its causative agent, the bacterial pathogen Plasmodiophora brassicae (Woronin 1878):
Ihre Endodermis oder Schutzscheide nämlich wird umgeben von einer Parenchymschicht, welche durch ihren Bau an die sogenannte secundäre oder Aussenschutzscheide erinnert, die nach den Untersuchungen von Ph. van Tieghem..... den Wurzeln einiger Coniferen zukommt. Diese Eigenthümlichkeit, die bis heutzutage als charakteristisches Merkmal blos für einige Coniferen gilt, ist, wie es aus meinen jetzigen Untersuchungen zu ersehen ist, auch bei den Kohlpflanzen vorhanden.
In den jungen Wurzeln der Kohlpflanzen besitzt, wie bei den Coniferen, jede Zelle der innersten, der Endodermis unmittelbar anliegenden Periblemschicht, an ihren radialen und Querwänden, eine sonderbare continuirliche Membranverdickung; dieselbe ragt in Form einer wulstartigen Ringleiste in's Lumen der Zelle hinein, wobei, was besonders hervorzuheben ist, die sämmtlichen Verdickungswülste aller Zellen dieser Periblemschicht stets einander innigst correspondiren
This text translates as:
Their endodermis or protective sheath is surrounded by a parenchymal layer, which, due to its construction, is reminiscent of the so-called secondary or outer protective sheath, and which according to studies by Van Tieghem..... occurs in the roots of some conifers. This peculiarity, which until now has been regarded as a characteristic feature of only a few conifers, is, as can be seen from my current investigations, also present in cabbage plants.
In the young roots of the cabbage plants, as in the conifers, every cell of the innermost layer of the cortex immediately adjacent to the endodermis has curious and continuous cell wall thickenings on its radial and transverse walls. These protrude into the lumen of the cell in the form of a series of bead-like bulges, and it should be emphasised, all the thickening bulges of all the cells of this peri-endodermal layer directly correspond.
An extensive investigation of the Brassicaceae by Van Tieghem (1887a) subsequently demonstrated that the phi thickenings are widely distributed in the family, and that their formation varied along the length of the primary root, forming near the tip in some species but considerably further back from the tip in other species. Following Van Tieghem’s study, surprisingly little research was conducted on phi thickenings in either Brassica or the wider Brassicaceae, a notable omission considering the family’s agricultural importance. The first modern images of phi thickenings were provided in a study of the effects of salt on the primary root of Raphanus sativus (radish), in which peculiar cell wall thickenings were induced in a cell layer outside the endodermis. Although described as xylem, the published images demonstrate that the structures are phi thickenings, supplemented by the reticulate network (Sect. 2.4) (Scialabba and Melati 1990). Subsequently, classical phi thickenings were observed in B. napus (Enstone et al. 2002), while phi thickenings could be induced in hydroponic cultures of B. oleracea (broccoli cultivar Marathon) through the addition of 80 mM salt (López-Pérez et al. 2007; Fernandez-Garcia et al. 2009).
We have used the Brassica root as a model system in which to study the induction, development, and function of phi thickenings (Aleamotuʻa et al. 2018). Three-dimensional imaging by confocal microscopy demonstrated that the lignified phi thickenings form a complex cage around the central vascular tissue of the Brassica root, and that these structures develop before the onset of suberisation of the endodermis. Confocal imaging of a Brassica napus roots double-labelled for lignin and cellulose with berberine and pontamine fast scarlet 4B, respectively, demonstrated the cage-like nature of the phi thickening array (Fig. 3a). Computer-generated reconstructed cross sections through a B. oleracea root ~10 mm from the root apex demonstrated three rings of cortical cells, a near complete ring of lignified phi thickenings in the inner cortex, a lignified Casparian strip in the endodermis, and commencement of metaxylem development (Fig. 3b). Intriguingly, in many roots that show extensive development of phi thickenings, individual cells were present in which both cellulose and lignin-labelling demonstrated that thickenings had not been induced (Fig. 3b, asterisk).
Confocal imaging of phi thickenings in a commercial cultivar of canola (Brassica napus). Roots were fixed, cleared, and then double-labelled for lignin stained with berberine and cellulose with pontamine. (a) Maximum projections of a root imaged near the root tip, showing phi thickenings (ϕ) and a faint Casparian strip (CS). (b) Computer-generated cross sections show phi thickenings (ϕ) in the inner cortex, immediately outside the endodermis (En). The reticulate network (RN) of lignified thickenings occurs only on the inner face of inner cortical cells. Some cells fail to undergo phi thickening development (*). Scale bar = 50 μm for both images. Ep epidermis, ϕ phi thickenings, RN reticulate network, En endodermis, X xylem
In our analyses of phi thickening induction and development, we replaced the hydroponic system of López-Pérez et al. (2007) and Fernandez-Garcia et al. (2009) with agar plates and have demonstrated that phi thickenings are induced by both salt and sucrose in the primary roots of B. oleracea and B. napus, and that within these two species, there were distinct variations in phi thickening induction between different cultivars (Aleamotuʻa et al. 2018). Moreover, we have recently investigated the mechanics of this induction pathway. Transfer of seedlings from uninduced conditions to inducing conditions causes the formation of a phi thickening network in as little as 24–48 h, and induction can also be triggered by several plant hormones (Aleamotuʻa et al., manuscripts in preparation) (Sect. 3).
Multiple advantages exist to study phi thickenings in Brassica. Although phi thickenings are common in the Brassicaceae (Van Tieghem 1887a; Aleamotuʻa et al. 2018), they are absent from the model species Arabidopsis thaliana (arabidopsis) (Aleamotuʻa et al. 2018), a species that has only a single layer of cortical cells. While the large collections of mutants and other genetic resources available within arabidopsis cannot be directly used to understand phi thickenings, the close relationship between arabidopsis and Brassica means that arabidopsis genomic data might be useful in furthering research into Brassica phi thickenings. Several further factors, however, make Brassica the best available experimental system for phi thickening research. Several thousand years of selective breeding have produced numerous different cultivars, and the collective genomes of these cultivars, referred to as the pangenome, show high variability. In one study in which nine different B. oleracea cultivars were sequenced, not only were more than four million single nucleotide polymorphisms (SNPs) identified, but nearly 20% of the ~60,000 genes identified were entirely absent in one or more of the cultivars (Golicz et al. 2016). More importantly, B. oleracea cultivars show wide variability in their ability to induce phi thickenings, with the broccoli Marathon cultivar inducing strongly, and with cultivars such as the kohlrabi Purple Vienna and the cabbage Golden Acre forming few if any thickenings under normal induction conditions (Aleamotuʻa et al. 2018). This variability may provide a route to dissecting the molecular pathways that lead to the formation of phi thickenings (Aleamotuʻa et al. 2019). Brassica roots also have another significant advantage for experiments in which to determine phi thickening functionality. Because thickenings can be induced by specific treatments, including osmotic stress and hormones (Sects. 3.1 and 3.5) (Aleamotuʻa et al., manuscripts in preparation), roots can be tested under identical conditions in the presence and absence of phi thickening.
2.4 Reticulate Networks in Roots of the Brassicaceae
Roots in the Brassicaceae show an interesting addition to the standard phi thickening pattern, this being the development of a reticulate network that is limited to the inner face of the inner cortical cells (Fig. 3b). We have used lignin staining and confocal microscopy to characterise the three-dimensional organisation of this structure in Brassica and other species (Aleamotuʻa et al. 2018). In B. oleracea, this reticulate network forms as a series of evenly spaced and delicate ridges, less than a micrometre wide and deep (Fig. 4a). Like the phi thickenings, this network initially develops as a cellulosic structure which then becomes lignified. However, while our confocal observations were the first published images of the reticulate network, the original description of this structure was by Woronin (1878) who wrote (in translation) that:
The characteristic, however, in the structure of these cells is that they are provided on their inner tangential wall with a very fine and delicate net-like thickening. This net originates from the ring of thickenings just described: it grows out of it, so to speak, and then spreads out quite regularly on the inner wall of the cell.
Van Tieghem (1887a) also observed similar structures in a range of different Brassicaceae including Sinapis alba (white mustard):
From each longitudinal band [of the conventional phi thickenings], a series of fine parallel strips extend across the inner, rounded edge of the cell. These bifurcate once or twice, and these branches then unite with branches from the opposite band so as to cover the internal face of the cells with a delicate network. All this network is lignified
Since these original descriptions, the reticulate network has only rarely been discussed in the literature, and in most instances the structural interpretations have been poor. These observations of a reticulate network, however, have always been limited to the Brassicaceae. In a study of salt-induced phi thickenings in radish roots, the reticulate network evident in longitudinal sections was described as xylem that had been induced outside the endodermis (Scialabba and Melati 1990). Similarly, light and electron microscopy images of thin sections of salt-induced phi thickenings in B. oleracea roots also identified structures on the inner face of the phi cells that were described as “wall ingrowths” (Fernandez-Garcia et al. 2009). The presence of a reticulate network has also been used to identify mature Brassica roots from field samples where wheat and canola had been grown, with scanning electron microscopy images highlighting the three-dimensional nature of the network (unpublished research, Margaret McCully and Rosemary White, CSIRO Agriculture, Canberra) (Fig. 4b).
Confocal imaging of the reticulate network in roots of the Brassicaceae. (a) Maximum projection of the lignified reticulate network in Brassica napus, stained with berberine. The reticulate network (RN) forms a delicate array on the inner face of the inner cortical cells, linking phi thickenings (ϕ). Several inner cortical cells failed to induce phi thickenings (asterisks). (b) SEM image of the reticulate network (RN) in Brassica napus. Image courtesy of Dr. Rosemary White and Dr. Margaret McCully, CSIRO Agriculture, Australia, with permission. (c, d) Confocal imaging of the modified reticulate network in Thlaspi caerulescens. Roots were fixed, cleared, and then double-stained for lignin (berberine) and cellulose (pontamine). (c) The modified reticulate network is a fenestrated, lignified sheet penetrated by small, round holes (arrow) thought to be pit-fields. Similar elongated pit-fields exist in the primary cell walls of the outer cortical cells (double-headed arrow) but at a lower frequency. (d) Computer-generated cross sections of a modified reticulate network (RN) across the entire inner face of the inner cortical cells. Scale bar in a = 50 μm for both images except b; scale bar in b = 20 μm. Ep epidermis, ϕ phi thickenings, RN reticulate network, En endodermis, X xylem
The patterns formed by the reticulate network can vary within and between species. We illustrated subtle differences in the networks formed by B. oleracea, B. napus, and S. alba (Aleamotuʻa et al. 2018), while Van Tieghem (1887a) described four different reticulate network patterns present within the many different genera within the Brassicaceae. We suggest that any attempt to understand the formation and function of phi thickenings also needs to understand the development and function of this Brassicaceae-specific reticulate network. There is, however, a major question to answer here: is the reticulate network specific to the Brassicaceae? As far as we can determine, there has been no work on primary root anatomy and development in other families within the order Brassicales. Based on recent molecular phylogenies, the families most closely related to the Brassicaceae are Cleomaceae and Capparaceae (genera including Cleome and Capparis (capers), respectively) (Edger et al. 2015). We recently initiated a study to determine whether species in these families contain phi thickenings and to investigate whether the reticulate network is specific to the Brassicaceae.
A role for the reticulate network in nutrient transport has been suggested. In both Thlaspi (Zelko et al. 2008) and Brassica (Fernández-García et al. 2014), the ridges of the reticulate network have been described as “wall ingrowths” with the resulting increase in plasma membrane surface area suggested to increase nutrient movement. Thus, the reticulate network was being directly compared to the wall ingrowths seen in transfer cells, the specialised cells that form at sites where plasma membrane transport of nutrients is rate limiting, and where highly complex networks of wall ingrowths significantly increase the surface area across which nutrient transport can occur (McCurdy et al. 2008). Three factors argue against this suggestion. First, the amount of extra plasma membrane formed through the presence of the simple and shallow wall ridges in the reticulate network in Thlaspi and Brassica would only be minimal. Second, the formation of transfer cell-like structures to aid in transport between the inner cortical cells and the apoplast between the inner cortex and endodermis would be a highly unusual location for transfer cells to develop. Not only are these inner cortical and endodermal cells well connected with plasmodesmata, making transfer cell formation redundant, the formation of the Casparian strip blocks trans-endodermal apoplastic trafficking. Third, the lignification of the reticulate network would reduce diffusion rates: if these structures were involved in nutrient transport, then lignification of these structures would not be expected to occur. The latter two lines of reasoning will be described in more detail below (Sect. 6.4).
If the standard reticulate network that forms on the inner face of the inner cortex does not normally function in either promoting membrane transport or serve to limit water and nutrient flows, what other functions might it perform? If the structures have any function at all, it is possible that this network may help reinforce the surface of the root as it undergoes secondary growth. During this process, cell divisions initiated in the pericycle, immediately inside the endodermis, are accompanied by the sloughing of the root cortex. This initially leaves the endodermis as the outermost cell layer, and the reticulate network thus forms the outer surface of the root.
Intriguing variations in this standard reticulate network can also occur in species that evolved the capacity to grow in extreme environments. For example, instead of a reticulate network, the heavy metal hyper-accumulator Thlaspi caerulescens, also known as Noccaea caerulescens (alpine pennycress), forms a lignified sheath across almost the entire inner face of the inner cortical cells that is continuous with the phi thickenings, a structure that has also been referred to as a peri-endodermal thickening (Zelko et al. 2008; Kováč et al. 2020). In an earlier study, this pattern had been described as two layers of endodermis, based on cell wall autofluorescence (van de Mortel et al. 2006), but analysis of the published images, and comparison to the images presented in the computer-generated cross section shown in Fig. 4d, suggests that the first layer of fluorescence to develop represents the inner of two cortical layers in Thlaspi, with the second layer developing inside this being the suberised endodermis. This reinterpretation was also recently suggested (Kováč et al. 2020). Similar patterns showing two rings of autofluorescent cell walls also occur in the related hyper-accumulator species T. goesingense (Zelko et al. 2008) and T. montana (Kutschera and Sobotnik 1992, as cited by Zelko et al. 2008). Furthermore, a similar “double endodermis” was also reported in the extreme halophyte Thellungiella halophila (saltwater cress) (Inan et al. 2004) although whether this layer was lignified or not remains unclear. On the basis that the reticulate network localised to the inner face of the inner cortex is widespread in the Brassicaceae, we suggest that the complete lignification of the inner cortical wall in several Brassicaceae species, referred to as peri-endodermal thickenings (Kováč et al. 2020), is simply a modification or elaboration of the reticulate network. As will be discussed in Sect. 6.4, however, this structure may function in the control of ion fluxes within the root.
3 Phi Thickening Induction in Roots
While most plant species do not develop phi thickenings, among the species that do, thickening formation is often subject to regulation by either abiotic or biotic stimuli. It is this induction of thickenings that forms circumstantial evidence that these structures play a role within the root. At least six different types of stimuli can induce thickenings among the different species where they have been identified, including water stress, flooding, biotic stress, heavy metals, hormones, and mechanical stimulation.
3.1 Water Stress
As discussed previously (Sect. 2.3), phi thickenings in the Brassicaceae can be induced by water stress. This effect was initially demonstrated by salt treatment in the roots of both radish (Scialabba and Melati 1990) and broccoli (López-Pérez et al. 2007; Fernandez-Garcia et al. 2009) but we have subsequently demonstrated a similar effect in a range of other Brassicaceae species. More importantly, sucrose also generated this response (Aleamotuʻa et al. 2018) which suggests that the effect is not due to salt toxicity per se, but water stress in general. This conclusion was confirmed recently when the rate of Brassica oleracea phi thickening induction was shown to be similar when plotted against the osmotic potential of salt, sucrose, and mannitol solutions, and when induction could be inhibited by the osmoprotectant glycine betaine (Aleamotuʻa et al., manuscript in preparation). Water stress has also been shown to induce the formation of phi thickenings in roots of the orchid Miltoniopsis (Idris and Collings 2019). Similarly, in Eriobotrya japonica (loquat, family Rosaceae), phi thickenings are also strongly induced in roots by drought stress (Pan et al. 2006).
3.2 Flooding
In contrast to thickenings being induced by water stress, thickenings can also be induced in some species by water-logging. In roots of Caesalpinia peltophoroides (sibipiruna, family Fabaceae) phi thickenings develop more strongly when the plants are flooded (Henrique et al. 2009), and so-called crescent thickenings, perhaps a modified form of phi thickenings were more common in Syzygium samarangense (wax apple, family Myrtaceae) roots grown in water-logged soils than under control conditions (Tuladhar et al. 2015). Phi thickenings and phi-thickening-like structures have also been demonstrated in numerous aquatic and semi-aquatic plant species associated with the development of aerenchyma, including the mangroves Rhizophora mangle (red mangrove, family Rhizophoraceae) (de Menezes 2006; Souza et al. 2014) and Avicennia marina (grey mangrove, family Acanthaceae) (Ashford and Allaway 1995; Allaway et al. 2001) and Bacopa monnierioides and Bacopa salzmannii (waterhyssops, family Plantaginaceae) (Bona and de Morretes 2003).
3.3 Biotic Stresses
In several cases, phi thickenings have been shown to develop following infection with fungi or oomycetes. According to Melville, Dryas integrifolia (mountain avens, family Rosaceae) roots contain few phi thickenings when uninfected, but extensive phi thickenings develop on infection from mycorrhizal fungi (Melville et al. 1987). The question must be asked, however, as to whether these induction events are caused directly by the fungi, rather than a response to the change in the way the root grows following infection. This question will be discussed in more detail below (Sect. 4).
3.4 Heavy Metals
A role for heavy metals in phi thickening induction has been suggested, but the evidence is inconclusive. The heavy metal accumulating species Thlaspi caerulescens develops a set of lignified cell walls on the inner face of the inner cortex that would appear to be a modified form of phi thickening and reticulate network (Sect. 2.4) (van de Mortel et al. 2006; Zelko et al. 2008; Kováč et al. 2020), but whether this is actually a response to the heavy metals or a constitutive growth pattern is unclear. While we have demonstrated that Thlaspi develops this set of lignified walls without the addition of any heavy metals, and only under water stress (Aleamotuʻa et al. 2018), our experiments have not addressed the question as to whether these cell walls are specifically induced as a response to the presence of toxic heavy metals. Further examination of this question is certainly warranted. Similarly, Zea mays (maize) has been reported to form phi thickening-like structures in its rhizodermis when grown in slaggy soils (Degenhardt and Gimmler 2000). However, it remains unclear whether these structures developed in response to heavy metals present, the high salt and pH of the soil, mechanical impedance, or a combination of some or all of these stresses.
3.5 Hormones
The induction of phi thickenings in B. oleracea roots is more complex than simply a response to water stress, as thickenings can be induced to different degrees by at least three classes of plant hormones (abscisic acid, gibberellic acid, jasmonic acid) at concentrations that do not overtly reduce the rate of root elongation. In contrast, other hormones, including auxin, cytokinin, ethylene, brassinosteroids, and salicylic acid, did not induce thickenings (Aleamotuʻa et al., manuscript in preparation). As far as we are aware, our observations represent the only example of phi thickening induction being shown to be caused by plant hormones.
3.6 Mechanical Stimulation
There are marked effects of growth media on the induction of phi thickenings, with these potentially related to mechanical interactions of the root with the surfaces surrounding them. Thickenings were not induced in Ceratonia siliqua (carob, family Fabaceae) roots when grown in perlite, thickenings were common when seedlings were transplanted to soil, an effect suggested to be caused by the mechanical impedance of the soil (Pratikakis et al. 1998). Similarly, Prunus avium (cherry) seedlings do not form phi thickenings when grown in agar culture but do so when transplanted to either soil or perlite (Soukup et al. 2004). Furthermore, in Brassica roots, a distinct correlation exists between the rate of root growth and the formation of phi thickenings (Aleamotuʻa et al., manuscript in preparation). However, maize roots grown in the presence of mechanical impedance did not form phi thickenings (Degenhardt and Gimmler 2000).
4 Biotic Interactions and Phi Thickenings
Links between phi thickening development and the interactions of roots with symbiotic fungi have been proposed. About 90% of plant species form associations with mycorrhizal fungi, and it has been hypothesised that phi thickenings may regulate fungal spread through the root. This hypothesis was developed from analysis of the dawn redwood (Metasequoia glyptostroboides) in which phi thickenings first develop near the root tip in cells immediately adjacent to the endodermis. Further from the root tip, more phi thickenings develop in multiple rings in cells further away from the endodermis. Concurrent with this outward development of the phi thickenings, mycorrhizal fungi which initially infect throughout the cortex, except for the cells adjacent to the endodermis, retreat towards the outer cortex. Thus, it is suggested that the phi thickenings prevent the fungus entering the central stele of the root and regulate their presence in the root cortex. This regulation was suggested to happen because the thickenings might help prevent the formation of the intracellular spaces that are important for fungal spread and by limiting the spread of carbohydrates through the cortical apoplast (Böcher 1964). Subsequently, several further studies have made similar conclusions. In Dryas integrifolia, few phi thickenings were present in control roots but thickenings were strongly induced by inoculation with the ectomycorrhizal fungus Hebeloma cylindrosporum which also caused the distortion of cortical cells (Melville et al. 1987). Similarly, penetration of the fungus Chloridium through Betula alleghaniensis (yellow birch, family Betulaceae) roots was blocked at the inner cortex by the formation of phi thickenings (Wilcox and Wang 1987). However, functional links between phi thickenings and endomycorrhizal fungi have also been questioned since the 10% of plants that lack mycorrhizal associations include the Brassicaceae, where phi thickenings are common. Furthermore, links between phi thickening formation and the development of mycorrhizal associations could not be detected in either the orchid Miltoniopsis (Idris and Collings 2019) nor Alnus glutinosa (black alder, family Betulaceae) (Massicotte et al. 1999). However, the absence of an apparent relationship between fungi and phi thickenings in some species does not invalidate interactions in other species.
5 Phi Thickenings: Mechanical Strengthening of the Plant Root
From their initial discovery, it has been assumed that phi thickenings mechanically strengthen the root, with Van Tieghem specifically stating that the “rectangular frame” of the phi thickenings would support the cells (Van Tieghem 1871), while a mechanical role for phi thickenings was even more strongly stated by Schwendener (1874) who described the thickenings “as devices against radial pressure forces”. In the following 150 years, numerous suggestions have been made regarding a mechanical role for phi thickenings in roots, but no experimental analyses have been published. Indeed, it remains unclear as to what “forces” the phi thickenings might be opposing. In this section, some of the suggestions regarding phi thickenings and mechanical forces will be discussed, followed by a summary of the rather sparse literature suggesting such roles. Finally, we make several suggestions regarding the way phi thickenings may strengthen the root, the role which we propose is the basic function of phi thickenings. In these discussions, however, it is important to remember that lignified walls are not the only contributing factor to the mechanical strength and stability of the plant. Turgor pressure, necessary for cell elongation (Pritchard 1994), is also a major contributing factor to the support of plants, notably in younger tissues where secondary growth has not yet commenced.
5.1 Historical Concepts
Numerous suggestions have been made concerning how phi thickenings might mechanically strengthen roots. Despite Schwenderer’s (1874) early suggestion for a role opposing radial forces, most recent suggestions have focused on the longitudinal strength of the root. Having studied Metasequoia roots, Böcher (1964) suggested that thickenings should strengthen the root lengthwise. However, having identified that phi thickenings are induced in the apex of carob roots by water stress, it was suggested that the phi thickenings might resist shrinkage of the roots growing in dry soil (Pratikakis et al. 1998), a concept based on Passioura (1988) who listed evidence for the possibility that roots undergo diurnal shrinking under low water potential. In reviewing the phi thickening literature, de Melo (2011) backed this suggestion, but also suggested an alternative whereby phi thickenings might generate a mechanical force that supports cells against the expansion of the central stele. In contrast, Gerrath et al. (2002) suggested that the location of phi thickenings in the region immediately behind the root cap was consistent with a mechanical role in strengthening the root to allow penetration in difficult conditions. It is this positional information with regard to phi thickening formation that we highlight in Sect. 5.3.
5.2 New Concepts
We recently identified two further aspects of root development that support the concept of a mechanical role for phi thickenings. The first example is the observation that phi thickenings are near universal in their orientation, forming bands in a tangential orientation linking the centre of the radial walls. However, the complex patterns of phi thickenings seen in orchid roots provide an exception to this pattern (Burr and Barthlott 1991; Idris and Collings 2015). Of more significance, however, is that some roots with significant aerenchyma can show radially oriented phi thickenings, as seen in mangroves (Ashford and Allaway 1995; Allaway et al. 2001; de Menezes 2006; Souza et al. 2014) and Bacopa (Bona and de Morretes 2003). We suggested that this unusual orientation might reflect different forces at play within the roots that contain aerenchyma (Aleamotuʻa et al. 2019). Aerenchyma are necessary in these aquatic species to allow for oxygen flow, but these air spaces reduce the mechanical strength of the root. Radially or partially radially oriented thickenings may help the cortex resist the compressive force of water pressure. However, most species that show aerenchyma lack phi thickening-like structures in their root cortex and instead form various cortical rings of reinforced tissue (Striker et al. 2007). These observations demonstrate that there may be different mechanical roles played by phi thickenings in different situations.
The second example is specific to the Brassicaceae where most species typically show a diarch organisation, with only two xylem strands being produced (Bancroft 1930). This means that as protoxylem forms, and then as the metaxylem undergoes exarch development to form a plate of xylem vessels across the root, the root is asymmetrically reinforced. However, the formation of a lignified ring of phi thickenings would provide symmetric reinforcement to the root (Aleamotuʻa et al. 2019). While turgor pressure will normally maintain both cell elongation and cell shape within the root, in periods of water stress the presence of phi thickenings may be structurally more important.
5.3 Phi Thickenings as a Mechanical Reinforcement in Plant Roots
The plant root performs multiple functions. Apart from the uptake of water and nutrients, roots are also responsible for anchoring the plant in the ground, meaning that root structure must balance these distinct roles. In considering the mechanical roles that phi thickenings might play within the root, it is necessary to first describe the different forces, tensions, and pressures that act on root tissues.
Young roots and stems show distinct patterns of vascular organisation. Within the stem, peripheral and discrete vascular bundles are composed of xylem and phloem, whereas root vascular tissue is organised into a central stele with alternating xylem and phloem. These differences in vascular patterning are taught as a component of basic plant sciences, but the reason(s) why these fundamentally different organisational states might have evolved are not discussed. These different patterns of organisation are, however, structurally important: in the structural mechanics of reinforced rods, the peripheral placement of reinforcement in the rod results in a larger quadratic moment and provides much higher resistance to flexing (Audoly and Pomeau 2010). Thus, the peripheral positioning of vascular bundles in the stem maximises mechanical strength and allows the stem to withstand the combination of compression, stretching, and torsional forces associated with wind and other disturbances (Fig. 5a). For roots, however, the central positioning of the vascular bundle will provide resistance to stretching forces associated with the wind and disturbances acting on the above ground tissues while minimising root resistance to bending. Thus, the centrally organised vascular tissue of the root makes the root more flexible, allowing it to buckle, and allowing the direction of root growth to be more readily adjusted (Fig. 5b). The centrally positioned vascular system might also provide the opportunity for more filtering of nutrients that are taken up by root hairs and the epidermis and allows for an even distribution of nutrients even if these are collected asymmetrically from the soil.
Stems and roots show different patterns of structural reinforcement related to the way the organs grow and undergo bending. We suggest that the formation of phi thickenings within the root apex functions to make the root mechanically stiffer. (a) In shoots, vascular bundles containing fibre cells and lignified xylem occur in the periphery. This reinforcement maximises the quadratic moment providing maximal resistance to bending. (b) In the apex of roots, lignified tissue is limited to protoxylem and developing metaxylem in the stele which is centrally positioned and surrounded by the lignified Casparian strip. This location of the vascular tissue minimises resistance to bending, but provides resistance to stretching and compression. (c) Many species develop lignified phi thickenings that form a framework around the central stele. For clarity, the lignified Casparian strip is not shown in this image. Our contention is that this network functions to stiffen the apex of the root, a location that is subject to high compressive forces associated with root growth and penetration through the soil
Young roots and stems undergoing primary growth also show tropistic bending responding to external stimuli such as light, gravity, touch, and so on. This bending occurs through differential cell elongation between the inner and outer sides of the bend, but the locations at which this bending occurs are different in roots and stems. In roots, bending occurs through differential elongation in the distal elongation zone, immediately behind the meristem. In stems that undergo bending, however, the location of the bending can occur one to several internodes below the shoot apical meristem. There are, therefore, important mechanical differences in these two different modes of bending: in stems, the displacement of the site of bending from the shoot apex can mean that bending also moves several leaves, and thus the stem needs considerable reinforcement to allow this to occur. In bending roots, however, the soil would prevent the mass movement of tissue as seen in stems, so bending is necessarily confined to the root apex.
Elongation in stems and roots is also associated with the development of stress asymmetries. If an elongating section of stem is halved lengthwise, the sections bow outwards because the epidermal layers, which characteristically contain thicker and often lignified cell walls, are under tension (Kutschera and Niklas 2007). In contrast, when elongating roots are cut lengthwise, the sections bend inwards as it is the central tissues that restrain growth, with the location of the most pronounced bending corresponding to the fastest elongating regions of the root (Pritchard 1994). However, Pritchard suggested, based on unpublished data, that it is not the vascular tissue at the centre of the root that restrains growth, but either the endodermis and/or the inner cortex.
The growing root also experiences forces that are not present in growing stems because root elongation requires penetration through the soil. These forces will cause compression of the root apex and may also result in bending or buckling of the root. Roots navigate a path through the soil directed by gravitational, nutritional, and hydrological stimuli, following a path of least resistance, but root growth is also determined by the mechanical properties of soil that depend on the soil constituents, moisture content, and degree of compaction. The compressive forces generated by elongation in the root tip will be transmitted back along the root, but will be highest in the regions of the root where elongation is occurring. This location is also the site where tropistic root bending will occur, and this is also where xylem reinforcement is limited, and where extensibility of the primary cell walls is highest (Pritchard 1994). Roots typically respond to increased soil hardness through lower growth rates and an increase in root diameter (Atwell 1993). However, several recent studies have investigated the dynamics of root penetration into hard media. For example, when Medicago truncatula (barrel medic) roots growing through agar impact a thicker agar substrate, they undergo deformation and buckling in the root elongation and differentiation zones (Silverberg et al. 2012). Similarly, three-dimensional imaging of Lens culinaris (lentils) roots showed buckling in root tips in response to compressive forces (Martins et al. 2020), while in Populus crosses (poplar), high resolution analysis demonstrated buckling following axial mechanical forces on the root tip (Bizet et al. 2018). In these examples, it is the un-reinforced parts of the root tip that typically show these bending and buckling responses.
The control or limitation of root buckling, and thus the mechanical strengthening of the root, may be an important role for phi thickenings based on the sites in which these structures develop. Multiple studies in different species show that phi thickening development can commence as cells leave the root elongation zone. Among the gymnosperms, this developmental pathway occurs in incense cedar (Wilcox 1962) and Ginkgo roots (Bonacorsi and Seago 2016), while in the angiosperms, the same developmental sequence occurs in cherry (Soukup et al. 2004), apple (Mackenzie 1979), and loquat roots (Nii et al. 2004; Pan et al. 2006) from the Rosaceae, carob roots from the Fabaceae (Pratikakis et al. 1998), and in Geranium (Scott and Whitworth 1928) and Brassica (Aleamotuʻa et al. 2018) roots. In these examples, the site of phi thickening formation would be consistent with the secondary cell wall of the phi thickenings being unable to elongate. This location also coincides with, or occurs very soon after, development of both the helically reinforced primary xylem that allows for stretching of the vessel elements and the initial formation of a lignified Casparian strip, but occurs prior to the suberisation of the endodermis. We suggest that the location of phi thickening development in the root apex is significant because it is here that maximum compressive forces develop during root growth, a location where structural reinforcement of the root is limited to the protoxylem.
We propose that the development of phi thickenings in the root apex strengthens the root, and that this is a response in the roots of species where thickenings form in response to stimuli that are linked to difficult and/or stressful growth conditions (Fig. 5c). Quantification of the area of phi thickenings in Brassica roots suggests that in the root tip, they can account for considerably more than half of the lignified tissue even after the formation of metaxylem (Aleamotuʻa et al. manuscript in preparation), so these structures can undoubtedly contribute significantly to root strength. Moreover, the positioning of the thickenings in the root cortex, rather than centrally within the stele as is the case for xylem vessels, would be expected to enhance the structural integrity of the root because their location provides for a large quadratic moment (Audoly and Pomeau 2010). These lignified phi thickenings in the root apex would also limit any contraction of the root due to reduced water potential, an effect that the helically reinforced protoxylem would not be able to counter. Thus, we consider that the likely outcome of phi thickening reinforcement of the root will be a stiffening of the root and a reduction in buckling in the elongation zone and differentiation zone, especially when the root hits obstacles or a more dense substrate. However, as with many aspects of phi thickening biology, a role for phi thickenings in modifying such buckling has not been investigated experimentally.
5.4 Conclusions
In discussing phi thickenings, Schwendener (1874) proposed that they play a mechanical role in strengthening the root, but qualified this statement by saying that it was a hypothesis which he had yet to scientifically investigate. Nearly 150 years later, any mechanical role that phi thickenings play within roots remains unclear. We suggest, however, that the principle role that phi thickenings play within roots is the mechanical reinforcement of the root and that in many cases, this is to prevent or control buckling of the root as it grows through difficult substrates. The evolutionary advantages of a system that enabled better penetration through soils are clear, as this would allow roots, and notably the primary root, to more effectively penetrate soil in adverse circumstances, thus enabling better establishment of young plants.
Furthermore, we note that the induction systems devised in Brassica oleracea roots, initially using hydroponics (López-Pérez et al. 2007; Fernandez-Garcia et al. 2009) and more recently simplified with the use of agar plates (Aleamotuʻa et al. 2018, Aleamotuʻa et al., manuscripts in preparation), provide an invaluable tool with which mechanical experiments might be conducted. Not only might the mechanical strength of the whole root be measured, in the presence and absence of phi thickenings, but live imaging penetration experiments analogous to those of Silverberg et al. (2012), Martins et al. (2020) and Bizet et al. (2018) might clarify whether thickenings do contribute to the control of shape in the root apex. Extensive protocols for measuring the strength of plant tissues have also recently been discussed, and although aimed at studying the biomechanics of stems, the concepts and tools might also be directly applied to root tissue (Shah et al. 2017). Similarly, direct measurements of the forces generated by the root tip as it penetrates through a substrate might also be measured using microfluidics. Such experiments have previously been conducted in smaller systems including pollen tubes (Nezhad et al. 2017) and fungal hyphae (Tayagui et al. 2017).
6 Phi Thickenings as an Apoplastic Barrier
Phi thickenings have been suggested to act as an apoplastic barrier because of the similarity in their location and, apparently, their structure when compared to the Casparian strip in the endodermis. This is notably the case for type I phi thickenings in the inner cortex where the thickening-containing cell layer is immediately adjacent to the endodermis. Both the endodermis and inner cortex develop bands of lignification, the Casparian strip and phi thickenings, respectively, that form complete rings around the radial cell walls, and which are aligned from cell-to-cell. This similarity has caused multiple, incorrect identifications in which phi thickenings in the inner cortex have been misinterpreted as the Casparian strip in the endodermis. This erroneous identification was a problem in multiple early publications on apple roots (Nightingale 1935; Stoutemyer 1937; Siegler and Bowman 1939) and remains a problem as seen in the roots of the gymnosperm Cunninghamia (Song et al. 2019). These apparent similarities in structure and positioning have meant that phi thickenings have been suggested to regulate solute and water transport into and out of the root’s central stele in a manner analogous to the Casparian strip (Mackenzie 1979).
6.1 The Casparian Strip and the Endodermis
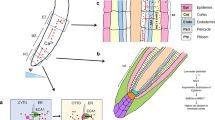
The formation of a functional endodermis is a multi-stage process, with both the development of a lignified Casparian strip, as well as the deposition of suberin lamellae into the cell wall, being required for a functional endodermis that limits the flow of water and ions into and out of the central stele of the root (Geldner 2013; Doblas et al. 2017) (Fig. 6).
Apoplastic and symplastic paths for the flow of water and solutes within the root. (a) In the very apex of the root, no apoplastic barriers to solute movement have formed, and uptake can be by apoplastic or symplastic pathways. (b) Formation of the Casparian strip (dark green) in the first stage of endodermal development prevents apoplastic flows through cell walls to the stele. (c) Suberisation (yellow) in the second stage of endodermal development prevents coupled trans-cellular flow across the endodermis. (d) Formation of phi thickenings within the root apex of some species will mechanically strengthen the root, but would be predicted to reduce solute movement to the endodermis and limit coupled trans-cellular flow. (e) In roots of certain Brassicaceae species growing under extreme conditions, the inner face of the inner cortex becomes lignified in the root apex. This lignification likely results in reduced access of water and solutes to channels and pumps in the plasma membrane of the endodermis, resulting in increased control over solute uptake. In some species, the endodermal cells might also undergo secondary wall deposition (not shown)
In the young root tip, water and solute movement from the root cortex to the developing stele can occur through either the apoplastic pathway, with diffusion through cell walls (Fig. 6a, red arrows), or through the symplastic pathway where transport occurs from cell-to-cell via plasmodesmata (blue arrows). Endodermal maturation begins with the deposition of the Casparian strip in a complete ring around the endodermal cells (Fig. 6b, green band). This deposition is a tightly coordinated and regulated process, which ensures that the Casparian strips of adjacent cells are fully aligned, and that all cells in the endodermis correctly form the band (Geldner 2013; Doblas et al. 2017). During Casparian strip development, the cell wall at the centre of the radial wall is lignified and the plasma membrane modified so that the membrane remains tightly appressed to the cell wall in a way that blocks the lateral diffusion of fluorescent dyes and proteins through the membrane (Alassimone et al. 2010). The development of the Casparian strip is the first step in controlling the movement of water and solutes from the root cortex into the central stele and limits transport to the symplastic pathway (Fig. 6b, blue arrows). This symplastic transport might, however, occur in one of the several ways. A fully symplastic pathway would be through the collective cytoplasm of the cortical, endodermal, and pericycle cells, all connected via plasmodesmata. Alternatively, plasma membrane pumps and channels might move solutes and water from the endodermal apoplast to endodermal symplast, with other channels and pumps moving material back into the apoplast on the inside of the Casparian strip. This process is sometimes referred to as the coupled trans-cellular pathway (Barberon et al. 2016) (Fig. 6b, magenta arrows). Various channels that localise specifically to either the inner or the outer face of the endodermal cells are now known (Bao et al. 2019).
In the second stage of endodermal development (Fig. 6c), layers of suberin, referred to as suberin lamellae, are deposited across the inner face of the entire endodermal cell wall. Suberin, a bio-polyester (Nawrath 2002; Graça 2015), forms a waterproof barrier around the endodermal cell, and reinforcing the block of transport through the wall from cell layer to cell layer provided by the Casparian strip, suberisation will also block transport between the apoplast and symplast (Geldner 2013). Thus, the coupled trans-cellular pathway involving channels and pumps in the endodermal plasma membrane is blocked by this second stage of endodermal development meaning that only trafficking through plasmodesmata will allow water and solutes to pass from the cortex to the pericycle (Fig. 6c, blue arrows).
6.2 Why Phi Thickenings Are Dissimilar to the Casparian Strip
Multiple experiments demonstrate the structural and functional differences between phi thickenings and the Casparian strip and endodermis. First, while both the Casparian strip and phi thickenings are lignified, only phi thickenings show substantial secondary cell wall deposition. Furthermore, the endodermis undergoes subsequent suberisation, a process which does not occur with phi thickenings. This difference has been demonstrated numerous times (Kroemer 1903; Scott and Whitworth 1928; Wilcox 1962; Mackenzie 1979; Peterson et al. 1981; Pratikakis et al. 1998; Fernandez-Garcia et al. 2009; Idris and Collings 2015). Second, there is no evidence for the differentiation of the plasma membrane adjacent to phi thickenings. For example, unlike the Casparian strip which holds tightly to the plasma membrane during plasmolysis (Bonnett 1968; Haas et al. 1976; Alassimone et al. 2010), no membrane domain specialisation occurs during plasmolysis of phi thickening-containing cells (Haas et al. 1976). Similarly, the modified reticulate network present in Thlaspi caerulescens does not hold the plasma membrane tightly during plasmolysis in that species (Kováč et al. 2020). Third, the Casparian strip and endodermis are constitutively formed in all vascular plants, forming a complete and unbroken ring around the stele (Geldner 2013), whereas in those plants that do form phi thickenings, thickening development is often incomplete. In maize, the phi thickenings induced by slaggy soil only occur in discrete patches within the root (Degenhardt and Gimmler 2000), whereas in Brassica, where complete rings of phi thickenings can be induced, gaps nonetheless will still often occur where individual cells fail to form thickenings (Figs. 3b and 4a, asterisks) (see also Table 1 in Fernandez-Garcia et al. 2009). These irregular gaps in phi thickening deposition would be sub-optimal for regulation of solute and water movements. Furthermore, while there is a continuous seal between the endodermis of the primary root and the endodermis of lateral roots in apple, gaps form in the phi thickening network at this junction (Weerdenburg and Peterson 1983) and also in the formation of the modified reticulate network in Thlaspi (Kováč et al. 2020). Finally, the formation of phi thickenings involves ordered deposition of cellulose within the cell wall, controlled by microtubules (Haas et al. 1976; Mackenzie 1979; Idris and Collings 2015, 2019), whereas there is no known relationship between the formation of the Casparian strip and the cytoskeleton.
The concept that phi thickenings might act as an apoplastic barrier has also been directly tested in numerous studies using fluorescent dyes that cannot pass through the plasma membrane and which, therefore, act as markers for apoplastic trafficking. In apple roots, the cell wall stain tinopal was shown to diffuse through phi thickenings adjacent to the endodermis (Peterson et al. 1981), with similar observations made of sub-epidermal phi thickenings in Pelargonium using tinopal (Peterson et al. 1981), calcofluor white (Perumalla et al. 1990), and berberine (Meyer and Peterson 2011). Furthermore, phi thickenings in roots of the orchid Miltoniopsis failed to block calcofluor movement (Idris and Collings 2019), while phi thickenings in maize roots did not block berberine flow (Degenhardt and Gimmler 2000). We have also conducted similar experiments showing that the cell wall stain propidium iodide, used elsewhere as an apoplastic tracer (Naseer et al. 2012), passes through phi thickenings in Brassica roots (unpublished data). Intriguingly, however, propidium iodide does not penetrate through the modified but heavily lignified reticulate network found in the inner cortex of Thlaspi (Kováč et al. 2020).
At first glance, therefore, these experiments suggest that phi thickenings do not act to regulate apoplastic transport, a conclusion we too suggested recently (Idris and Collings 2019). However, exceptions to this conclusion exist. The most direct evidence that phi thickenings can regulate apoplasmic flow comes from Brassica oleracea roots, where the uptake of the heavy metal lanthanum, which acts as an apoplastic tracer and can be directly visualised by electron microscopy, was reduced by the presence of salt-induced phi thickenings (Fernandez-Garcia et al. 2009). Furthermore, Brassica roots in which phi thickenings had been induced were also found to have reduced hydraulic conductivity and decreased apoplastic flow of water into the roots (López-Pérez et al. 2007).
Can these two different sets of experimental evidence be reconciled? As Peterson et al. (1981) noted in the conclusion to their initial study showing the free access of dye through phi thickenings, their results do not exclude the possibility that phi thickenings reduce rates of water and solute movement through the apoplast. An understanding of the structure of the Casparian strip and a consideration of the evolutionary implications of phi thickening biology suggest that any differences in interpretations derived from these data sets can be resolved.
6.3 An Evolutionary Perspective on Phi Thickenings
Phi thickenings are an ancient evolutionary advance in land plants, conserved in gymnosperms, and found in both the monocots and eudicots in the angiosperms (Aleamotuʻa et al. 2019). Furthermore, a surprisingly detailed fossil exists record for phi thickenings in gymnosperms (Basinger 1981; Millay et al. 1987) with the oldest fossils from the Cordaitales, a sister group to conifers, dating from the late Carboniferous period about 300 million years ago (Lignier 1906; Strullu-Derrien et al. 2009; Césari et al. 2012). Phi thickenings are not, however, known in the ferns. In contrast to this, the endodermis has been suggested to have evolved around 400 million years ago (Geldner 2013) although being in essentially all vascular plants including the ferns, its origins would predate the split between the ferns and the spermatophytes that is now dated to between 420 and 450 million years ago (Morris et al. 2018). Thus, the evolution of the Casparian strip and endodermis predates the development of phi thickenings.
Unlike the Casparian strip and endodermis, phi thickenings are not essential to plants, and most species do not form them. Nevertheless, the retention of phi thickenings within these different taxa implies that they play some important function(s). However, if we assume that phi thickenings are important, then why do only some species contain them? And more interestingly, why in many species are phi thickenings not constitutively present within a root, instead being inducible in response to stress? It is tempting to suggest that if phi thickenings were such an important characteristic to have been conserved during evolution, then all roots should show these structures, just as all roots in land plants retain an endodermis and Casparian strip.
When phi thickenings are considered in this evolutionary context, a simple solution presents itself. The reason that phi thickenings are not constitutively produced in all plant roots is that there is some downside, or negative consequences for roots to form phi thickenings, with the plant having to balance the presumed mechanical advantages of inducing phi thickenings with any disadvantages that their presence causes. We suggest two possible ways in which phi thickenings might be disadvantageous. First, thickened and lignified secondary walls are energetically expensive to produce, as noted previously (Gerrath et al. 2002). A more likely explanation, however, is that phi thickenings act to reduce water and solute uptake. This regulation would be different from that seen with the Casparian strip where the very tight control of nutrient movement forces symplastic uptake of nutrients. Instead, the presence of phi thickenings in the cortical cell walls would slow the diffusion of water and nutrients through the cell wall. While this slowed uptake might be helpful in specific circumstances, for example, in roots growing in extreme environments that are high in salt or toxic metals (Sects. 3.4 and 6.4), the basic function of the root is nutrient and water uptake. Thus, decreases in the diffusion of water and ions through the cortical apoplast would presumably be detrimental. For this concept to be valid, however, one would need to assume that a major pathway for nutrient uptake is through the apoplast, even after the formation of the Casparian strip. However, the coupled trans-cellular apoplastic route for water and solute trafficking into the central stele (Barberon et al. 2016) that requires the presence of specific transporters in the plasma membrane of endodermal cells (Bao et al. 2019) demonstrates this possibility.
The concept that phi thickenings slow the uptake of nutrients is consistent with both physiological data and the anatomy of roots that have phi thickenings. In Brassica roots, salt-induced phi thickenings have been reported to reduce hydraulic conductivity of the root (López-Pérez et al. 2007) and uptake of the heavy metal apoplastic tracer lanthanum (Fernandez-Garcia et al. 2009). More importantly, the site of formation of phi thickenings in most species in which their developmental sequence has been investigated is consistent with the physiology of water uptake which occurs predominantly in the root apex, from where protoxylem begins to differentiate through to the differentiation zone where endodermal suberisation commences (see Fig. 5.8 in Kramer 1983). This location is also the region in which phi thickenings are formed in the majority of roots in which the developmental sequence of phi thickening deposition has been identified. As discussed in Sect. 5.3, phi thickenings characteristically develop in the root apex concurrent with or soon after the formation of the Casparian strip and protoxylem, and prior the formation of extensive metaxylem (for example, Wilcox 1962; Mackenzie 1979; Soukup et al. 2004; Bonacorsi and Seago 2016; Aleamotuʻa et al. 2018). After the development of the Casparian strip (stage I of endodermal development, Fig. 6b), uptake of water and nutrients into the root can occur by either the symplastic pathway or the coupled trans-cellular route. If phi thickenings are induced within the root apex prior to suberisation of the endodermis, they will result in a mechanically strengthened root in which the coupled trans-cellular pathway of water and nutrient uptake into the central stele is significantly reduced (Fig. 6d). Thus, the phi thickenings can act in a similar way to other apoplastic barriers such as the Casparian strip and hypodermis that occur within the root, where lignified cells reduce apoplastic flows (Schreiber et al. 1999). We contend that this is not, however, the normal function of the phi thickenings, but a negative consequence of their presence.
6.4 The Specific Case of Brassicaceae Growing in Extreme Conditions
In the preceding section, we proposed that any role that phi thickenings might play in the regulation of water and solute uptake occurs solely as a by-product of their role in mechanically strengthening the root. However, as noted in the introduction (Sect. 1.6), specific cases exist where phi thickenings have been re-tooled to play new and different roles within the root. Modifications to the reticulate network in some Brassicaceae species are an example of such re-tooling.
Certain Brassicaceae species that can grow in extreme conditions lignify much of the inner face of the inner cortex. As this cell wall develops in the location where the reticulate network normally develops in the Brassicaceae, we view this structure, sometimes described as a peri-endodermal thickenings, as a modification of the reticulate network. These patterns occur in several heavy metal hyper-accumulating Thlaspi species (van de Mortel et al. 2006; Zelko et al. 2008; Aleamotuʻa et al. 2018; Kováč et al. 2020) and the extreme halophile Thellungiella halophila (Inan et al. 2004). A role for this modified reticulate network in regulating heavy metal uptake was proposed based on the observation that Thlaspi arvense, a close relative of Thlaspi caerulescens that does survive on heavy metals, lacks phi thickenings and the modified reticulate network (Zelko et al. 2008), and recent physiological evidence has provided strong support for the concept (Kováč et al. 2020).
The development of modified reticulate network formation in Thlaspi roots occurs prior to the suberisation of the endodermis (Kováč et al. 2020). This lignified cell wall would limit flows of solutes through the outer endodermal cell wall and limit movement of material through the endodermis to a solely symplastic route (Fig. 6e). Three pieces of experimental evidence are consistent with this model. First, the apoplastic tracer propidium iodide that passes through phi thickenings in Brassica roots (our unpublished data) does not pass through the modified reticulate network in Thlaspi (Kováč et al. 2020). Second, energy-dispersive X-ray (EDX) analysis by electron microscopy shows that heavy metal ions accumulate in the cortical layers in Thlaspi roots compared to the central stele (Kováč et al. 2020). And third, the high density of pit-fields present within the cell wall linking the inner cortex and the endodermis, compared to the lower density of pit-fields present in outer cortical walls, is also consistent with trafficking reliant solely in a symplastic pathway (Fig. 4c) (Aleamotuʻa et al. 2018).
These observations suggest that the modified reticulate network has a specific role that is not mechanical in nature. Moreover, as many Brassicaceae species have evolved the capacity to grow in toxic environments, undergoing local speciation events in the presence of heavy metals (Baker 1981; Reeves et al. 2018), we predict that one aspect of coping with toxic metals in many of these species will be the modification of the reticulate network in order to reduce solute uptake by the root apex.
These developments would be typical of the strategies that are used to cope with growing in difficult environments. Species growing on soils containing heavy metals are often being grouped as excluders and hyper-accumulators (Baker 1981; Reeves et al. 2018), with the examples of the Brassicaceae in Thlaspi described above being hyper-accumulator species in which the heavy metals accumulate in the above ground organs of the plant. The fact that these species can survive even in the presence of high concentrations of otherwise toxic heavy metal ions does not mean that they lack mechanisms that might minimise ion uptake. Many plant species growing in soils containing high levels of toxic metals develop novel root anatomies. For example, phi thickenings and additional cortical lignification occur in the selenium hyper-accumulator Cardamine hupingshanensis (Brassicaceae) (Xiang et al. 2019), while in metal-hyper-accumulating genotypes of Senecio coronatus (family Asteraceae), novel structures unrelated to phi thickenings develop in the inner cortex but these structures are absent in non-hyper-accumulating genotypes (Mesjasz-Przybyłowicz et al. 2007). Similarly, Salix spp. (willow) clones that have higher tolerance to the heavy metal cadmium show increased development of suberised and lignified exodermal and endodermal layers (Lux et al. 2004). In conclusion, the re-tooling of the reticulate network to provide some type of barrier to ion movements through the root tip seems to have occurred in several Brassicaceae species although the precise mechanism through which this might function remains unclear. Nevertheless, we believe that further investigation of phi thickenings and the reticulate network in extremophile species of the Brassicaceae, whether heavy metal excluders of hyper-accumulators, is certainly warranted.
6.5 Conclusions
A role for phi thickenings in regulating water and solute uptake in the manner of the Casparian strip, as previously proposed (Mackenzie 1979), seems to be a highly unlikely function for phi thickenings. Instead, we suggest that phi thickenings might reduce water and solute uptake as a by-product of their presence within the root, and that this downregulation is balanced against the mechanical strengthening that the thickenings provide to the root. In specific case, however, phi thickenings, or more specifically the reticulate network associated with the phi thickenings in the Brassicaceae, may have evolved into a distinct, lignified barrier that acts in conjunction with the Casparian strip to provide filtering of solutes.
7 Future Directions
In the 150 years since phi thickenings were first illustrated (Van Tieghem 1871), and despite the structures having been identified in a wide range of species, the role(s) of these enigmatic structures within the root remains unclear. In this review, we suggest that the primary role of the phi thickenings is mechanical, but that these structures have most likely been re-tooled by evolution to perform different roles in different species.
To date, the study of phi thickenings has been the preserve of plant anatomists and, occasionally, plant physiologists, cataloguing species in which they are found, and identifying stimuli that might cause their formation. The future for understanding phi thickening functions, however, will lie with modern genetic and inter-disciplinary science, and to this end we have identified four systems that we consider the most suitable for investigating functionality. The genetic diversity present within Brassica oleracea and B. napus populations and the ability to reliably trigger phi thickening formation through hormonal treatments suggest that the developmental pathway through which thickenings form might be readily identified. We have previously discussed the concept of an as yet unidentified master regulator for phi thickening development, a transcription factor(s) that turns on the formation of the multiple cell wall formation pathways required to make functional, spatially coordinated phi thickenings (Aleamotuʻa et al. 2019). Similar master regulators exist for other secondary cell walls such as xylem development (Oda et al. 2010) and pathways regulating development of other novel cell walls such as transfer cells have been identified (Nguyen et al. 2017; Offler and Patrick 2020). The identification of similar regulators and pathways of phi thickening development through genetic approaches will be important, and technologies such as single cell sequencing (Denyer et al. 2019; Jean-Baptist et al. 2019), proteomics (Petricka et al. 2012), and genome wide association studies (GWAS), already routinely conducted in B. napus populations (Gacek et al. 2017; Raman et al. 2019), may all prove of value. Meanwhile, multiple different mechanical strength tests might be performed on Brassica roots with induced thickenings (Shah et al. 2017), and these results might be checked by mathematically modelling the phi thickening cell walls (Geitmann and Ortega 2009, Bidhendi and Geitmann 2018).
Considering the limited body of research investigating phi thickenings over the last 150 years, are these structures of sufficient significance to warrant further investigations in light of the many other challenges facing plant sciences? We argue that if phi thickenings do help stiffen the root tips of species from angiosperms and gymnosperms, then this attribute must be significant. Moreover, the induction of these structures in response to various stresses also argues for their functional importance. A mechanism enabling enhanced penetration of roots into soil, particularly in adverse conditions, thus facilitating access to water and nutrients to support plant growth (Padilla and Pugnaire 2007), clearly has considerable importance to agriculture.
References
Alassimone J, Naseer S, Geldner N (2010) A developmental framework for endodermal differentiation and polarity. Proc Natl Acad Sci U S A 107:5214–5219
Aleamotuʻa M, Tai YT, McCurdy DW, Collings DA (2018) Developmental biology and induction of phi thickenings by abiotic stress in roots of the Brassicaceae. Plants 7:47
Aleamotuʻa M, McCurdy DW, Collings DA (2019) Phi thickenings in roots: novel secondary wall structures responsive to biotic and abiotic stresses. J Exp Bot 70:4631–4641
Allaway WG, Curran M, Hollington LM, Ricketts MC, Skelton NJ (2001) Gas space and oxygen exchange in roots of Avicennia marina (Forssk.) Vierh. var. australasica (Walp.) Moldenke ex N. C. Duke, the grey mangrove. Wetl Ecol Manag 9:211–218
Ashford AE, Allaway WG (1995) There is a continuum of gas space in young plants of Avicennia marina. Hydrobiologia 295:5–11
Atwell BJ (1993) Response of roots to mechanical impedance. Environ Exp Bot 33:27–40
Audoly B, Pomeau Y (2010) Elasticity and geometry: from hair curls to the non-linear response of shells. Oxford University Press, Oxford
Baker AJM (1981) Accumulators and excluders - strategies in the response of plants to heavy metals. J Plant Nutr 3:643–654
Bancroft H (1930) The arborescent habit in angiosperms. A review (continued). New Phytol 29:227–275
Bao Z, Bai J, Cui H, Gong C (2019) A missing link in radial ion transport: ion transporters in the endodermis. Front Plant Sci 10:713
Barberon M, Vermeer JEM, De Bellis D, Wang P, Naseer S, Andersen TG, Humbel BM, Nawrath C, Takano J, Salt DE, Geldner N (2016) Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164:447–459
Basinger JF (1981) The vegetative body of Metasequoia milleri from the middle Eocene of southern British Columbia. Can J Bot 59:2379–2410
Bergendal D (1883) Undersökningar öfver Geraniaceernas byggnad tillika med några jemförande blickar på andra Gruinaler. Lunds Uni Års-skrift 19:23–134
Bidhendi AJ, Geitmann A (2018) Finite element modeling of shape changes in plant cells. Plant Physiol 176:41–56
Bizet F, Bengough AG, Hummel I, Bogeat-Triboulot MB, Dupuy LX (2018) 3D deformation field in growing plant roots reveals both mechanical and biological responses to axial mechanical forces. J Exp Bot 67:5605–5614
Böcher TW (1964) Morphology of the vegetative body of Metasequoia glybtostroboides. Dansk Bot Arkiv 24:1–70
Bona C, de Morretes BL (2003) Anatomy of roots of Bacopa salzmanii (Benth.) Wettst. Ex Edwall and B. monnierioides (Cham.) Robinson (Scrophulariaceae) in aquatic and terrestrial environments. Acta Bot Brasilica 17:155–170
Bonacorsi NK, Seago JL (2016) Root development and structure in seedlings of Ginkgo biloba. Am J Bot 103:355–363
Bonnett HT (1968) The root endodermis: fine structure and function. J Cell Biol 37:199–206
Brundrett MC, Enstone DE, Peterson CA (1988) A berberine-aniline blue fluorescent staining procedure for suberin, lignin and callose in plant tissue. Protoplasma 146:133–142
Burr B, Barthlott W (1991) On a velamen-like tissue in the root cortex of orchids. Flora 185:313–323
Césari SN, Busquets P, Méndez-Bedia I, Colombo F, Limarino CO, Cardó R, Gallastegui G (2012) A late Paleozoic fossil forest from the southern Andes, Argentina. Palaeogeogr Palaeoclimatol Palaeoecol 333-334:131–147
de Bary A (1877) Vergleichende Anatomie der Vegetationsorgane der Phanerogamen und Farne. Wilhelm Engelmann, Leipzig
de Melo HC (2011) Espessamento em fi de parede celular. Hoehnea 38:1–7
de Menezes NL (2006) Rhizophores in Rhizophora mangle: an alternative interpretation of so-called “aerial roots”. An Acad Bras Cienc 78:213–226
de Oliviera Pires MF, Semir J, de Pinna GFDAM, Felix LP (2003) Taxonomic separation of the genera Prosthechea and Encyclia (Laeliinae: Orchidaceae) using leaf and root anatomical features. Bot J Linn Soc 143:293–303
Degenhardt B, Gimmler H (2000) Cell wall adaptations to multiple environmental stresses in maize roots. J Exp Bot 51:595–603
Denyer T, Ma X, Kelsey S, Scacchi E, Nieselt K, Timmermans MCP (2019) Spatiotemporal developmental trajectories in the Arabidopsis root revealed using high-throughput single-cell RNA sequencing. Dev Cell 48:840–852
Doblas VG, Geldner N, Barberon M (2017) The endodermis, a tightly controlled barrier for nutrients. Curr Opinion Plant Biol 39:136–143
Edger PP, Heidel-Fischerd HM, Bekaerte M, Rota J, Glöckner G, Platts AE, Heckel DG, Der JP, Wafula EK, Tang M, Hofberger JA, Smithson A, Hall JC, Blanchette M, Bureau TE, Wright SI, de Pamphilis CW, Schranz ME, Barker MS, Conant GC, Wahlberg N, Vogel H, Pires JC, Wheat CW (2015) The butterfly plant arms-race escalated by gene and genome duplications. Proc Natl Acad Sci U S A 112:8362–8366
Enstone DE, Peterson CA, Ma F (2002) Root endodermis and exodermis: structure, function, and responses to the environment. J Plant Growth Regul 21:335–351
Fernandez-Garcia N, Lopez-Perez L, Hernandez M, Olmos E (2009) Role of phi cells and the endodermis under salt stress in Brassica oleracea. New Phytol 181:347–360
Fernández-García N, López-Berenguer C, Olmos E (2014) Role of phi cells under abiotic stress in plants. In: Morte A, Varma A (eds) Root engineering. Springer, Heidelberg, pp 23–37
Gacek K, Bayer PE, Bartkowiak-Broda I, Szala L, Bocianowski J, Edwards E, Batley J (2017) Genome-wide association study of genetic control of seed fatty acid biosynthesis in Brassica napus. Front Plant Sci 7:2062
Geitmann A, Ortega JKE (2009) Mechanics and modeling of plant cell growth. Trends Plant Sci 14:467–478
Geldner N (2013) The endodermis. Ann Rev Plant Biol 64:531–558
Gerrath JM, Covington L, Doubt J, Larson DW (2002) Occurrence of phi thickenings is correlated with gymnosperm systematics. Can J Bot 80:852–860
Golicz AA, Bayer PE, Barker GC, Edger PP, Kim HR, Martinez PA, Chan CKK, Severn-Ellis A, McCombie WR, Parkin IAP, Paterson AH, Pires JC, Sharpe AG, Tang H, Teakle GR, Town CD, Bailey J, Edwards D (2016) The pangenome of an agriculturally important crop plant Brassica oleracea. Nat Commun 7:13390
Graça J (2015) Suberin: the biopolyester at the frontier of plants. Front Plant Sci 3:62
Haas DL, Carothers ZB, Robbins RR (1976) Observations on the phi-thickenings and Casparian strips in Pelargonium roots. Am J Bot 63:863–867
Henrique PC, Alves JD, Goulart PFP, Deuner S, Silveira NM, Zanandrea I, de Castro EM (2009) Características fisiológicas e anatômicas de plantas de sibipiruna submetidas à hipoxia. Cienc Rural 40:70–76
Idris NA, Collings DA (2015) The life of phi: the development of phi thickenings in roots of the orchids of the genus Miltoniopsis. Planta 241:489–506
Idris NA, Collings DA (2019) The induction and roles played by phi thickenings in orchid roots. Plants 8:574
Inan G, Zhang Q, Li P, Wang Z, Coo Z, Zhang H, Zhang C, Quist TM, Goodwin SM, Zhu J, Shi H, Damsz B, Charbaji T, Gong Q, Ma S, Fredericksen M, Galbraith DW, Jenks MA, Rhodes D, Hasegawa PM, Bohnert HJ, Joly RJ, Bressan RA, Zhu JK (2004) Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles. Plant Physiol 135:1718–1737
Jean-Baptist J, McFaline-Figueroa JL, Alexandre CM, Dorrity MW, Saunders L, Bubb KL, Trapnell C, Fields S, Queitsch C, Cuperus JT (2019) Dynamics of gene expression in single root cells of Arabidopsis thaliana. Plant Cell 31:993–1011
Joca TAC, de Oliveira DC, Zotz G, Winkler U, Moreira ASFP (2017) The velamen of epiphytic orchids: variation in structure and correlations with nutrient absorption. Flora 230:66–74
Klein J (1872a) Weitere Beiträge zur Anatomie junger Coniferen-Wurzeln. Flora 55:25–30
Klein J (1872b) Zur Anatomie junger Coniferen-Wurzeln. Flora 55:81–86
Klein J (1872c) Zur Anatomie junger Cöniferen-Wurzeln. Flora 55:103
Kováč J, Lux A, Soukup M, Weidinger M, Gruber D, Lichtscheidl I, Vaculík M (2020) A new insight on structural and some functional aspects of peri-endodermal thickenings, a specific layer in Noccaea caerulescens roots. Ann Bot 126:423–434
Kramer PJ (1983) Water relations of plants. Academic Press, New York
Kroemer K (1903) Wurzelhaut Hypodermis und Endodermis der Angiospermenwurzel. Biblio Bot 12:1–159
Kutschera U, Niklas KL (2007) The epidermal-growth-control theory of stem elongation: an old and new perspective. J Plant Physiol 164:1395–1409
Kutschera L, Sobotnik M (1992) Wurzelatlas mitteleuropäischer grünlandpflanzen. Band 2 Pteridiopohyta und Dicotyledonae (Magnoliopsida) Teil 2 Anatomie. Gustav Fischer, Stuttgart
Lignier MO (1906) Radiculites reticulatus, radicelle fossile de Séquoïnée. Bull Soc Bot France 53:193–201
López-Pérez L, Fernández-García N, Olmos E, Carvajal M (2007) The phi thickening in roots of broccoli plants: an acclimation mechanism to salinity? Int J Plant Sci 168:1141–1149
Lux A, Šottníková A, Opatrná J, Greger M (2004) Differences in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Physiol Plantarum 120:537–545
Mackenzie KAD (1979) Development of the endodermis and phi layer of apple roots. Protoplasma 100:21–32
Martins AD, O’Callaghan F, Bengough AG, Loades KW, Pasqual M, Kolb E, Dupuy LX (2020) The helical motions of roots are linked to avoidance of particle forces in soil. New Phytol 225:2356–2367
Massicotte HB, Melville LH, Peterson RL, Unestam T (1999) Comparative studies of ectomycorrhiza formation in Alnus glutinosa and Pinus resinosa with Paxillus involutus. Mycorrhiza 8:229–240
McCurdy DW, Patrick JW, Offler CE (2008) Wall ingrowth formation in transfer cells: novel examples of localized wall deposition in plant cells. Curr Opinion Plant Biol 11:653–661
Melville LH, Massicotte HB, Peterson RL (1987) Ontogeny of early stages of ectomycorrhizae synthesized between Dryas integrifolia and Hebeloma cylindrosporum. Bot Gaz 148:332–341
Mesjasz-Przybyłowicz J, Barnabas A, Przybyłowicz W (2007) Comparison of cytology and distribution of nickel in roots of Ni-hyperaccumulating and non-hyperaccumulating geneotypes of Senecio coronatus. Plant Soil 293:61–78
Meyer CJ, Peterson CA (2011) Casparian bands occur in the periderm of Pelargonium hortorum stem and root. Ann Bot 107:591–598
Millay MA, Taylor TN, Taylor EL (1987) Phi thickenings in fossilised plants from Antarctica. IAWA Bull 8:191–200
Morris JL, Puttick MN, Clark JW, Edwards D, Kenrick P, Pressel S, Wellman CH, Yang Z, Schneider H, Donoghue PCJ (2018) The timescale of early land plant evolution. Proc Natl Acad Sci U S A 115:e2274–e2283
Naseer S, Lee Y, Lapierre C, Franke R, Nawrath C, Geldner N (2012) Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proc Natl Acad Sci U S A 109:10101–10106
Nawrath C (2002) The biopolymers cutin and suberin. Arabidopsis Book 1:e0021
Nezhad AS, Naghavi M, Packirisamy M, Bhat R, Geitmann A (2017) Quantification of cellular penetrative forces using lab-on-a-chip technology and finite element modeling. Proc Natl Acad Sci U S A 110:8093–8098
Nguyen TTS, Greaves T, McCurdy DW (2017) Heteroblastic development of transfer cells is controlled by the microRNA miR156/SPL module. Plant Physiol 173:1676–1691
Nicolai O (1865) Das Wachsthum der Wurzel. Schr König Phys-Ökon Gesell Königsberg 7:33–78
Nightingale GT (1935) Effects of temperature on growth, anatomy, and metabolism of apple and peach roots. Bot Gaz 96:581–639
Nii N, Pan CX, Ogawa Y, Cui SM (2004) Anatomical features of the cell wall ingrowth in the cortical cells outside the endodermis and the development of the Casparian Stip in loquat trees. J Jap Soc Hort Sci 73:411–414
Oda Y, Idaho Y, Kondo Y, Fukuda H (2010) Wood cell-wall structure requires local 2D-microtubule assembly by a novel plasma membrane-anchored protein. Curr Biol 20:P1197–P1202
Offler CE, Patrick JW (2020) Transfer cells: what regulates the development of their intricate wall labyrinths? New Phytol 228:427–444
Olatunji OA, Nengim RO (1980) Occurrence and distribution of tracheoidal elements in the Orchidaceae. Bot J Linn Soc 80:357–370
Padilla FM, Pugnaire FI (2007) Rooting depth and soil moisture control Mediterranean woody seedling survival during drought. Funct Ecol 21:489–495
Pan CX, Nakao Y, Nii N (2006) Anatomical development of phi thickening and the Casparian strip in loquat roots. J Jap Soc Hort Sci 75:445–449
Passioura JB (1988) Water transport in and to roots. Ann Rev Plant Physiol Plant Mol Biol 39:245–265
Perumalla CJ, Peterson CA, Enstone DE (1990) A survey of angiosperm species to detect hypodermal Casparian bands. I. Roots with a uniseriate hypodermis and epidermis. Bot J Linn Soc 103:93–112
Peterson CA, Cholewa E (1998) Structural modifications of the apoplast and their potential impact on ion uptake. Z. Z Pflanzenernähr Bodenkd 161:521–531
Peterson CA, Perumalla CJ (1990) Survey of angiosperm species to detect hypodermal Casparian bands. II. Roots with a multiseriate hypodermis or epidermis. Bot J Linn Soc 103:113–125
Peterson CA, Emanuel ME, Weerdenburg CA (1981) The permeability of phi thickenings in apple (Pyrus malus) and geranium (Pelargonium hortorum) roots to an apoplastic fluorescent dye tracer. Can J Bot 59:1107–1110
Petricka JJ, Schauer MA, Megraw M, Breakfield NW, Thompson JW, Georgiev S, Soderblom EJ, Ohler U, Moseley MA, Grossniklaus U, Benfey PN (2012) The protein expression landscape of the Arabidopsis root. Proc Natl Acad Sci U S A 109:6811–6818
Pratikakis E, Rhizopoulou S, Psaras GK (1998) A phi layer in roots of Ceratonia siliqua L. Bot Acta 111:93–98
Pritchard J (1994) The control of cell expansion in roots. New Phytol 127:3–26
Raman H, Raman R, Qiu Y, Yadav AS, Sureshkumar S, Borg L, Rohan M, Wheeler D, Owen O, Menz I, Balasubramanian S (2019) GWAS hints at pleiotropic roles for FLOWERING LOCUS T in flowering time and yield-related traits in canola. BMC Genomics 20:636
Reeves RD, van der Ent A, Baker AJM (2018) Global distribution and ecology of hyperaccumulator plants. In: van der Ent A, Echevarria G, Baker AJM, Morel JL (eds) Agromining: farming for metals. Springer, Heidelberg
Reinke J (1873) Morphologische Abhandlungen. Englemann, Leipzig
Riedhart JM, Guard AT (1957) On the anatomy of the roots of apple seedlings. Bot Gaz 118:191–194
Russow E (1875) Betrachtungen über das Leitbündel-und Grundgewebe aus vergleichend morphologischem und phylogenetischem Geschichtspunkt. von Schnakenburg, Dorpat
Schreiber L, Hartmann K, Skrabs M, Zeier J (1999) Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. J Exp Bot 50:1267–1280
Schwendener S (1874) Das mechanische Princip in anatomischen Bau der Monocotylen: mit vergleichenden Ausblicken auf übrigen Pflanzenklassen. Engelmann, Leipzig
Scialabba A, Melati MR (1990) The effect of NaCl on growth and xylem differentiation of radish seedlings. Bot Gaz 151:516–521
Scott LI, Whitworth AB (1928) On a structural peculiarity of the exodermis of the root of Pelargonium. Proc Leeds Philos Lit Soc Sci Sect 1:312–317
Shah DU, Reynolds TPS, Ramage MH (2017) The strength of plants: theory and experimental methods to measure the mechanical properties of stems. J Exp Bot 68:4497–4516
Siegler EA, Bowman JJ (1939) Anatomical studies of root and shoot primordia in 1-year apple roots. J Agric Res 58:795–803
Silverberg JL, Noar RD, Packer MS, Harrison MJ, Henley CJ, Cohen I, Gerbode SJ (2012) 3D imaging and mechanical modeling of helical buckling in Medicago truncatula plant roots. Proc Natl Acad Sci U S A 109:16794–16799
Song C, Shen W, Du L, Wen J, Li R (2019) Development and chemical characterization of Casparian strips in the roots of Chinese fir (Cunninghamia lanceolata). Trees 33:827–836
Soukup A, Mala J, Hrubcova M, Kalal J, Votrubova O, Cvikrova M (2004) Differences in anatomical structure and lignin content of roots of pedunculate oak and wild cherry-tree plantlets during acclimation. Biol Plantarum 48:481–489
Souza IC, Morozesk M, Duarte ID, Bonomo MM, Rocha LD, Furlan LM, Arrivabene HP, Monferran MV, Matsumoto ST, Milanez CRD, Wunderlin DA, Fernandes MN (2014) Matching pollution with adaptive changes in mangrove plants by multivariate statistics. A case study, Rhizophora mangle from four neotropical mangroves in Brazil. Chemosphere 108:115–124
Stoutemyer VT (1937) Regeneration in various types of apple wood. Iowa St Coll Agr Res Sta Res Bull 19:308–352
Striker GG, Insausit P, Grimoldi AA, Vega AS (2007) Trade-off between root porosity and mechanical strength in species with different types of aerenchyma. Plant Cell Environ 30:580–589
Strullu-Derrien C, Rioult JP, Strullu DG (2009) Mycorrhizas in upper carboniferous Radiculites-type cordaitalean rootlets. New Phytol 182:561–564
Tayagui A, Sun Y, Collings DA, Garrill A, Nock V (2017) An elastomeric micropillar platform for the study of protrusive forces in hyphal invasion. Lab Chip 17:3643–3653
Tuladhar A, Ohtsuka S, Nii N (2015) Anatomical study on wax apple (Syzygium samarangense) roots under long-term water-logged conditions. Trop Agr Develop 59:1–6
van de Mortel JE, Almar Villanueva L, Schat H, Kwekkeboom J, Coughlan S, Moerland PD, Ver Loren van Themaat E, Koornneef M, Aarts MG (2006) Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol 142:1127–1147
Van Tieghem P (1871) Recherches sur la symetrie de structure des plantes vasculaires. Ann Sci Nat Bot 13:3–315
Van Tieghem P (1887a) Sur le réseau sus-endodermique de la racine des cruciferes. Bull Soc Bot France 34:125–131
Van Tieghem P (1887b) Sur le réseau sus-endodérmique de la racine des rosacées. Bull Soc Bot France 34:221–223
Van Tieghem P (1887c) Sur le réseau sus-endodermique de la racine des caprifoliacées. Bull Soc Bot France 34:251–253
Van Tieghem P (1888a) Sur le réseau sus-endodermique de la racine chez les légumineuses et les éricacées. Bull Soc Bot France 35:273
Van Tieghem P (1888b) Sur le réseau de soutien de l’ecoré de la racine. Ann Sci Nat Bot Ser 7(8):375–378
Van Tieghem P, Monal (1888) Sur le réseau sous-épidermique de la racine des géraniacées. Bull Soc Bot France 35:274
Weerdenburg CA, Peterson CA (1983) Structural changes in phi thickenings during primary and secondary growth in roots. 1. Apple (Pyrus malus) Rosaceae. Can J Bot 61:2570–2576
Wilcox H (1962) Growth studies of the root of incense cedar, Libocedrus decurrens I. the origin and development of primary tissues. Am J Bot 49:221–236
Wilcox HE, Wang CJK (1987) Mycorrhizal and pathological associations of dematiaceous fungi in roots of 7-month-old tree seedlings. Can J For Res 17:884–899
Woronin M (1878) Plasmodiophora brassicae, Urheber der Kophpflanzenhernie. Jahrb Wiss Bot 1:548–574
Xiang J, Ming J, Yin H, Zhu Y, Li Y, Long L, Ye Z, Wang H, Wang X, Zhang F, Yang Y, Yang C (2019) Anatomy and histochemistry of the roots and shoots in the aquatic selenium hyperaccumulator Cardamine hupingshanensis (Brassicaceae). Open Life Sci 14:318–326
Zelko I, Lux A, Czibula K (2008) Difference in the root structure of hyperaccumulator Thlaspi caerulescens and non-hyperaccumulator Thlaspi arvense. Int J Environ Pollut 33:123–132
Acknowledgements
M.A. is supported by a University of Newcastle International Doctoral Scholarship. Research into phi thickenings in the laboratories of DC and DMcC has been supported by research grants from the Faculty of Science, University of Newcastle, with research into orchid roots supported by grant number 316.17 to DC from the Australian Orchid Foundation. We thank Rosemary White (CSIRO Agriculture, Canberra) and Olivier Buzzi (University of Newcastle) for discussions and advice and Thomas Martin (University of Western Australia) for assistance with translations.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Collings, D.A., Aleamotuʻa, M., McCurdy, D.W. (2020). Phi Thickenings: Their History, Current Status and Role(s) in Mechanically Strengthening the Plant Root. In: Lüttge, U., Cánovas, F.M., Risueño, MC., Leuschner, C., Pretzsch, H. (eds) Progress in Botany Vol. 83. Progress in Botany, vol 83. Springer, Cham. https://doi.org/10.1007/124_2020_51
Download citation
DOI: https://doi.org/10.1007/124_2020_51
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-12781-6
Online ISBN: 978-3-031-12782-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)