Abstract
People suffering from obesity and associated metabolic disorders including diabetes are increasing exponentially around the world. Adipose tissue (AT) distribution and alteration in their biochemical properties play a major role in the pathogenesis of these diseases. Emerging evidence suggests that AT heterogeneity and depot-specific physiological changes are vital in the development of insulin resistance in peripheral tissues like muscle and liver. Classically, AT depots are classified into white adipose tissue (WAT) and brown adipose tissue (BAT); WAT is the site of fatty acid storage, while BAT is a dedicated organ of metabolic heat production. The discovery of beige adipocyte clusters in WAT depots indicates AT heterogeneity has a more central role than hither to ascribed. Therefore, we have discussed in detail the current state of understanding on cellular and molecular origin of different AT depots and their relevance toward physiological metabolic homeostasis. A major focus is to highlight the correlation between altered WAT distribution in the body and metabolic pathogenesis in animal models and humans. We have also underscored the disparity in the molecular (including signaling) changes in various WAT tissues during diabetic pathogenesis. Exercise-mediated beneficial alteration in WAT physiology/distribution that protects against metabolic disorders is evolving. Here we have discussed the depot-specific biochemical adjustments induced by different forms of exercise. A detailed understanding of the molecular details of inter-organ crosstalk via substrate utilization/storage and signaling through chemokines provide strategies to target selected WAT depots to pharmacologically mimic the benefits of exercise countering metabolic diseases including diabetes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In the last few decades, obesity and its related disorders have taken the form of a pandemic affecting more than two billion people worldwide. Obesity is a physiological state with complex metabolic alterations impacting multiple organ systems of the body. Epidemiological studies have shown that obesity worsens the conditions of several other diseases including Type2 diabetes mellitus (T2DM), cardiovascular diseases, stroke, and even cancers (Kyrou et al. 2018). Obesity originates from a misbalance in the utilization and storage of energy substrates (lipids and sugars) that alter the canonical mechanisms in WAT and other tissues associated with the progression of T2DM and other metabolic disorders (Ormazabal et al. 2018; Romieu et al. 2017). Among mammals, the major site of energy storage is WAT which is distributed in different parts of the body as discrete depots. Apart from white, another major type of AT found in mammals is termed BAT. In addition to energy storage, WAT is shown to meet some other physiological needs such as physical protection as shock absorption and insulation as blubber layer (Choe et al. 2016). While WAT primarily serves as an energy storage organ, BAT serves as fat utilization site in converting the chemical energy of substrates into heat that is employed to maintain internal body temperature. Except for being fatty, these two AT (WAT and BAT) have nothing in common; they differ in developmental lineage, morphological appearance, texture, cellular biochemistry as well as physiological function (Billon and Dani 2012). The activities of these two tissues have been shown to greatly influence the whole-body metabolic rate of mammals including humans and as obvious this topic has attracted significant research attention. Interestingly, some recent studies have indicated the possibility of interconversion between BAT and WAT (Lee et al. 2014a), but whether this switching is partial or complete as well as its mechanism is not fully defined.
The structural and functional heterogeneity of various AT sites has also generated an idea that these adipocytes can group as distinct fat depots other than being pure WAT or BAT. One such transitional form of adipocyte cluster is termed as “Beige” adipocytes discovered in subcutaneous WAT (sWAT) depots in rodents as well as in humans (Brown 2020; Sidossis et al. 2015). Also, beige adipocyte abundance and degree of beiging depend upon physiological signals that vary across WAT depots (Romieu et al. 2017; Rabiee 2020).Their transient appearance and disappearance of beige adipocytes are highly correlated with whole-body metabolic demand (energy surplus and deficient states) (Rabiee 2020). It is suggested that the beige adipocytes are recruited for thermogenesis within WAT and are induced by external stimuli like cold, exercise (Valgas et al. 2019; Phillips 2019; Rowland et al. 2015). BAT was traditionally considered as a thermo active metabolic sink, but after the discovery of beige cells, WAT is also being proposed to provide such a site. The seesaw equilibrium of energy storage–energy utilization lies greatly in functional capacities of AT depots. Therefore, different adipocytes including BAT, WAT, and beige have been targeted by pharmacological agents to enhance energy consumption and counter metabolic diseases (González et al. 2017; Thyagarajan and Foster 2017). In mammals, WAT depots govern metabolic homeostasis by influencing nutrient mobilization and thermogenesis mediated by several signaling pathways including insulin (Chait and den Hartigh 2020). Interestingly, exercise or elevated physical activity status directly influences WAT physiology including its beiging in different depots (Dewal and Stanford 2019). Recent studies have identified several adipocytes, myokines, and hepatocytes mediating functional crosstalk between fat tissue and muscle during various physiological and/or pathological states (Dewal and Stanford 2019; Rodríguez et al. 2017). It has been proposed that pharmacological activation of WAT or skeletal muscle (SkM) function mimicking exercise can retard metabolic diseases (Yu et al. 2021; Piccirillo 2019; Olesen et al. 2014; Cabrero et al. 2001). However, WAT heterogeneity is an important aspect that can affect the outcome by a pharmacological agent and may be a major cause of not being able to effectively enhance whole-body energy status. In obese individuals, these depot-specific differences transform into fat distribution patterns that also display gender-based variations implying T2DM (Jensen 2008; Karastergiou et al. 2012). Therefore, the differential role of WAT depots needs more detailed investigation to gain insight in selective targeting of some individual WAT depots. Here, we are trying to highlight the structure/function of different WAT depots and their biochemical and physiological roles in metabolic diseases.
2 Heterogeneity of Fat Depots: Morphology, Molecular Variability, and Differentiation
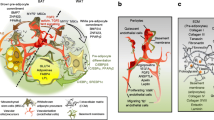
Adipocytes are localized distinctly as aggregated masses termed as AT or depot in different parts of the mammalian body as shown in Fig. 1. Moreover, in the AT the relative abundance of components such as preadipocytes, endothelial cells, macrophages, lymphocytes, blood vessels, and loose connective tissue varies across the different depots (Frese et al. 2016). Due to this, each depot displays uniqueness in their protein expression profile, texture, shape, and 3-dimensional arrangement. This process is regulated developmentally as well as in a gender-specific manner (Rodgers and Sferruzzi-Perri 2021; Keuper and Jastroch 2021).The initial biogenesis of the major adipocytes (white, brown, and beige) occurs during perinatal development from the mesenchymal stem cells (MSCs) regulated by a set of common transcription factors (TFs) (Chooi et al. 2019; Harms and Seale 2013). MSCs undergo differentiation and become committed preadipocytes that are unique for white and brown lineages (Fig. 2). This initial adipogenic differentiation program is regulated by PPARγ that is induced by C/EBP family members, especially C/EBP-β and C/EBP-δ (Ambele et al. 2020). During the latter part of adipogenesis, C/EBP-α plays a key role in maintaining PPARγ expression and both these TFs cooperatively modulate the expression of adipocyte-specific genes involved in lipid metabolism, storage, and cytokine secretion (Moseti et al. 2016). Despite the similarity in early adipogenesis, each of the adipose depots shows remarkable variability in several attributes.
Temporal and spatial heterogeneity in AT depots in rodents and humans. In the neonatal stages of both cases, BAT is prominent (interscapular BAT being the most conspicuous one) and WAT is a minor component, just starting to grow. However, in the adult stages, WAT depots are more abundant and distinct. In human adults, BAT is qualitative and quantitatively much minor than in adult rodents. WAT is broadly categorized into visceral AT (vWAT, primarily around visceral organs) and subcutaneous AT (sWAT, located beneath the skin). The vWAT can be subdivided into gonadal (as epididymal in males and periovarian in females), mesenteric, perirenal (prWAT, around kidneys), and retroperitoneal. The sWAT can be further classified as anterior sWAT, found between the cervical and axillae regions including the interscapular WAT (isWAT) and posterior sWAT, spreading from dorsolumbar to the gluteal region encompassing inguinal (iWAT). Other minor BAT depots are distinctly shown, although in most cases these sites are closely integrated with the surrounding organs. Created withBioRender.com
Scheme showing pathways and processes involved in white-beige-brown fat/adipocyte interconversions. The BAT and WAT depots have a distinct developmental origin. While BAT is close to skeletal muscle, WAT is close to smooth muscle in their cellular lineage. PRDM16 is the key transcription factor that determines the differentiation of mesenchymal stem cell precursors into BAT lineage. Another transcription factor “ZFP423” helps in the induction of commitment toward WAT cellular lineage. The molecular markers of mature BAT, WAT, and beige adipocytes are shown in blue fonts. The possibility of interconversion of BAT to WAT and vice versa has been proposed and several stimuli and transcription factors/cytokines are shown suggested to mediate this process. Created withBioRender.com. Factors regulating adipocytes differentiation are represented in colored box as such: Zinc finger proteins  , Cytokines
, Cytokines  , angiogenesis factor
, angiogenesis factor  , thermogenic gene
, thermogenic gene  , transcription coactivators for energy metabolism
, transcription coactivators for energy metabolism  , transcription factor for differentiation
, transcription factor for differentiation  , cell signaling pathway regulators
, cell signaling pathway regulators  , and precursor cell markers
, and precursor cell markers 
2.1 Not All White Adipose Tissue (WAT)s Are Physiologically Identical
The unique MSCs that generate white adipocytes during development do not express the key TF, Myf5 (Fig. 2). The commitment in these MSCs is induced by bone morphogenetic protein (BMP) family cytokines (especially BMP2 and BMP4) by the modulation of the SMAD pathway, converting them into preadipocytes. It is interesting to point out that BMP4 is capable of the conversion of brown adipocytes to white in the BAT (Qian et al. 2013; Denton et al. 2019). While zinc finger protein (ZFP) 423, T-cell-specific factor 7 like 1 (TCF7l), and early B cell factor (EBF) 1 positively induce white adipogenesis; contrastingly ZFP521 and WNT1-inducible signaling pathway protein (WISP) 2 negatively regulate the process (Addison et al. 2014; Shao et al. 2016a; Cristancho et al. 2011; Hammarstedt et al. 2013; Gupta et al. 2010). ZFP423 drives early-stage adipogenesis by SMAD and BMP pathways and suppressing factors like EBF2, Prdm16 in the white adipocyte precursors (Addison et al. 2014; Shao et al. 2016a; Gupta et al. 2010). ZFP423 also regulated EBF1 by the formation of a heterodimer, which is inhibited by ZFP521 and WISP2 retarding the adipogenesis process. TCF7l1 promotes adipogenesis by repressing the WNT pathway and cell structural genes while enhancing the expression of PPARγ (Cristancho et al. 2011). C/EBPβ works in close association with PPAR to promote WAT adipogenesis (Rosen et al. 1999). Another protein called secreted frizzled-related protein 4 (SFRP4) reduces commitment toward brown adipocyte lineage and mediates white adipogenesis in a depot-specific manner; while positively in vWAT and eWAT, negatively in iWAT (Guan et al. 2018, 2021). White adipocytes also express receptors for several hormones like insulin, glucagon, catecholamines, and glucocorticoids mediating interorgan-crosstalk regulating energy homeostasis (Kuo et al. 2015). The mature white adipocytes are typified by the expression of some transcriptional genes and genes for lipid droplets associated proteins (TCF2, ASC-1, RIP140, HOXC8, PLIN1, and TLE3) (Giordano et al. 2016; Shijun et al. 2020; Ussar et al. 2014; Onogi et al. 2020; Inagaki et al. 2016; Nanduri 2021; Ma et al. 2015).
Depending on the location in the body WAT has been categorized as visceral AT (vWAT, primarily around visceral organs) and subcutaneous AT (sWAT, located below the skin). Types of WAT are shown in Fig. 1. Rodents, especially mice have been used as a model for studying whole-body energy homeostasis. Interestingly, the distributions of WAT, as well as its sexual dimorphism, do differ between humans and rodents. In humans, the anterior sWAT has been distinguished based on depth as superficial subcutaneous WAT (ssWAT) or deep subcutaneous WAT (dsWAT), which is absent in rodents (Chusyd et al. 2016). The posterior sWAT in the human body is mainly localized in the abdomen, buttocks, and thighs and has been considered to be analogous to the iWAT of rodents. In women, sWAT is more conspicuous and vWAT is lesser than men (Demerath et al. 2007; Després et al. 2000). Further, in obese women, the adipocytes in anterior sWAT undergo hypertrophy, as opposed to hyperplasia in the posterior sWAT during weight gain (Jensen et al. 1989). Due to this, women and men after becoming obese display characteristic pear and apple body shapes, respectively (Karastergiou et al. 2012; Bloor and Symonds 2014). In contrast, rodents do not exhibit a clear sexual dimorphism (Chusyd et al. 2016). Additionally, rodent sWAT is separated from dermal AT by a smooth muscle layer whereas; in humans, the sWAT is continuous with dermal AT (Luong et al. 2019). The mass of perigonadal and peritoneal vWAT depots in comparison to body weight is higher in rodents than humans. It has been observed that rodent females tend to accumulate more fat in posterior sWAT while women accumulate more in anterior sWAT (Chusyd et al. 2016). So, the different WAT depots show distinct features which can imply metabolic pathogenesis in a gender-specific manner (Keuper and Jastroch 2021).
2.2 Brown Adipose Tissue (BAT): House of Futile Mitochondria
In contrast to WAT, the BAT is a primary thermogenic organ that is highly vascularized with a lesser amount of loose connective tissue. BAT is abundant in several clades of eutherian mammals especially during neonatal stages and hibernation (Tapia et al. 2018); but, is found in most rodents throughout their life (Cannon and Nedergaard 2004). The brown adipocytes are characterized by their multilocular appearance due to small lipid droplets and the presence of numerous cristae-dense mitochondria that express UCP1 abundantly in the inner membrane (Ikeda et al. 2018; Michurina et al. 2021). Its function is coordinated through β-adrenergic stimulation and synergistic inputs from various endocrine mediators, especially the thyroid and steroids. The other proteins hallmarking BAT are FGF21, ZIC1, BMP7, PRDM16, CIDEA, PGC1α, Eva1, EBF3, Hspb1, P2RX5, and PAT2 (Harms and Seale 2013; Ussar et al. 2014; Rockstroh et al. 2015; Sharp et al. 2012; Waldén et al. 2012). The BAT in neonatal rodents can be considered as classical BAT with relatively uniform small lipid droplets and numerous mitochondria with highly abundant UCP1 expression. Postnatally, however, UCP1 and Tfam expression is gradually decreased, indicating the reduction of mitochondrial abundance, along with the increase in lipid droplet size (Liu et al. 2020). Moreover, mitochondrial activity, protein synthesis, and metabolism were higher in neonates compared to adult BAT (Liu et al. 2020). In rodents, the major BAT depot is located interscapular under the skin, whereas in humans its abundance is reduced during early life (Nedergaard et al. 2007). Interestingly, recent PET studies revealed the presence of BAT in limited quantities discretely in interscapular, supraclavicular, cervical, axillary, periaortic, peri-vertebral, and suprarenal areas in the human adults and that even respond to cold exposure (Ogawa et al. 2018).
The BAT adipocytes originate from myogenic precursor MSCs that express Myf5, EN1, and PAX7, thus a distinct cellular lineage than WAT (Fig. 2) (Wang and Seale 2016). During early gastrulation, in these progenitor cells, two factors, namely, Ewing sarcoma (EWS) and its binding partner Y-box binding protein 1 (YBX1) are upregulated, which activate BMP7 expression inducing commitment for BAT adipogenesis by modulating the key TFs such as PRDM16, PGC1α, PPARγ, C/EBPβ (Park et al. 2013). PRDM16 in partnership with EBF2 repress myogenesis by downregulating the expression of MyoD, myogenin in Myf5-expressing preadipocytes (Wang et al. 2014). Post-natal development and regulation of BAT are much more complex due to the involvement of several reported factors such as FOXC2 (Forkhead box C2, a member of forkhead family protein), BMP8B, TAF7L (TATA-binding protein-associated factor 7L), FGF9 (fibroblast growth factor) (Xue et al. 2008; Zhou et al. 2013, 2014; Whittle Andrew et al. 2012; Cederberg et al. 2001; Mueller 2016; Shamsi et al. 2020). This early differentiation of BAT requires sympathetic activation and is reliant on mitochondrial biogenesis and the expression of thermogenic genes. While FOXC2 works through β-adrenergic-cAMP-Protein kinase A (PKA) signaling cascade (Cederberg et al. 2001), BMP8B mediates its effect through the SMADs/p38 MAPK pathway (Whittle Andrew et al. 2012). Interestingly, loss of TAF7L has been shown to cause activation of myocyte factors in BAT (Zhou et al. 2014), whereas loss of FGF9 affects UCP1 expression leading to impaired BAT development and thermogenesis (Shamsi et al. 2020). Further, differentiated BAT is highly responsive to caloric availability and plays a critical role in metabolic disorders.
2.3 Beige Fat: A Recent Discovery
In the last decade, exciting discoveries identified specialized preadipocytes in some of the WAT depots that can acquire BAT-like features and have been termed as “Beige adipocytes” (Fig. 2) (Wu et al. 2012). The stimuli for inducing beige fat can be external like cold, exercise, PPARγ agonists or internal such as immune function, chronic β-adrenergic response, and cancer cachexia (Arroyave et al. 2020; Chang et al. 2019; Markussen et al. 2017; Petruzzelli et al. 2014). The appearance of beige adipocytes and increased vascularization are suggested as the two major attributes of WAT-to-beige conversion (Harms and Seale 2013). These adipocytes express LHX8, Cox7a, PAT2, and P2RX5 similar to BAT and show low-level UCP1 expression associated with accelerated mitochondrial biogenesis as well as other thermogenic proteins like PGC1α (Ussar et al. 2014; Di Franco et al. 2014; Fang et al. 2020). In addition, beige adipocytes are unique in expressing proteins specific to themselves, not found in BAT or WAT. Such beige markers are TBX1, TMEM26, CD137, Epsti1, Ear2, SP100, CD40, CITED1, and CAR4 (Wu et al. 2012; Garcia et al. 2016; Wang et al. 2016; De Jong et al. 2015). The beige adipocytes cluster predominantly in the iWAT and anterior sWAT in mice, whereas gluteofemoral sWAT and supraclavicular area in humans (Luong et al. 2019).
The origin of beige adipocytes has been a topic of hot debate. Studies have also claimed that these cells are derived from (1) smooth muscle cell precursors ((Long et al. 2014), (Tran et al. 2012)) or (2) white adipocyte precursors (Wu et al. 2012; Garcia et al. 2016) or (3) transdifferentiated directly from existing white adipocytes (Barbatelli et al. 2010). Cold exposure is suggested to induce beiging in rodents by the activation of pro-opiomelanocortin (POMC)-expressing neurons increasing sympathetic tone in WAT that recruit PRDM16, PGC1α (Zhu et al. 2016; Contreras et al. 2014; Lee et al. 2014b). Cold exposure and cAMP upregulated a transcriptional co-partner of PGC-1α named interferon regulatory factor (IRF) 4. IRF 4 acts as a dominant transcriptional effector of thermogenesis and beiging in adipocytes (Kong et al. 2014). Adding to this, in vitro studies and shotgun proteomics analysis revealed that cold exposure also induces PKA-dependent proteasomal degradation of homeobox protein 10 (HOXC10, negative regulator of beiging) thereby promoting browning in white adipocytes (Tan et al. 2021). Similarly, Kruppel-like factor 11 (KLF11) is a target of PPARγ, as well as a cofactor of PPARγ super-enhancers of beiging, leading to increased mitochondrial oxidative capacity in rosiglitazone-induced beiging in human adipocytes (Loft et al. 2015). Whereas Foxp1 directly represses β3-AR transcription thereby playing the role of the master repressor of browning and thermogenesis (Liu et al. 2019). Interestingly, several immunomodulators (both cytokines and cells) have been shown to influence beiging thereby linking energy metabolism with immunity (Ding et al. 2016; Villarroya et al. 2018; Lv et al. 2016; Lee et al. 2015; Rao et al. 2014). WAT browning is determined by an equilibrium between pro-inflammatory [inducible nitric oxide synthase (iNOS), TNFα, IL6, and MCP-1] and anti-inflammatory cytokines (Rao et al. 2014; Cawthorn et al. 2007). Other immune cells (eosinophils and ILC2) induce M2 macrophage to produce anti-inflammatory cytokines mediating beiging, which is also modulated by meteorin-like hormone (METRNL) secreted from SkM and adipocytes (Lee et al. 2015; Rao et al. 2014). ILC2 cells induce beiging by the differentiation of PDGFRα+ (smooth muscle) precursors through recruitment of eosinophils or via the secretion of methionine-enkephalin (Met-Enk) in white adipocytes (Lee et al. 2015; Brestoff et al. 2015; Man et al. 2017). A recent study shows that a pro-inflammatory cytokine, TNF super family protein 14 (TNFSF14) attenuates WAT adipogenesis and beige adipocyte differentiation by blocking JNK signaling, thereby playing a key role in diverting energy in favor of immune activation. Its deficiency caused diet-induced obesity, glucose intolerance, InR in the KO mouse model suggesting it as a regulator of AT homeostasis (Kou et al. 2019). Other cytokines originating from different organs can also influence beige adipocyte development include BDNF (CNS), TGF-β (immune cells), FGF21 (liver), betatrophin (WAT), ANF (heart), suggesting that beige fat tissues are versatile regulators of body energy equilibrium (Kajimura et al. 2015; Liao et al. 2020; Luce et al. 2020; Kleiner et al. 2012; Wang and Yang 2017). Therefore, inducing beiging to treat metabolic disorders has been an attractive weapon and several different approaches for its application are being tested.
3 BAT as a Coordinating Center of Metabolism
Since the discovery of activatedable BAT in adult humans’ extensive studies have been performed to define its role in health and disease. However, the mechanistic understanding of the BAT function comes primarily from studies in rodents, where BAT is abundant during adulthood (Cannon and Nedergaard 2004). BAT mainly relies on UCP1-mediated heat production in mitochondria as shown in Fig. 3. Recent studies suggest BAT also possesses noncanonical futile cycling mechanisms like creatine and Ca2+ (via SERCA2b) (Kazak et al. 2015; Bertholet et al. 2017). BAT function is mainly regulated by norepinephrine (NE) and thyroid both during cold and diet-induced thermogenesis. Interestingly, UCP1 is activated in the BAT by free FA and succinate while it is inhibited by purine nucleotides (Fromme et al. 2018; Fedorenko et al. 2012). In addition to thermogenesis, BAT is important in the regulation of energy expenditure, glucose (substrate) utilization, reliving oxidative stress thereby protecting against obesity and diabetes (Jung et al. 2021; Carpentier et al. 2018; Fernández-Verdejo et al. 2019; McNeill et al. 2020; Lee et al. 2019). In rodents, BAT has been demonstrated to have a very high capacity for utilizing both lipid and glucose. BAT also has high rates of de novo lipogenesis with some lipid storage capacity (Sanchez-Gurmaches et al. 2018; Townsend and Tseng 2014). The lipid reserve in the BAT can be mobilized by NE and recruited for NST in coordination with several factors like PGC, insulin, thyroid. PGC1 (α and β) downregulates lipogenesis and promotes mitochondrial biogenesis priming the BAT for NST and energy utilization (Kim et al. 2018; Worsch et al. 2018). Insulin also plays an important role in substrate fluxes into the BAT and this response is blunted during metabolic disorders (Smith et al. 2018).
BAT as a playground of substrate utilization and molecular metabolic processes. In the BAT depots, receptors for glucose, lipid, and BCAA uptake are abundantly expressed mediating substrate uptake and utilization. Glut1 (induced by mTOR pathway) and Glut4 (induced by insulin signaling) mediate glucose uptake, while several receptors for different lipids are found on brown adipocyte membrane such as SR-BI (scavenger receptor class B type I), LDLR (LDL Receptor), FATP (Fatty acid transporters), CD36 (cluster of differentiation 36). BCAA utilization is mediated by LAT1 (L-type amino acid transporter 1) located on the plasma membrane and SLC25A44 (Solute Carrier Family 25 Member 44) located on the mitochondria. Thyroid, NE, and insulin are major hormones that influence the substrate metabolism of brown adipocytes. Both thyroid and NE induce UCP1 expression via transcriptional upregulation and function in the BAT. Insulin signaling, on the other hand, enhances glucose uptake via Glut4, which is essential to support elevated BAT metabolism and UCP1-mediated heat production. Abbreviations: D2: type 2 deiodinase; TRE: a thyroid or T3 response element; CRE: cAMP response element; PPRE: PPAR response element; ATGL: Adipose triglyceride lipase; modLDL: modified LDL; VLSC: very long-chain acyl-CoA-synthetase. Created with BioRender.com
3.1 Amino Acids as Substrate
During the scarce availability of sugars, amino acids play a major role in energy metabolism. Studies in rodents show that amino acids can be used as metabolic (anaplerotic) substrates by the BAT (Carpentier et al. 2018). During cold exposure, a specialized protein called SLC25A44 is expressed in BAT mitochondria that facilitate uptake of amino acids, more specifically the branched-chain amino acids (BCAAs) (Yoneshiro et al. 2019, 2021). BAT mitochondria can also use the BCAAs to generate heat (McNeill et al. 2020; Cannavino et al. 2021). Reduced BCAAs uptake by BAT has been suggested to be associated with obesity and T2DM (White et al. 2021; Bloomgarden 2018).
3.2 BAT as a Sugar Sink
Activated BAT has been shown to uptake a significant amount of glucose and reduces serum glucose both in rodents and humans serving as a “Sugar sink” (Bloomgarden 2018; Sandoval and D'Alessio 2015). Studies suggest NE-induced β3-adrenoceptor-stimulated acute glucose uptake to depend on cAMP-mediated rapid de novo synthesis of GLUT1 and its translocation to the plasma membrane by mTORC2 (Chernogubova et al. 2004; Mukaida et al. 2017). Other studies report that glucose disposal into BAT is via postprandial activation of the RalA-glut4-axis, which might be altered in obesity and diabetes (Karunanithi et al. 2014; Olsen et al. 2014). Depending on the physiological state glucose inside the brown adipocytes can enter either anabolic or catabolic pathways (McNamara 1991). While at rest, it might enter anabolic pathways such as lipogenesis, during the cold challenge it would enter catabolic pathways leading to heat production via UCP1 in mitochondria (Schlein et al. 2021; Boon et al. 2014). Interestingly, the glucose uptake in BAT is enhanced by hypothalamic nuclei (ARC and POMC) via the secretion of α-MSH that exerts its effects by acting on the sympathetic innervations in the BAT (Han et al. 2021; Labbé et al. 2015; Timper and Brüning 2017). Further, BAT glucose utilization is closely associated with Rev-Erbα circadian rhythm that regulates Glut4 and UCP1 functions (Heyde et al. 2021; Lee et al. 2016). Other studies suggest that expanded BAT mass can provide a sink for the excess of glucose in the body and compensate for InR (Virtanen et al. 2005; Mitrou et al. 2009; Bernardis 1985).
3.3 Lipid Clearance by BAT
The BAT has also been proposed as a sink for lipid substrates, as it has been found that the rate of lipid uptake into BAT coincides with plasma lipid metabolism and clearance of triglycerides (Hauton et al. 2009; Hoeke et al. 2016). Studies have demonstrated that BAT-mediated lipid utilization is regulated at two levels: one, by plasma levels of NEFA, triglyceride-rich lipoproteins (TRL) like chylomicrons, VLDL those are mostly synchronized with circadian rhythm; second, by the activity of local mediators of lipid utilization in BAT-like LPL activity, CD36, and angiopoietin-like 4 (ANGPTL4) (Hoeke et al. 2016; Singh et al. 2018; Bartelt et al. 2011). Higher BAT lipid uptake affects vascular lipoprotein homeostasis protecting hyperlipidemia and the development of other cardiovascular diseases (Shao et al. 2016b; Berbée et al. 2015). Many studies confirm that the reduction of intracellular triglyceride content in the BAT during acute cold exposure is independent of age and diabetic status, influencing body insulin sensitivity (Remie et al. 2021; Iwen et al. 2017; Hanssen et al. 2016). Based on these observations it has been suggested that browning can be recruited for clearance of FFA in systemic circulation to ameliorate the progression of the T2DM phenotype (Crandall and Wahl 2021).
4 WAT: More Than an Inert Fat Storage Site
Different WAT depots have distinct functions not necessarily energy storage, like the fat layers in the skin and around internal organs are primarily intended to be shock absorbers and/or connective tissue. But, it is true in mammals that WAT is the major organ of fat storage capable of up-taking both FFA and glucose from the plasma and reserve fat during energy surplus (fed) states. During conditions of high energy demand such as cold, exercise, and low energy intake (starvation), WAT releases FFA. So, switching from fed to the fasted state, WAT becomes a lipid buffering site: during fed state lipid flux into WAT increases, whereas in fasted state lipid efflux predominates (Ruge et al. 2009). In addition to lipid storage and remobilization, WAT has several important functions such as shock absorption, insulation, hormone/cytokine secretion (Rondinone 2006; Zwick et al. 2018). Through the cytokines (adipokines), WAT influences the function of many organs including the brain, heart, and liver (Rondinone 2006; Castillo-Armengol et al. 2019). Therefore, WAT metabolism is closely associated with whole-body energy status and plays a critical role in InR and the progression of metabolic syndrome.
4.1 Fat Remobilization
Retrieval of stored lipids in WAT is facilitated largely by perilipin 1 and hormone-sensitive lipase (HSL) regulated by insulin and catecholamines (Frühbeck et al. 2014). Perilipin 1 coats the lipid droplet in adipocytes and serves as a physical barrier protecting them from breakdown by HSL, thereby regulating lipid metabolism (Moore et al. 2005). Studies show that loss of perilipin 1 action leads to increased basal lipolysis and reduction of WAT size. The phosphorylation of perilipin 1 by cAMP-dependent PKA facilitates HSL translocation to the lipid droplet promoting lipolysis and release of FA (Holm 2003). Elevation of perilipin1 expression has been found in people with obesity, without significant correlation with peripheral InR (Pinhel et al. 2017). Reduced adipose O-GlcNAc transferase (OGT) increases O-GlcNAcylation of perilipin1 that inhibits lipolysis in eWAT and promotes diet-induced obesity (Yang et al. 2020). On the other hand, reduced HSL function (haploinsufficiency or inhibitor treatment) improves insulin-stimulated lipogenesis in WAT in mice models and human-derived primary adipocytes (Girousse et al. 2013). This de novo lipogenesis along with reduced lipolysis reshapes FA uptake in the WAT, which also increases glucose uptake thereby minimizing the systemic load inducing whole-body insulin sensitivity in coordination with other peripheral organs like the liver and SkM (Solinas et al. 2015). Lipolysis of WAT and subsequent release of NEFA is also dependent on the action of LPL and ANGPTL4. LPL located at capillary endothelium hydrolyzes triacylglycerol (TAG)-rich plasma lipoproteins to glycerol and NEFAs depending on tissue nutritional status and also is regulated by hormones. ANGPTL4 inhibits LPL and its expression correlates with alterations in circulating lipids both in mouse models and humans. Further, fat remobilization from the WAT is regulated via CNS-derived hormones like the GH, which modulates FA metabolism in two ways: (1) by increasing glycerol production and (2) through decreasing the amount of FA reconversion to triglyceride (Goodman 1988; Møller and Jørgensen 2009). Overall, blunted lipolysis and lipogenesis from glucose in WAT are major factors in protecting against InR and pathogenesis of T2DM.
4.2 Vascularization of WAT
According to the bodily energy demand, the lipid fluxes from/to the WAT require appropriate vascularization (Choe et al. 2016). It is observed mostly that sWAT has a higher vascularization capacity than that of vWAT in humans (Caputo et al. 2021). The original blood vessels in the WAT depots are formed during embryonic stages by vasculogenesis from the mesodermal angioblasts. In contrast, neovascularization of adult WAT is more closely regulated by the involvement of pro- and anti-angiogenic factors, which is tuned to lipid flux to the WAT. This process involves primarily two types of progenitor cells; one for new endothelial cell generation and the other (pericytes) for generation of smooth muscle and supporting cells blood vessels in the WAT depots are formed during embryonic stages by vasculogenesis from the mesodermal angioblasts (Corvera and Gealekman 2014). While, physiological stresses that cause lipid efflux from WAT like cold, exercise, starvation promote neovascularization; conditions of lipid influx like obesity induce the reduction of capillary density in both vWAT and sWAT (Fan et al. 2021; Fuster et al. 2016). The factor most highlighted as WAT angiogenesis regulator is VEGF-A, which is also suggested in WAT browning independent of IL-4R activation (Park et al. 2017). While adipocyte-specific overexpression of VEGF-A promotes vascularization, the depletion of VEGF-A in the adipocytes reduces vascularization in mouse model leading to impaired insulin sensitivity inducing inflammation (Corvera and Gealekman 2014; Sun et al. 2012). Intriguingly, it has been observed that upon significant reduction of capillary density in the WAT, larger blood vessels are upregulated potentially due to the elevated local hypoxia and induction of HIF1 (Gaspar and Velloso 2018). In fat from obese individual and mouse models, capillary density is often reduced which is also associated with increased levels of endogenous angiogenic inhibitors in WAT such as pigment epithelium-derived factor (PEDF), angiostatin, endostatin (Cheng and Ma 2015). In addition, endothelial cell activation in the capillaries of WAT is observed during obesity, which catalyzes the recruitment of immune cells like macrophages and T-cells (Cho et al. 2007; Leung et al. 2018). Therefore, WAT vascularization serves as a connecting link between the pathogenesis of metabolic diseases and the immune system of the individual.
4.3 Browning of WAT
It has been reported that selective WAT depots are prone to browning; while, abdominal omental vWAT (oWAT) in humans, sWAT in rodents (Cleal et al. 2017). Several physiological stimuli have been proposed to induce browning such as exercise and cold adaptation (Wang and Seale 2016; Arroyave et al. 2020; Chang et al. 2019; Markussen et al. 2017; Petruzzelli et al. 2014). Recent studies have identified several exercise-induced myokines such as irisin, myostatin, METRNL, and β-amino isobutyric acid (BAIBA) that are suggested to cause WAT browning mostly in mice models (Rodríguez et al. 2017; Rao et al. 2014; Murphy et al. 2020; Roberts et al. 2014; Maalouf and El Khoury 2019). Metabolic benefits of WAT browning have been highlighted, which include increased glucose utilization and reduction in adiposity. A major regulator of WAT browning, PRDM16, has also been shown to influence the metabolic demand of the beige adipocytes via promoting futile Ca2+-cycling through the SERCA2b-RyR2 pathway (Ikeda et al. 2017). WAT browning induced by cold adaptation has been difficult to be defined as lipolysis-mediated changes can also produce similar WAT phenotypes (Schreiber et al. 2017). Studies show that WAT browning is controlled by neurons in the hypothalamus involved in the regulation of the caloric status of the body. While POMC and RIP-Cre neurons promote, agouti-related peptide (AgRP) neurons usually retard the browning of iWAT and vWAT (Wang et al. 2018; Bi and Li 2013; Dodd et al. 2015; Ruan et al. 2014). Stress-induced WAT browning is complex as well as interesting because of its association with neuro-hormonal factors and metabolic diseases. The HPA and HPT axes along with cytokine regulators like IL4, IL6, and IL13 have been suggested to critically influence WAT browning (Stephens and Wand 2012; Fekete and Lechan 2014; Reinehr 2010). This process of browning is more complex due to the further involvement of hormones such as insulin, leptin, IGF1, catecholamines (Dodd et al. 2015; Boucher et al. 2016; Yasmeen et al. 2018). During the progression of obesity, most of these factors are altered reducing WAT browning (Chen et al. 2017; Ye 2013; Bose et al. 2009). Chronic β-adrenergic stimulus enhances glycogen accumulation, glycogen turnover in sWAT, which is driving UCP1 expression and thermogenesis via the ROS mediated p38MAPK pathway (Keinan et al. 2021). Interestingly, an experiment mimicking WAT browning by ectopic overexpression of UCP1 in sWAT was shown to improve insulin sensitivity and whole-body glucose homeostasis providing evidence for beneficial effects of browning (Poher et al. 2015).
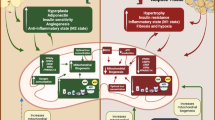
4.4 Pathological Changes in WAT Distribution
Different WAT depots having discrete functions can have differential fat storage capacity. It is believed that metabolic disorders during energy surplus start after storing capacity of preferentially fat-storing WATs is exhausted causing the recruitment of the alternate sites not primarily meant for energy storage (Lanktree et al. 2010; Akinci et al. 2018; Fiorenza et al. 2011). The altered WAT distribution also has significant metabolic consequences and is classified into two main types: lipodystrophy (including lipoatrophy) and ectopic adiposity. Lipodystrophy is defined as a lack of adipocyte expandability with reduced lipid accumulation capacity in the adipocytes; ectopic adiposity describes the condition when fat accumulation is found in tissues other than WAT like SkM, liver, kidney, and pancreas (Bombardier et al. 2013; Purcell and Taylor 2019; Guebre-Egziabher et al. 2013; Chung and Qi 2019; Singh et al. 2017; van der Zijl et al. 2011). Lipodystrophy can be observed in any fat depot iWAT, vWAT, etc. These conditions may arise due to either the dysregulation of storage (including substrate uptake) or the secretion of fat from the adipocytes (Lim et al. 2020). Obviously, in most obese individuals the fat-storing capacity of adipocytes is already exhausted leading to hypertriglyceridemia along with higher fatty substances in circulation that induce ectopic adiposity (Laclaustra et al. 2007). On the other hand, lipodystrophy is closely associated with altered adipokine (leptin and adiponectin) production leading to impaired InR in the skeletal muscle and liver that is often associated with reduced energy expenditure (Fiorenza et al. 2011). The hepatic and SkM lipodeposition shows similarity while the deposition of lipid in the pancreas during pathogenesis slightly differs in humans compared to rodents (Pajed et al. 2021; Yki-Järvinen 2002). The pancreas is more susceptible than the liver as 20-folds higher lipid infiltration is observed after 15 weeks of HFD feeding in mice. This fat infiltration to the pancreas is associated with de novo lipogenesis and the accumulation of unsaturated fatty acids. In contrast, the fat deposition in the human pancreas is more extensive encompassing both exocrine and endocrine parts. This differential fat accumulation enriches the paracrine effects of leptin and adipokines in the proximity of pancreatic islet leading to altered insulin secretion (Pinnick et al. 2008). Lipodystrophy patients usually have a lower circulating level of leptin and beneficial effects of leptin replacement have been reported (Oral et al. 2002). The increased ectopic adiposity is often associated with an increase in systemic FFA, diacylglycerol (DAG), and ceramide that promotes T2DM (Pararasa et al. 2015).
5 Altered WAT Function in Diabetic Pathogenesis
During diabetic pathogenesis, the various WAT depots undergo several key alterations both in human and animal models. This as a cause or an effect can be debatable, but the changes in WAT overlap with the progression of diabetic pathogenesis from a quite early stage indicating a cause.
5.1 Insulin Signaling Is a Major Determiner of WAT
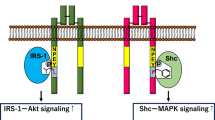
Obviously, the insulin signaling pathway is heavily impacted; several other cytokines also exhibit marked changes that lead to altered glucose and lipid homeostasis in WAT. Interestingly in humans, the expression of IR, IR substrate 2 (IRS-2), p85, Glycogen synthase kinase 3 (GSK-3), mitogen-activated protein kinase (MEK), and ERK is higher in vWAT than sWAT, whereas IRS-1 and AKT show equivalent expression (Laviola et al. 2006). Negative regulators of insulin signaling such as inositol phosphate (IP) 7 and mTOR working in concert with S6K and Grb10 also play a critical role in WAT function (Hsu et al. 2011; Yu et al. 2011). Insulin along with IGF-1 increases IP7 that reduces AKT-signaling, while mTOR along with S6K reduces insulin signaling by altering phosphorylation of IRS and Grb10. The action of these pathways is profoundly altered during metabolic imbalance due to stress, high-fat-diet (HFD), high sugar diet, and obesity that impacts the pathogenesis of T2DM. It has been shown that insulin-mediated anti-lipolytic effects differ in various fat depots; sWAT being more responsive than vWAT, suggesting a less robust intracellular insulin signaling pathway (Perrini et al. 2003; Giorgino et al. 2005; Perrini et al. 2008). Insulin signaling is initiated in WAT adipocyte upon insulin binding to its receptor via IRS-1 and IRS-2 along with the Shc proteins (i.e., p66Shc, p52Shc, and p46Shc) that recruit downstream signaling cascade as shown in Fig. 4 (Li et al. 2019). The speed of insulin action varies across WAT depots. It has been shown that intravenous insulin administration induces phosphorylation of IR and the p85 regulatory site of PI3K within 6 min in vWAT much higher than in the sWAT. While receptor phosphorylation comes back to baseline in vWAT within 30 min, it remains high in sWAT (Li et al. 2019). The next signaling protein AKT undergoes differential phosphorylation upon insulin administration. In the vWAT (especially oWAT), Ser-473 and Thr-308 sites of AKT become phosphorylated at a faster rate than in the sWAT on insulin injection (Li et al. 2019). Similarly, phosphorylation of GSK-3α, ERK-1, and ERK-2 was found to be higher within a few minutes of insulin injection in the vWAT compared to sWAT. In addition to phosphorylation, an increase in protein expression of insulin signaling intermediates like PI3K, MEK was shown to be more pronounced in vWAT than sWAT.
Schematic diagram showing pathways and their alterations in the WAT adipocyte. In the WAT depots, insulin signaling ensures glucose uptake via transcriptional activation in the nucleus as well as the regulation of Glut4-mediated glucose uptake by regulating the function of the RAL-A/RAC1/Rab10 complex. Upon insulin binding to its receptor on the WAT adipocyte two major pathways, namely, AKT and MAPK pathways are activated. The AKT pathway is the most abundant in WAT insulin signaling that is regulated mostly via phosphorylation and substrates mainly glucose. Lipid load on WAT adipocyte also influences AKT pathway and some lipids like palmitate induce ER stress that may have multiple effects such as transcriptional changes, inflammation, autophagy. Points of dysfunction during T2DM are shown by “red circle with a white minus sign ( )” and blunted (red) arrows, while that are points of negative regulation during physiological states is shown by “red circle with a black minus sign (
)” and blunted (red) arrows, while that are points of negative regulation during physiological states is shown by “red circle with a black minus sign ( )” and blunted (black) arrows. The signaling steps that are activated during normal physiological states are shown by “green circle with a black plus sign (
)” and blunted (black) arrows. The signaling steps that are activated during normal physiological states are shown by “green circle with a black plus sign ( ).” Abbreviations: AS160: AKT substrate of 160 kDa, RAC1: Rac Family Small GTPase 1, TSC1/2: Tuberous sclerosis proteins 1/2,4EBP1: Eukaryotic translation initiation factor 4E-binding protein 1. Created with BioRender.com
).” Abbreviations: AS160: AKT substrate of 160 kDa, RAC1: Rac Family Small GTPase 1, TSC1/2: Tuberous sclerosis proteins 1/2,4EBP1: Eukaryotic translation initiation factor 4E-binding protein 1. Created with BioRender.com
It is commonly observed that WAT adipocytes from obese people and mice models exhibit impaired insulin signaling resulting in poor Glut4 translocation, thus glucose uptake (Freidenberg et al. 1988). Surprisingly, the initial signaling events of insulin receptor tyrosine kinase activity in adipocytes from obese insulin-resistant patients are normal (de Mutsert et al. 2018), suggesting an alteration in downstream intracellular signaling. Weaker association of IRS-1 to PI3K in obese individual-derived adipocytes upon insulin action is suggested as a major cause of impaired insulin signaling. Few other alterations suggested for insulin signaling in the adipocytes upon obesity are; changes in protein expression of p85α subunit, impaired AKT phosphorylation. Interestingly, gender-specific differences in WAT depot insulin signaling were reported. Epidemiological studies showed that in men, Swat in the abdominal part and vWAT are associated with InR to a similar extent; whereas, in women, it is particularly vWAT (Björnholm et al. 2002). Adipocytes isolated from sWAT of obese women exhibit markedly impaired IRS-1 associated PI3K activity, while increased IRS-2 associated PI3K activity. Further, a reduction in protein expression of Glut4 (37%) and p85α-subunit of PI3K (55%) was observed in obese women compared to lean subjects (Boura-Halfon and Zick 2009).
5.2 Signals Opposing Insulin Action Are Equally Important
Inhibitory regulation of insulin signaling is also equally important that is primarily governed via inhibitory Ser/Thr phosphorylation of IR, IRS-1, and -2. These pathways are recruited in the adipocytes by factors like cytokines, fatty acids, hyperglycemia, and insulin itself via the activation of multiple kinases (JNK, hTBC1, and MAPK) (Davis et al. 2000; Gao et al. 2002; Zhang et al. 2008; Geraldes and King 2010; Hilton et al. 2000). Atypical PKC-ζ also reduces insulin signaling via Ser-phosphorylation of IRS-1 and Thr-34 phosphorylation of AKT, thereby blocking its translocation to the plasma membrane (Geraldes and King 2010; Goldstein et al. 1998). Some transmembrane phosphatases including protein tyrosine phosphatase (PTP) 1B dephosphorylate activated IR and IRS proteins thereby deterring insulin signaling (Emamgholipour et al. 2020). The role of PTP1B is demonstrated by the finding that PTP1B KO shows improved IR phosphorylation and resistance to HFD-induced obesity and associated InR (Holt and Siddle 2005). Some cytoplasmic adaptor proteins like Grb10 and Grb14 have been shown to decrease IR activity by preventing access of substrates to the activated receptors (Youngren 2007; Smith et al. 2007; Liu et al. 2014). Grb10 overexpression in adipocytes results in impaired growth, glucose intolerance, and InR. Upregulation in Grb14 expression was found in AT of insulin-resistant animal models and type-2 diabetic patients (Béréziat et al. 2002; Errico 2018). Interestingly, insulin signaling is down-regulated by the suppressor of cytokine signaling (SOCS) proteins, especially SOCS1 and SOCS3 (Hilton et al. 2000; Rui et al. 2002). Their expression is increased in WAT during obesity and they induce InR via either the inhibition of the tyrosine kinase activity of the IR or targeting the IRS proteins to degradation (Rui et al. 2002; Palanivel et al. 2012). However, the overexpression of SOCS3 alone in pre-eWAT causes local InR, but not sufficient to cause systemic InR (Shi et al. 2006; Sleeman et al. 2005).
Obesity and InR in WAT are also found to be initiated via some other intermediary signaling pathways. Enhanced PERK and IRE1α activity in WAT of obese mice is suggested to cause JNK and IKK activation inducing Ser307-phosphorylation of IRS-1. Protein phosphatases (PPs) are important regulators of rate-limiting enzymes in glucose and lipid metabolism in the WAT, including glycogen synthase, hormone-sensitive lipase, acetyl CoA carboxylase. A protein phosphatase – PHLPP1 impairs AKT and glycogen synthase kinase 3 (GSK3) activities in adipocytes, resulting in decreased glycogen synthesis and glucose uptake. Upregulated PHLPP1 has been observed in WAT from obese and diabetic patients that correlate with reduced AKT2 phosphorylation. Lipid phosphatases regulate insulin signaling by modulating PIP3 levels which are dephosphorylated by PTEN, thus antagonizing PI3K signaling in adipocytes. Consistently, the deletion of PTEN in mice AT increases insulin sensitivity. A subunit of PI3K called p85α has been shown to enhance PTEN activity regulating both generation and degradation of PIP3. Another phosphatase called SH2 domain-containing inositol 5-phosphatases (SHIP) 2 is ubiquitously expressed and plays a role in insulin signaling in WAT through the AKT pathway (Tang et al. 2005).
5.3 Metabolites May Have a More Critical Role in WAT Regulation
The regulation of WAT function by substrates (glucose and lipids) and metabolites has also been studied in recent decades. Elevated circulating levels of FFAs are observed in obesity and induce activation of JNK, IKK, PKC, and IRS-1 Ser307 phosphorylation in the WAT (Davis et al. 2009). Among the FFAs, palmitate (16:0), DAG, and ceramide have a critical role in InR. In WAT, palmitate causes InR by inducing JNK activation and ER stress (Guo et al. 2007; VandeKopple et al. 2017), while DAG by inducing the activation of PKCh which inhibits PI3K, whereas ceramides by activating PP2A and PKCf that inhibit insulin signaling. Interestingly, the induced anomalies of fat metabolism in the WAT depots increase FFA flux to non-adipose tissues that amplify dyslipidemia, hepatic steatosis, and peripheral tissue InR. Recent studies in cultured adipocytes suggest that NF-κB signaling downregulates PPARγ that impairs triglyceride storage. This can occur through the expressional regulation of triglyceride metabolism enzymes such as phosphoenolpyruvate carboxykinase (PEPCK), fatty acid synthase (FAS), Acyl-CoA synthetase (ACS), lipoprotein lipase (LPL), and proteins associated with lipid droplet including CIDEA, FSP27, perilipin, and HILPDA (Shijun et al. 2020; Ahmadian et al. 2013; Morigny et al. 2021; Foretz et al. 2005).
5.4 Altered Chemokines and Adipomyokines in Diabetes
Many adipokines have been identified in recent decades and shown to affect WAT pathophysiology including the progression of T2DM. Several types of immune cells reside within WAT and contribute to adipokine secretion that plays a critical role in pathological conditions. Adiponectin (primarily produced by sWAT) enhances glucose and fat use in SkM as well as adipocytes and its reduced circulatory level is associated with obesity (Cătoi et al. 2014). Omentin-1 is produced primarily by vWAT correlates with InR, oxidative stress, and chronic inflammation in morbidly obese patients (Li et al. 2008). Vaspin (visceral adipose tissue-derived serine protease inhibitor) is another newly defined adipokine that reduces InR and metabolic disorders (Ruigrok et al. 2021). Another adipokine, Leptin, is known to affect substrate utilization in the SkM and nutrient sensing in the brain, thereby influencing whole-body energy consumption and InR (Gerrits et al. 2012). Apart from these adipokines, several cytokines are also produced by several other tissues but still are considered to be adipokines and contribute to InR in the WAT. Such ubiquitous adipokines are PAI-1, resistin, BMP, NRG-4, FGF21, SFRP5, visfatin; which can affect the function of other tissues in addition to WAT (Feijóo-Bandín et al. 2020; Christian 2015). Interestingly, resident immune cells (macrophages, T-cells, neutrophils, etc.) secrete cytokines like MCP-1, IL1β, TNF-α that affect the inflammation of WAT during obesity and associated T2DM (Panee 2012; Mazur-Bialy et al. 2017). These cytokines act through several pathways in the WAT including the activation of Ser/Thr phosphatases and SOCS3, decreasing IRS-1, expressional regulation of GLUT4, and PPARγ. MCP-1 secreted by macrophages attracts monocytes into WAT causing macrophage accumulation and InR (Mazur-Bialy et al. 2017). Further, some adipokines are also substantially secreted by muscles and are now classified as adipomyokines such as IL-6, TNF-α, irisin (Luo et al. 2020; Bal et al. 2017a). These muscle-derived cytokines affect substrate (glucose and fatty acid) fluxes into/out of adipocytes, influence mitochondrial metabolism, and modulate insulin action in WAT. The role of adipomyokines in muscle-AT crosstalk during physiological challenges (Bal et al. 2017b; Sahu et al. 2019), like cold, exercise, starvation, and pathological states such as obesity and T2DM needs more detailed understanding.
6 Why Does Exercise Improve WAT Metabolism?
It is well documented that exercise, both acute and chronic, enhances cardiac output and muscular activities. However, the way different forms of exercise work on WAT metabolism is still not fully explained (Pedersen 2017a). Exercise may impact WAT function in two ways: first, by creating an energy demand it stimulates WAT to undergo lipolysis releasing of FFAs; second, by affecting other organ function it modulates circulatory cytokines (hepatokines and cardiokines) levels that indirectly affect WAT physiology.
6.1 Exercise-Induced Myokines
Acute bout of exercise increases the secretion of Interleukin-6 (IL-6), irisin, BAIBA, IL-15, and METRONL that signals to the WAT. Acute exercise of 60 min increases IL-6 output depending on the intensity and continues to be released post-exercise (Carey et al. 2006). In WAT (mainly sWAT), IL-6 is involved in glucose utilization and AMPK-mediated fat remobilization (Steensberg et al. 2003). Intriguingly, IL-6 induces mononuclear immune cells to produce IL-10 (Opp et al. 1995), which retard the synthesis of pro-inflammatory cytokines such as TNF-a thereby reducing the IR of WAT (Tsuchiya et al. 2014). The high-intensity acute exercise was shown to induce higher irisin production compared to low-intensity (Löffler et al. 2015). Irisin is suggested to cause mitochondrial biogenesis in WAT and PGC1α-dependent browning in both mice and humans. Reports showed a positive association between circulating irisin and BMI along with improved glucose homeostasis by both acute exercise and training (Crujeiras et al. 2014; Stengel et al. 2013; Boström et al. 2012). A study by Rodríguez et al. showed that leptin crosstalk with irisin differentially in fat and SkM. This antagonizes the thermogenic mechanism of irisin in sWAT while promoting SkM myogenesis during exercise. It suggests that higher leptin concentration in obesity hinders irisin’s role in sWAT although physical exercise is applied (Rodríguez et al. 2015). Interestingly, the rate of IL-6 release from muscle is retarded upon long-term exercise; other myokines are suggested to mediate the beneficial effects of chronic exercise in WAT. Different exercise training has been found to have differential effects on WAT in various individuals, which may depend on myokines secretion. Basal irisin level was increased following long-term resistance training, while simple aerobic training had no effects (Kim et al. 2016; Stautemas et al. 2019). A novel myokine, BAIBA, was shown to increase following 30 min of acute exercise (Stautemas et al. 2018; Riechman et al. 2004) as well as 16–20 weeks of aerobic exercise training only in the normal subjects compared to the sedentary and obese individuals (Roberts et al. 2014; Stautemas et al. 2019). However, sedentary subjects can also increase circulating BAIBA upon regular exercise reducing WAT mass (Roberts et al. 2014). The secretion of other myokines, IL-15, in acute vs. chronic physical activity is unclear as opposing results have been published. While Riechman et al. showed transient increase following acute resistance exercise and no change with age training, few other studies showed no change upon sub-maximal acute exercise and increase in basal IL-15 level following long-term endurance training (Riechman et al. 2004; Rinnov et al. 2014; Ostrowski et al. 1998). The elevated level of IL-15 in trained humans has been suggested to induce lipolysis of visceral fat thereby regulating abdominal obesity (Pedersen 2017b). IL-15 influences WAT physiology by decreasing lipid deposition in preadipocytes, adiponectin secretion, and TNFα secretion (especially in patients with low-grade chronic inflammation) (Carbó et al. 2001; Sánchez-Jiménez and Alvarado-Vásquez 2013). METRNL production in muscles was shown to be increased by a single bout of downhill treadmill-running exercise in both mice and humans (Rao et al. 2014). Both aerobic and resistance training increase circulating METRNL levels that induce WAT browning and reduce adiposity (Rao et al. 2014; Bae 2018; Amano et al. 2020).
6.2 Exercise-Induced Chemokines from Other Organs
Exercise affects the function of other organs like the adrenal, heart, liver, and pancreas to produce factors that have been shown to indirectly influence WAT physiology. Acute resistance exercise induces epinephrine production that is known to cause lipolysis of WAT, especially ipWAT and sWAT/ ingWAT in both humans and mice. Studies in humans have identified follistatin as an exercise-inducible hepatokine that is produced during recovery from an exercise bout. Follistatin enhances the expression of thermogenic markers in WAT and also reduces the production of myostatin that lowers WAT mass (Braga et al. 2014; Allen et al. 2008). The liver also produces FGF21 and Soluble Fibronectin type III domain-containing 4 (sFNDC4) upon both acute and chronic exercise. FGF21 is known to decrease body weights by a reduction in WAT mass leading to improved whole-body insulin sensitivity (Sarruf et al. 2010; Coskun et al. 2008). On the other hand, sFNDC4 binds to its G-Protein coupled Receptor (GPR) 116 in the WAT (mostly iWAT) that is suggested to improve insulin tolerance in prediabetic mice (Georgiadi et al. 2021). Recent studies have described a few other hepatokines like Activin-E, Growth differentiation factor 15 (GDF15), ANGPTL6, Lipocalin 13; that modulate WAT metabolism-regulating fat mass and weight gain. Heart with the greater load during exercise secretes cardiokines like atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP). Acute exercise with the highest workload causes a twofold increase in ANP, while ~30% increase in BNP secretion (Barletta et al. 1998). Both ANP and BNP are shown to induce lipolysis and AT remodeling enhancing lipid mobilization in human sWAT. Surprisingly, long-term exercise training induces no rise in BNP secretion, whereas rise in ANP continues although at a reduced rate. The physiological rise in ANP and BNP levels is impaired in the case of overweight and obese individuals, which is however improved by endurance exercise training (Lafontan et al. 2005). Interestingly, the kidney also participates in this exercise-induced inter organ-crosstalk by secreting erythropoietin that promotes vascularization in sWAT in trained runners after prolonged exercise (Bodary et al. 1999; Schwandt et al. 1991). Hence, increased physical activity exerts its beneficial effects by decreasing total, truncal, and limb AT, reducing triglyceride levels, increasing HDL cholesterol levels that in turn improve peripheral insulin sensitivity in humans, especially important for obese patients.
7 Outlook and Future Direction
In humans, adipose depot-specific differences are documented that are influenced by age, gender, genetic predisposition, and environmental factors. These inter- and intra-depot heterogeneity also drastically modulate embryonic development, cellular composition, and whole-body phenotype of the offspring. Studies have shown a depot-dependent disparity in physiology (vascularity), metabolic function (nutrient uptake and clearance), and endocrine function. The sWAT is lipolytically less active with high insulin sensitivity unlike vWAT excepting oWAT. Hence, the sWAT plays a protective role in the nutrient surplus state, whereas vWAT is associated with central obesity and InR. The literature supports the differential role of WAT depots in physiological and pathological conditions that can be targeted for weight management and potentially counter metabolic disorders. In light of emerging research on the impact of different forms of exercise on different fat depots as well as ectopic fat accumulation can be expected to be beneficial to halt the progression of T2DM. Further understanding into molecular details of exercise as an antioxidant and anti-inflammatory agent can provide better targets to future pharmacological agents.
Unlike appreciated by many in the field WAT depots can be categorized into more subtypes than merely sWAT and vWAT. In most mammals including rodents and humans, the unique anatomical and physiological roles of epicardial WAT, eWAT, and oWAT depots are being unraveled by recent discoveries. One of the interesting aspects is that different WAT depots originate from diverse precursor cells, which are regulated by many endocrine agents and growth factors. The mechanistic details of preadipocytes differentiation in different WAT depots are not well understood and insight on this aspect will help in defining the role of distinct WAT depots in the progression of metabolic syndrome. Similarly, the post-natal expansion of WAT should be studied to understand the role of vascularity and epigenetic effect in the different WAT depots. These studies will help in better delineating the mechanism behind lipodystrophy and lipoatrophy.
Increasing energy expenditure has been proposed as an attractive target to counter obesity and to some extent T2DM. Although classically energy-dispensing properties of BAT were being suggested as the main target, the discovery of beige adipocytes has brought WAT onto the center stage. However, distinct WAT depots display the differential ability to undergo beiging, which may mean that not all WAT can be pharmacologically targeted to similar extents. Further, health outcomes (both obesity and T2DM) of pharmacological targeting of different WAT depots need to be carefully evaluated. In traditional medicine, plant-derived agents have been used to target different WAT depots especially for T2DM that needs to be reassessed with modern biomedical research approaches.
In the literature, the two terms beiging and browning of WAT have been used very loosely and, in many cases, interchangeably. However, “Beiging” is a transitional state due to pharmacological intervention and/or external stimuli, while browning of WAT is a more durable conversion primarily due to sustained pathological state. Hence, the requisite conditions and therefore the molecular mechanism must be different. Research should be conducted for clarification of the distinction between beiging and browning of WAT to understand whether beiging can be used to modulate nutrient metabolism in a regulated manner.
Emerging facts suggest obesity-associated inflammation is mainly caused by cytokines released by resident immune cells in AT; integrating the immune system with glucose utilization. The imbalances of pro-inflammatory and anti-inflammatory cytokines worsen the insulin signaling in various fat depots and SkM. A vivid understanding of these cytokines will provide much-needed insight into the genesis of metabolic imbalance leading to obesity. Further, cytokines from other organs (especially hepatokine and cardiokine) describe the influence of other organs in the WAT substrate cycling. Future studies should be addressed to unravel the molecular details of inter-organ cytokine crosstalk and might provide strategies to target lipid mobilization in selected WAT depots and the suppression of ectopic fat deposition.
Abbreviations
- AgRP:
-
Agouti-related peptide
- AKT1:
-
A strain k thymoma/transforming protein kinase 1
- ANF:
-
Atrial natriuretic factor
- ANGPTL4:
-
Angiopoietin-like 4
- ANP:
-
Atrial natriuretic peptide
- ARC:
-
Arcuate nucleus
- AT:
-
Adipose tissue
- BAIBA:
-
β-aminoisobutyric acid
- BAT:
-
Brown adipose tissue
- BCAAs:
-
Branched-chain amino acids
- BDNF:
-
Brain-derived neurotrophic factor
- BMP:
-
Bone morphogenetic protein
- BNP:
-
B-type natriuretic peptide
- C/EBP:
-
CCAAT/enhancer binding proteins
- CAR4:
-
Carbonic anhydrase
- CD:
-
Cluster of differentiation
- CIDEA:
-
Cell death-inducing DFFA-like effector A
- CNS:
-
Central nervous system
- Cox:
-
Cytochrome c oxidase
- DAG:
-
Diacylglycerol
- dsWAT:
-
Deep subcutaneous WAT
- Ear2:
-
Eosinophil-associated ribonuclease A-2
- EBF:
-
Empty body fat
- EGR1:
-
Early growth response 1
- EN1:
-
Engrailed-1
- Epsti1:
-
Epithelial stromal interaction 1
- Eva1:
-
Epithelial V-like antigen 1
- eWAT:
-
Epididymal white adipose tissue
- EWS:
-
Ewing sarcoma
- FGF9:
-
Fibroblast growth factor 9
- FNDC4:
-
Fibronectin type III domain-containing 4
- FOXC2:
-
Forkhead box C2
- FSP27:
-
Fat-specific protein 27
- GDF15:
-
Growth differentiation factor 15
- GLUT1:
-
Glucose transporter protein type 1
- GPR:
-
G-protein coupled receptor
- Grb10:
-
Growth factor receptor bound protein 10
- GSK-3:
-
Glycogen synthase kinase 3
- HIF1:
-
Hypoxia-inducible factor
- HSL:
-
Hormone-sensitive lipase
- Hspb1:
-
Small heat shock protein beta-1
- hTBC1:
-
Human TBC1 isoform
- IKK:
-
IκB kinase
- IL6:
-
Interleukin 6
- ILC2:
-
Innate lymphoid type 2 cells
- InR:
-
Insulin resistance
- IR:
-
Insulin receptor
- IRE1α:
-
Inositol-requiring transmembrane kinase endoribonuclease-1α
- IRF 4:
-
Interferon regulatory factor 4
- IRS-2:
-
IR substrate 2
- iWAT:
-
Inguinal WAT
- JNK:
-
c-Jun N-terminal kinase
- KLF11:
-
Kruppel-like factor 11
- LHX8:
-
LIM Homeobox8
- MCP-1:
-
Monocyte chemoattractant protein-1
- MEK:
-
Mitogen-activated protein kinase
- Met-Enk:
-
Methionine-enkephalin
- METRNL:
-
Meteorin-like hormone
- MSCs:
-
Mesenchymal stem cells
- mTOR:
-
Mechanistic target of rapamycin
- Myf5:
-
Myogenic factor 5
- MyoD:
-
Myoblast determination protein
- NE:
-
Norepinephrine
- NRG-4:
-
Neuregulin 4
- OGT:
-
O-GlcNAc transferase
- P2RX5:
-
P2X purinoceptor5
- PAI-1:
-
Plasminogen activator inhibitor 1
- PAT:
-
Phosphate acetyl transferase
- PAX7:
-
Paired box gene 7 protein
- PDGFR:
-
Platelet-derived growth factor receptor
- PEDF:
-
Pigment epithelium-derived factor
- PEPCK:
-
Phosphoenol pyruvate carboxy kinase
- PERK:
-
PKR-like ER protein kinase
- PET:
-
Positron emission tomography
- PGC1α:
-
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PI3K:
-
Phosphoinositide 3-kinase
- PKA:
-
Protein kinase A
- PKC:
-
Protein kinase C
- POMC:
-
Proopiomelanocortin
- PRDM:
-
PR domain zinc finger protein
- PTEN:
-
Phosphatase and tensin homolog
- ROS:
-
Reactive oxygen species
- RyR:
-
Ryanodine receptor
- S6K:
-
S6 kinase beta-1
- SERCA:
-
Sarco/endoplasmic reticulum Ca2+-ATPase
- SFRP4:
-
Secreted frizzled-related protein 4
- SFRP5:
-
Soluble frizzled-related protein 5
- SHIP:
-
SH2 domain-containing inositol 5-phosphatases
- SkM:
-
Skeletal muscle
- SLC25A44:
-
Solute carrier family 25 member 44
- SOCS:
-
Suppressor of cytokine signaling
- SP100:
-
Sp100 nuclear antigen
- TAF7L:
-
TATA-binding protein-associated factor 7L
- Tcf7l:
-
T-cell-specific factor 7 like 1
- Tfam:
-
Mitochondrial transcription factor A
- TGF-β:
-
Transforming growth factor beta
- TLR:
-
Toll like receptor
- TMEM26:
-
Transmembrane protein 26
- TNFSF14:
-
Tumor necrosis factor superfamily member14
- TNFα:
-
Tumor necrosis factor α
- UCP1:
-
Uncoupling protein 1
- VEGF-A:
-
Vascular endothelial growth factor A
- WAT:
-
White adipose tissue
- WISP:
-
WNT1-inducible signaling pathway protein
- WNT:
-
Wingless-related integration site
- YBX1:
-
Y-box binding protein 1
- ZFP:
-
Zinc finger protein
- ZIC1:
-
Zinc finger protein of the cerebellum 1
References
Addison WN, Fu MM, Yang HX, Lin Z, Nagano K, Gori F et al (2014) Direct transcriptional repression of Zfp423 by Zfp521 mediates a bone morphogenic protein-dependent osteoblast versus adipocyte lineage commitment switch. Mol Cell Biol 34(16):3076–3085
Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M et al (2013) PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med 19(5):557–566
Akinci B, Sahinoz M, Oral E (2018) Lipodystrophy syndromes: presentation and treatment
Allen DL, Cleary AS, Speaker KJ, Lindsay SF, Uyenishi J, Reed JM et al (2008) Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am J Physiol Endocrinol Metab 294(5):E918–EE27
Amano Y, Nonaka Y, Takeda R, Kano Y, Hoshino D (2020) Effects of electrical stimulation-induced resistance exercise training on white and brown adipose tissues and plasma meteorin-like concentration in rats. Physiol Rep 8(16):e14540
Ambele MA, Dhanraj P, Giles R, Pepper MS (2020) Adipogenesis: a complex interplay of multiple molecular determinants and pathways. Int J Mol Sci 21(12):4283
Arroyave F, Montaño D, Lizcano F (2020) Adipose tissue browning for the treatment of obesity and metabolic diseases. CellR4 8:2877
Bae JY (2018) Aerobic exercise increases meteorin-like protein in muscle and adipose tissue of chronic high-fat diet-induced obese mice. Biomed Res Int 2018:6283932
Bal NC, Maurya SK, Pani S, Sethy C, Banerjee A, Das S et al (2017a) Mild cold induced thermogenesis: are BAT and skeletal muscle synergistic partners? Biosci Rep 37(5)
Bal NC, Singh S, Reis FCG, Maurya SK, Pani S, Rowland LA et al (2017b) Both brown adipose tissue and skeletal muscle thermogenesis processes are activated during mild to severe cold adaptation in mice. J Biol Chem 292(40):16616–16625
Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K et al (2010) The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 298(6):E1244–E1E53
Barletta G, Stefani L, Del Bene R, Fronzaroli C, Vecchiarino S, Lazzeri C et al (1998) Effects of exercise on natriuretic peptides and cardiac function in man. Int J Cardiol 65(3):217–225
Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K et al (2011) Brown adipose tissue activity controls triglyceride clearance. Nat Med 17(2):200–205
Berbée JF, Boon MR, Khedoe PPS, Bartelt A, Schlein C, Worthmann A et al (2015) Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun 6(1):1–11
Béréziat V, Kasus-Jacobi A, Perdereau D, Cariou B, Girard J, Burnol A-F (2002) Inhibition of insulin receptor catalytic activity by the molecular adapter Grb14. J Biol Chem 277(7):4845–4852
Bernardis LL (1985) Ventromedial and dorsomedial hypothalamic syndromes in the weanling rat: is the “center” concept really outmoded? Brain Res Bull 14(6):537–549
Bertholet AM, Kazak L, Chouchani ET, Bogaczyńska MG, Paranjpe I, Wainwright GL et al (2017) Mitochondrial patch clamp of beige adipocytes reveals UCP1-positive and UCP1-negative cells both exhibiting futile creatine cycling. Cell Metab 25(4):811–22.e4
Bi S, Li L (2013) Browning of white adipose tissue: role of hypothalamic signaling. Ann N Y Acad Sci 1302(1):30–34
Billon N, Dani C (2012) Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem Cell Rev Rep 8(1):55–66
Björnholm M, Al-Khalili L, Dicker A, Näslund E, Rössner S, Zierath J et al (2002) Insulin signal transduction and glucose transport in human adipocytes: effects of obesity and low calorie diet. Diabetologia 45(8):1128–1135
Bloomgarden Z (2018) Diabetes and branched-chain amino acids: what is the link? J Diabetes 10(5):350–352
Bloor ID, Symonds ME (2014) Sexual dimorphism in white and brown adipose tissue with obesity and inflammation. Horm Behav 66(1):95–103
Bodary PF, Pate RR, Wu QF, Mcmillan GS (1999) Effects of acute exercise on plasma erythropoietin levels in trained runners. Med Sci Sports Exerc 31(4):543–546
Bombardier E, Smith IC, Gamu D, Fajardo VA, Vigna C, Sayer RA et al (2013) Sarcolipin trumps beta-adrenergic receptor signaling as the favored mechanism for muscle-based diet-induced thermogenesis. FASEB J 27(9):3871–3878
Boon MR, Khedoe PPS, Hoeke G, Kooijman S, Dijk W, Kersten S et al (2014) Brown adipose tissue internalizes fatty acids by selective delipidation of lipoproteins rather than by uptake of lipoproteins, Turning up the heat: role of brown adipose tissue, p 53
Bose M, Oliván B, Laferrère B (2009) Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr Opin Endocrinol Diabetes Obes 16(5):340–346
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC et al (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481(7382):463–468
Boucher J, Softic S, El Ouaamari A, Krumpoch MT, Kleinridders A, Kulkarni RN et al (2016) Differential roles of insulin and IGF-1 receptors in adipose tissue development and function. Diabetes 65(8):2201–2213
Boura-Halfon S, Zick Y (2009) Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab 296(4):E581–EE91
Braga M, Reddy ST, Vergnes L, Pervin S, Grijalva V, Stout D et al (2014) Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J Lipid Res 55(3):375–384
Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF et al (2015) Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519(7542):242–246
Brown AC (2020) Brown adipocytes from induced pluripotent stem cells-how far have we come? Ann N Y Acad Sci 1463(1):9–22
Cabrero À, Alegret M, Sánchez RM, Adzet T, Laguna JC, Vázquez M (2001) Bezafibrate reduces mRNA levels of adipocyte markers and increases fatty acid oxidation in primary culture of adipocytes. Diabetes 50(8):1883–1890
Cannavino J, Shao M, An YA, Bezprozvannaya S, Chen S, Kim J et al (2021) Regulation of cold-induced thermogenesis by the RNA binding protein FAM195A. Proc Natl Acad Sci 118(23):e2104650118
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84(1):277–359
Caputo T, Tran V, Bararpour N, Winkler C, Aguileta G, Trang K et al (2021) Anti-adipogenic signals at the onset of obesity-related inflammation in white adipose tissue. Cell Mol Life Sci 78
Carbó N, López-Soriano JN, Costelli P, Alvarez B, Busquets SL, Baccino FM et al (2001) Interleukin-15 mediates reciprocal regulation of adipose and muscle mass: a potential role in body weight control. Biochim Biophys Acta 1526(1):17–24
Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G et al (2006) Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55(10):2688–2697
Carpentier AC, Blondin DP, Virtanen KA, Richard D, Haman F, Turcotte ÉE (2018) Brown adipose tissue energy metabolism in humans. Front Endocrinol 9:447
Castillo-Armengol J, Fajas L, Lopez-Mejia IC (2019) Inter-organ communication: a gatekeeper for metabolic health. EMBO Rep 20(9):e47903
Cătoi AF, Suciu Ş, PÂrvu AE, Copăescu C, Galea RF, Buzoianu AD et al (2014) Increased chemerin and decreased omentin-1 levels in morbidly obese patients are correlated with insulin resistance, oxidative stress and chronic inflammation. Clujul Med 87(1):19
Cawthorn W, Heyd F, Hegyi K, Sethi J (2007) Tumour necrosis factor-α inhibits adipogenesis via a β-catenin/TCF4 (TCF7L2)-dependent pathway. Cell Death Differ 14(7):1361–1373
Cederberg A, Grønning LM, Ahrén B, Taskén K, Carlsson P, Enerbäck S (2001) FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 106(5):563–573. https://doi.org/10.1016/S0092-8674(01)00474-3
Chait A, den Hartigh LJ (2020) Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med 7(22)
Chang JC, Durinck S, Chen MZ, Martinez-Martin N, Zhang JA, Lehoux I et al (2019) Adaptive adipose tissue stromal plasticity in response to cold stress and antibody-based metabolic therapy. Sci Rep 9(1):8833
Chen W, Balland E, Cowley MA (2017) Hypothalamic insulin resistance in obesity: effects on glucose homeostasis. Neuroendocrinology 104(4):364–381
Cheng R, Ma J-x (2015) Angiogenesis in diabetes and obesity. Rev Endocr Metab Disord 16(1):67–75
Chernogubova E, Cannon B, Bengtsson T (2004) Norepinephrine increases glucose transport in brown adipocytes via β3-adrenoceptors through a cAMP, PKA, and PI3-kinase-dependent pathway stimulating conventional and novel PKCs. Endocrinology 145(1):269–280
Cho C-H, Jun Koh Y, Han J, Sung H-K, Jong Lee H, Morisada T et al (2007) Angiogenic role of LYVE-1–positive macrophages in adipose tissue. Circ Res 100(4):e47–e57
Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB (2016) Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol 7(30)
Chooi YC, Ding C, Magkos F (2019) The epidemiology of obesity. Metabolism 92:6–10. https://doi.org/10.1016/j.metabol.2018.09.005
Christian M (2015) Transcriptional fingerprinting of “browning” white fat identifies NRG4 as a novel adipokine. Adipocytes 4(1):50–54
Chung LH, Qi Y (2019) Lipodystrophy – a rare condition with serious metabolic abnormalities, Rare diseases. IntechOpen
Chusyd DE, Wang D, Huffman DM, Nagy TR (2016) Relationships between rodent white adipose fat pads and human white adipose fat depots. Front Nutr 3:10
Cleal L, Aldea T, Chau Y-Y (2017) Fifty shades of white: understanding heterogeneity in white adipose stem cells. Adipocyte 6(3):205–216. https://doi.org/10.1080/21623945.2017.1372871
Contreras GA, Lee Y-H, Mottillo EP, Granneman JG (2014) Inducible brown adipocytes in subcutaneous inguinal white fat: the role of continuous sympathetic stimulation. Am J Physiol Endocrinol Metab 307(9):E793–E7E9
Corvera S, Gealekman O (2014) Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim Biophys Acta 1842(3):463–472
Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y et al (2008) Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149(12):6018–6027
Crandall JP, Wahl RL (2021) Perspectives on Brown adipose tissue imaging: insights from preclinical and clinical observations from the last and current century. J Nucl Med 62(Suppl 2):34S–43S
Cristancho AG, Schupp M, Lefterova MI, Cao S, Cohen DM, Chen CS et al (2011) Repressor transcription factor 7-like 1 promotes adipogenic competency in precursor cells. Proc Natl Acad Sci 108(39):16271–16276
Crujeiras AB, Pardo M, Arturo RR, Santiago NC, Zulet MA, Martínez JA et al (2014) Longitudinal variation of circulating irisin after an energy restriction-induced weight loss and following weight regain in obese men and women. Am J Hum Biol 26(2):198–207
Davis R, Aguirre V, Uchida T, Yenush L, White MF (2000) The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J Biol Chem 275(12):9047–9054
Davis J, Gabler N, Walker-Daniels J, Spurlock M (2009) The c-Jun N-terminal kinase mediates the induction of oxidative stress and insulin resistance by palmitate and toll-like receptor 2 and 4 ligands in 3T3-L1 adipocytes. Horm Metab Res 41(07):523–530
De Jong J, Larsson O, Cannon B, Nedergaard J (2015) A stringent validation of mouse adipose tissue identity markers. Am J Physiol Endocrinol Metab 308. https://doi.org/10.1152/ajpendo.00023.2015
de Mutsert R, Gast K, Widya R, de Koning E, Jazet I, Lamb H et al (2018) Associations of abdominal subcutaneous and visceral fat with insulin resistance and secretion differ between men and women: the Netherlands epidemiology of obesity study. Metab Syndr Relat Disord 16(1):54–63
Demerath EW, Sun SS, Rogers N, Lee M, Reed D, Choh AC et al (2007) Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity 15(12):2984–2993
Denton NF, Eghleilib M, Al-Sharifi S, Todorčević M, Neville MJ, Loh N et al (2019) Bone morphogenetic protein 2 is a depot-specific regulator of human adipogenesis. Int J Obes (Lond) 43(12):2458–2468
Després J-P, Couillard C, Gagnon J, Bergeron J, Leon AS, Rao D et al (2000) Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the health, risk factors, exercise training, and genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol 20(8):1932–1938
Dewal RS, Stanford KI (2019) Effects of exercise on brown and beige adipocytes. Biochim Biophys Acta 1864(1):71–78
Di Franco A, Guasti D, Mazzanti B, Ercolino T, Francalanci M, Nesi G et al (2014) Dissecting the origin of inducible brown fat in adult humans through a novel adipose stem cell model from adipose tissue surrounding pheochromocytoma. J Clin Endocrinol Metabol 99(10):E1903–E1E12
Ding X, Luo Y, Zhang X, Zheng H, Yang X, Yang X et al (2016) IL-33-driven ILC2/eosinophil axis in fat is induced by sympathetic tone and suppressed by obesity. J Endocrinol 231(1):35
Dodd GT, Decherf S, Loh K, Simonds Stephanie E, Wiede F, Balland E et al (2015) Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 160(1):88–104
Emamgholipour S, Ebrahimi R, Bahiraee A, Niazpour F, Meshkani R (2020) Acetylation and insulin resistance: a focus on metabolic and mitogenic cascades of insulin signaling. Crit Rev Clin Lab Sci 57(3):196–214
Errico J (2018) Insulin resistance, glucose metabolism, inflammation, and the role of neuromodulation as a therapy for type-2 diabetes. Neuromodulation:1565–1573
Fan Z, Turiel G, Ardicoglu R, Ghobrial M, Masschelein E, Kocijan T et al (2021) Exercise-induced angiogenesis is dependent on metabolically primed ATF3/4+ endothelial cells. Cell Metab 33(9):1793–807.e9
Fang W, Deng Z, Benadjaoud F, Yang D, Yang C, Shi G-P (2020) Regulatory T cells promote adipocyte beiging in subcutaneous adipose tissue. FASEB J 34(7):9755–9770
Fedorenko A, Lishko PV, Kirichok Y (2012) Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151(2):400–413
Feijóo-Bandín S, Aragón-Herrera A, Moraña-Fernández S, Anido-Varela L, Tarazón E, Roselló-Lletí E et al (2020) Adipokines and inflammation: focus on cardiovascular diseases. Int J Mol Sci 21(20):7711
Fekete C, Lechan RM (2014) Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev 35(2):159–194
Fernández-Verdejo R, Marlatt KL, Ravussin E, Galgani JE (2019) Contribution of brown adipose tissue to human energy metabolism. Mol Aspects Med 68:82–89
Fiorenza CG, Chou SH, Mantzoros CS (2011) Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol 7(3):137–150
Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A et al (2005) Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes 54(5):1331–1339
Freidenberg G, Reichart D, Olefsky J, Henry R (1988) Reversibility of defective adipocyte insulin receptor kinase activity in non-insulin-dependent diabetes mellitus. Effect of weight loss. J Clin Invest 82(4):1398–1406
Frese L, Dijkman PE, Hoerstrup SP (2016) Adipose tissue-derived stem cells in regenerative medicine. Transfus Med Hemother 43(4):268–274
Fromme T, Kleigrewe K, Dunkel A, Retzler A, Li Y, Maurer S et al (2018) Degradation of brown adipocyte purine nucleotides regulates uncoupling protein 1 activity. Molecular Metabolism 8:77–85
Frühbeck G, Méndez-Giménez L, Fernández-Formoso J-A, Fernández S, Rodriguez A (2014) Regulation of adipocyte lipolysis. Nutr Res Rev 27(1):63–93
Fuster JJ, Ouchi N, Gokce N, Walsh K (2016) Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res 118(11):1786–1807
Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ et al (2002) Serine phosphorylation of insulin receptor substrate 1 by inhibitor κB kinase complex. J Biol Chem 277(50):48115–48121
Garcia RA, Roemmich JN, Claycombe KJ (2016) Evaluation of markers of beige adipocytes in white adipose tissue of the mouse. Nutr Metab 13(1):24
Gaspar JM, Velloso LA (2018) Hypoxia inducible factor as a central regulator of metabolism–implications for the development of obesity. Front Neurosci 12:813
Georgiadi A, Lopez-Salazar V, Merahbi RE, Karikari RA, Ma X, Mourão A et al (2021) Orphan GPR116 mediates the insulin sensitizing effects of the hepatokine FNDC4 in adipose tissue. Nat Commun 12(1):2999
Geraldes P, King GL (2010) Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 106(8):1319–1331
Gerrits AJ, Gitz E, Koekman CA, Visseren FL, van Haeften TW, Akkerman JWN (2012) Induction of insulin resistance by the adipokines resistin, leptin, plasminogen activator inhibitor-1 and retinol binding protein 4 in human megakaryocytes. Haematologica 97(8):1149–1157
Giordano A, Frontini A, Cinti S (2016) Convertible visceral fat as a therapeutic target to curb obesity. Nat Rev Drug Discov 15(6):405–424
Giorgino F, Laviola L, Eriksson JW (2005) Regional differences of insulin action in adipose tissue: insights from in vivo and in vitro studies. Acta Physiol Scand 183(1):13–30
Girousse A, Tavernier G, Valle C, Moro C, Mejhert N, Dinel A-L et al (2013) Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol 11(2):e1001485
Goldstein BJ, Ahmad F, Ding W, Li P-M, Zhang W-R (1998) Regulation of the insulin signalling pathway by cellular protein-tyrosine phosphatases. Mol Cell Biochem 182(1):91–99
González N, Moreno-Villegas Z, González-Bris A, Egido J, Lorenzo Ó (2017) Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc Diabetol 16(1):44
Goodman HM (1988) The role of growth hormone in fat mobilization. Designing foods, animal product options in the marketplace. National Academy Press, Washington
Guan H, Zhang Y, Gao S, Bai L, Zhao S, Cheng XW et al (2018) Differential patterns of secreted frizzled-related protein 4 (SFRP4) in adipocyte differentiation: adipose depot specificity. Cell Physiol Biochem 46(5):2149–2164
Guan H, Zheng H, Zhang J, Xiang A, Li Y, Zheng H et al (2021) Secreted frizzled-related protein 4 promotes brown adipocyte differentiation. Exp Ther Med 21(6):637
Guebre-Egziabher F, Alix PM, Koppe L, Pelletier CC, Kalbacher E, Fouque D et al (2013) Ectopic lipid accumulation: a potential cause for metabolic disturbances and a contributor to the alteration of kidney function. Biochimie 95(11):1971–1979
Guo W, Wong S, Xie W, Lei T, Luo Z (2007) Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am J Physiol Endocrinol Metab 293(2):E576–EE86
Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM et al (2010) Transcriptional control of preadipocyte determination by Zfp423. Nature 464(7288):619–623
Hammarstedt A, Hedjazifar S, Jenndahl L, Gogg S, Grünberg J, Gustafson B et al (2013) WISP2 regulates preadipocyte commitment and PPARγ activation by BMP4. Proc Natl Acad Sci 110(7):2563–2568
Han Y, Xia G, Srisai D, Meng F, He Y, Ran Y et al (2021) Deciphering an AgRP-serotoninergic neural circuit in distinct control of energy metabolism from feeding. Nat Commun 12(1):1–16
Hanssen MJ, van der Lans AA, Brans B, Hoeks J, Jardon KM, Schaart G et al (2016) Short-term cold acclimation recruits brown adipose tissue in obese humans. Diabetes 65(5):1179–1189
Harms M, Seale P (2013) Brown and beige fat: development, function and therapeutic potential. Nat Med 19(10):1252–1263
Hauton D, Coney AM, Egginton S (2009) Both substrate availability and utilisation contribute to the defence of core temperature in response to acute cold. Comp Biochem Physiol A Mol Integr Physiol 154(4):514–522
Heyde I, Begemann K, Oster H (2021) Contributions of white and brown adipose tissues to the circadian regulation of energy metabolism. Endocrinology 162(3):bqab009
Hilton D, Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Van Obberghen E (2000) SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem 275(21):15985–15991
Hoeke G, Kooijman S, Boon MR, Rensen PC, Berbée JF (2016) Role of brown fat in lipoprotein metabolism and atherosclerosis. Circ Res 118(1):173–182
Holm C (2003) Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans 31(6):1120–1124
Holt LJ, Siddle K (2005) Grb10 and Grb14: enigmatic regulators of insulin action – and more? Biochem J 388(2):393–406
Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D et al (2011) The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 332(6035):1317–1322
Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M et al (2017) UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med 23(12):1454–1465
Ikeda K, Maretich P, Kajimura S (2018) The common and distinct features of brown and beige adipocytes. Trends Endocrinol Metab 29(3):191–200. https://doi.org/10.1016/j.tem.2018.01.001
Inagaki T, Sakai J, Kajimura S (2016) Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat Rev Mol Cell Biol 17(8):480–495
Iwen KA, Backhaus J, Cassens M, Waltl M, Hedesan OC, Merkel M et al (2017) Cold-induced brown adipose tissue activity alters plasma fatty acids and improves glucose metabolism in men. J Clin Endocrinol Metabol 102(11):4226–4234
Jensen MD (2008) Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 93(11 Suppl 1):S57–S63
Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles J (1989) Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest 83(4):1168–1173
Jung SM, Doxsey WG, Le J, Haley JA, Mazuecos L, Luciano AK et al (2021) In vivo isotope tracing reveals the versatility of glucose as a brown adipose tissue substrate. Cell Rep 36(4):109459
Kajimura S, Spiegelman Bruce M, Seale P (2015) Brown and beige fat: physiological roles beyond heat generation. Cell Metab 22(4):546–559
Karastergiou K, Smith SR, Greenberg AS, Fried SK (2012) Sex differences in human adipose tissues – the biology of pear shape. Biol Sex Differ 3(1):13
Karunanithi S, Xiong T, Uhm M, Leto D, Sun J, Chen X-W et al (2014) A Rab10: RalA G protein cascade regulates insulin-stimulated glucose uptake in adipocytes. Mol Biol Cell 25(19):3059–3069
Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P et al (2015) A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163(3):643–655
Keinan O, Valentine JM, Xiao H, Mahata SK, Reilly SM, Abu-Odeh M et al (2021) Glycogen metabolism links glucose homeostasis to thermogenesis in adipocytes. Nature 599(7884):296–301
Keuper M, Jastroch M (2021) The good and the BAT of metabolic sex differences in thermogenic human adipose tissue. Mol Cell Endocrinol 533:111337
Kim H-J, Lee H-J, So B, Son JS, Yoon D, Song W (2016) Effect of aerobic training and resistance training on circulating irisin level and their association with change of body composition in overweight/obese adults: a pilot study. Physiol Res 65(2):271
Kim J, Park MS, Ha K, Park C, Lee J, Mynatt RL et al (2018) NT-PGC-1α deficiency decreases mitochondrial FA oxidation in brown adipose tissue and alters substrate utilization in vivo. J Lipid Res 59(9):1660–1670. https://doi.org/10.1194/jlr.M085647
Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J et al (2012) FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26(3):271–281
Kong X, Banks A, Liu T, Kazak L, Rao RR, Cohen P et al (2014) IRF4 is a key thermogenic transcriptional partner of PGC-1α. Cell 158(1):69–83
Kou Y, Liu Q, Liu W, Sun H, Liang M, Kong F et al (2019) LIGHT/TNFSF14 signaling attenuates beige fat biogenesis. FASEB J 33(2):1595–1604
Kuo T, McQueen A, Chen T-C, Wang J-C (2015) Regulation of glucose homeostasis by glucocorticoids. Adv Exp Med Biol 872:99–126
Kyrou I, Randeva HS, Tsigos C, Kaltsas G, Weickert MO (2018) Clinical problems caused by obesity. Endotext
Labbé SM, Caron A, Lanfray D, Monge-Rofarello B, Bartness TJ, Richard D (2015) Hypothalamic control of brown adipose tissue thermogenesis. Front Syst Neurosci 9:150
Laclaustra M, Corella D, Ordovas JM (2007) Metabolic syndrome pathophysiology: the role of adipose tissue. Nutr Metab Cardiovasc Dis 17(2):125–139
Lafontan M, Moro C, Sengenes C, Galitzky J, Crampes F, Berlan M (2005) An unsuspected metabolic role for atrial natriuretic peptides. Arterioscler Thromb Vasc Biol 25(10):2032–2042
Lanktree MB, Johansen CT, Joy TR, Hegele RA (2010) A translational view of the genetics of lipodystrophy and ectopic fat deposition. Prog Mol Biol Transl Sci 94:159–196
Laviola L, Perrini S, Cignarelli A, Natalicchio A, Leonardini A, De Stefano F et al (2006) Insulin signaling in human visceral and subcutaneous adipose tissue in vivo. Diabetes 55(4):952–961
Lee Y-H, Mottillo EP, Granneman JG (2014a) Adipose tissue plasticity from WAT to BAT and in between. Biochim Biophys Acta 1842(3):358–369
Lee Y-H, Jung Y-S, Choi D (2014b) Recent advance in brown adipose physiology and its therapeutic potential. Exp Mol Med 46(2):e78
Lee M-W, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC et al (2015) Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160(1–2):74–87
Lee P, Bova R, Schofield L, Bryant W, Dieckmann W, Slattery A et al (2016) Brown adipose tissue exhibits a glucose-responsive thermogenic biorhythm in humans. Cell Metab 23(4):602–609
Lee JH, Park A, Oh K-J, Lee SC, Kim WK, Bae K-H (2019) The role of adipose tissue mitochondria: regulation of mitochondrial function for the treatment of metabolic diseases. Int J Mol Sci 20(19):4924
Leung OM, Li J, Li X, Chan VW, Yang KY, Ku M et al (2018) Regulatory T cells promote apelin-mediated sprouting angiogenesis in type 2 diabetes. Cell Rep 24(6):1610–1626
Li Q, Chen R, Moriya J, Yamakawa J, Sumino H, Kanda T et al (2008) A novel adipocytokine, visceral adipose tissue-derived serine protease inhibitor (vaspin), and obesity. J Int Med Res 36(4):625–629
Li S, Huang Q, Zhang L, Qiao X, Zhang Y, Tang F et al (2019) Effect of CAPE-pNO2 against type 2 diabetes mellitus via the AMPK/GLUT4/GSK3beta/PPARalpha pathway in HFD/STZ-induced diabetic mice. Eur J Pharmacol 15(853):1–10
Liao Z-Z, Qi X-Y, Wang Y-D, Li J-Y, Gu Q-Q, Hu C et al (2020) Betatrophin knockdown induces beiging and mitochondria biogenesis of white adipocytes. J Endocrinol 245(1):93–100
Lim K, Haider A, Adams C, Sleigh A, Savage D (2020) Lipodystrophy: a paradigm for understanding the consequences of “overloading” adipose tissue. Physiol Rev
Liu M, Bai J, He S, Villarreal R, Hu D, Zhang C et al (2014) Grb10 promotes lipolysis and thermogenesis by phosphorylation-dependent feedback inhibition of mTORC1. Cell Metab 19(6):967–980
Liu P, Huang S, Ling S, Xu S, Wang F, Zhang W et al (2019) Foxp1 controls brown/beige adipocyte differentiation and thermogenesis through regulating β3-AR desensitization. Nat Commun 10(1):5070
Liu J, Zhang C, Zhang B, Sheng Y, Xu W, Luo Y et al (2020) Comprehensive analysis of the characteristics and differences in adult and newborn brown adipose tissue (BAT): newborn BAT is a more active/dynamic BAT. Cell 9(1):201
Löffler D, Müller U, Scheuermann K, Friebe D, Gesing J, Bielitz J et al (2015) Serum irisin levels are regulated by acute strenuous exercise. J Clin Endocrinol Metabol 100(4):1289–1299
Loft A, Forss I, Siersbaek MS, Schmidt SF, Larsen AS, Madsen JG et al (2015) Browning of human adipocytes requires KLF11 and reprogramming of PPARgamma superenhancers. Genes Dev 29(1):7–22
Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X et al (2014) A smooth muscle-like origin for beige adipocytes. Cell Metab 19(5):810–820
Luce M, Barba C, Yi D, Mey A, Roussel D, Bres E et al (2020) Accumulation of natriuretic peptides is associated with protein energy wasting and activation of browning in white adipose tissue in chronic kidney disease. Kidney Int 98(3):663–672
Luo Y, Qiao X, Ma Y, Deng H, Xu CC, Xu L (2020) Disordered metabolism in mice lacking irisin. Sci Rep 10(1):1–10
Luong Q, Huang J, Lee KY (2019) Deciphering white adipose tissue heterogeneity. Biology 8(2):23
Lv Y, Zhang S-Y, Liang X, Zhang H, Xu Z, Liu B et al (2016) Adrenomedullin 2 enhances beiging in white adipose tissue directly in an adipocyte-autonomous manner and indirectly through activation of M2 macrophages. J Biol Chem 291(45):23390–23402
Ma X, Lee P, Chisholm DJ, James DE (2015) Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy. Front Endocrinol 6:1
Maalouf G-E, El Khoury D (2019) Exercise-induced irisin, the fat browning myokine, as a potential anticancer agent. J Obes 2019:6561726
Man K, Kutyavin VI, Chawla A (2017) Tissue immunometabolism: development, physiology, and pathobiology. Cell Metab 25(1):11–26
Markussen L, Isidor M, Breining P, Andersen E, Rasmussen N, Petersen L et al (2017) Characterization of immortalized human brown and white pre-adipocyte cell models from a single donor. PLoS One 12:e0185624
Mazur-Bialy AI, Pocheć E, Zarawski M (2017) Anti-inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. Int J Mol Sci 18(4):701
McNamara J (1991) Regulation of adipose tissue metabolism in support of lactation. J Dairy Sci 74(2):706–719
McNeill BT, Morton NM, Stimson RH (2020) Substrate utilization by brown adipose tissue: what’s hot and what’s not? Front Endocrinol 11(785)
Michurina SS, Stafeev IS, Menshikov MY, Parfyonova YV (2021) Mitochondrial dynamics keep balance of nutrient combustion in thermogenic adipocytes. Mitochondrion 59:157–168
Mitrou P, Boutati E, Lambadiari V, Maratou E, Papakonstantinou A, Komesidou V et al (2009) Rates of glucose uptake in adipose tissue and muscle in vivo after a mixed meal in women with morbid obesity. J Clin Endocrinol Metabol 94(8):2958–2961
Møller N, Jørgensen JOL (2009) Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 30(2):152–177
Moore H-PH, Silver RB, Mottillo EP, Bernlohr DA, Granneman JG (2005) Perilipin targets a novel pool of lipid droplets for lipolytic attack by hormone-sensitive lipase. J Biol Chem 280(52):43109–43120
Morigny P, Boucher J, Arner P, Langin D (2021) Lipid and glucose metabolism in white adipocytes: pathways, dysfunction and therapeutics. Nat Rev Endocrinol 17:1–20
Moseti D, Regassa A, Kim W-K (2016) Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int J Mol Sci 17(1):124
Mueller E (2016) Browning and graying: novel transcriptional regulators of brown and beige fat tissues and aging. Front Endocrinol 7:19
Mukaida S, Evans BA, Bengtsson T, Hutchinson DS, Sato M (2017) Adrenoceptors promote glucose uptake into adipocytes and muscle by an insulin-independent signaling pathway involving mechanistic target of rapamycin complex 2. Pharmacol Res 116:87–92
Murphy RM, Watt MJ, Febbraio MA (2020) Metabolic communication during exercise. Nat Metab 2(9):805–816
Nanduri R (2021) Epigenetic regulators of white adipocyte browning. Epigenomes 5
Nedergaard J, Bengtsson T, Cannon B (2007) Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol
Ogawa Y, Abe K, Sakoda A, Onizuka H, Sakai S (2018) FDG-PET and CT findings of activated brown adipose tissue in a patient with paraganglioma. Eur J Radiol Open 5:126–130
Olesen J, Gliemann L, Biensø R, Schmidt J, Hellsten Y, Pilegaard H (2014) Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. J Physiol 592(8):1873–1886
Olsen JM, Sato M, Dallner OS, Sandström AL, Pisani DF, Chambard J-C et al (2014) Glucose uptake in brown fat cells is dependent on mTOR complex 2–promoted GLUT1 translocation. J Cell Biol 207(3):365–374
Onogi Y, Khalil AEMM, Ussar S (2020) Identification and characterization of adipose surface epitopes. Biochem J 477(13):2509–2541
Opp M, Smith E, Hughes T Jr (1995) Interleukin-10 (cytokine synthesis inhibitory factor) acts in the central nervous system of rats to reduce sleep. J Neuroimmunol 60(1-2):165–168
Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P et al (2002) Leptin-replacement therapy for lipodystrophy. N Engl J Med 346(8):570–578
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA (2018) Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol 17(1):122
Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK (1998) A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol 513(3):889–894
Pajed L, Taschler U, Tilp A, Hofer P, Kotzbeck P, Kolleritsch S et al (2021) Advanced lipodystrophy reverses fatty liver in mice lacking adipocyte hormone-sensitive lipase. Commun Biol 4(1):323
Palanivel R, Fullerton MD, Galic S, Honeyman J, Hewitt KA, Jorgensen SB et al (2012) Reduced Socs3 expression in adipose tissue protects female mice against obesity-induced insulin resistance. Diabetologia 55(11):3083–3093
Panee J (2012) Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine 60(1):1–12
Pararasa C, Bailey CJ, Griffiths HR (2015) Ageing, adipose tissue, fatty acids and inflammation. Biogerontology 16(2):235–248
Park JH, Kang HJ, Kang SI, Lee JE, Hur J, Ge K et al (2013) A multifunctional protein, EWS, is essential for early brown fat lineage determination. Dev Cell 26(4):393–404
Park J, Kim M, Sun K, An YA, Gu X, Scherer PE (2017) VEGF-A–expressing adipose tissue shows rapid beiging and enhanced survival after transplantation and confers IL-4–independent metabolic improvements. Diabetes 66(6):1479–1490
Pedersen BK (2017a) Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest 47(8):600–611
Pedersen BK (2017b) Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest 47(8):600–611
Perrini S, Laviola L, Belsanti G, Cignarelli A, Cuscito M, Neri V, et al. Differences of insulin signalling in human adipose tissue depots in vivo. 2003
Perrini S, Laviola L, Cignarelli A, Melchiorre M, De Stefano F, Caccioppoli C et al (2008) Fat depot-related differences in gene expression, adiponectin secretion, and insulin action and signalling in human adipocytes differentiated in vitro from precursor stromal cells. Diabetologia 51(1):155–164
Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J et al (2014) A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab 20(3):433–447
Phillips KJ (2019) Beige fat, adaptive thermogenesis, and its regulation by exercise and thyroid hormone. Biology 8(3):57
Piccirillo R (2019) Exercise-induced myokines with therapeutic potential for muscle wasting. Front Physiol 10(287)
Pinhel MAS, Noronha NY, Nicoletti CF, Quinhoneiro DCG, Oliveira BAP, Cortes-Oliveira C et al (2017) Comparison of gene expression profile between blood cells and white adipose tissue of patients with obesity. Nutr Hosp 34(3):603–607
Pinnick KE, Collins SC, Londos C, Gauguier D, Clark A, Fielding BA (2008) Pancreatic ectopic fat is characterized by adipocyte infiltration and altered lipid composition. Obesity 16(3):522–530
Poher A-L, Veyrat-Durebex C, Altirriba J, Montet X, Colin DJ, Caillon A et al (2015) Ectopic UCP1 overexpression in white adipose tissue improves insulin sensitivity in Lou/C rats, a model of obesity resistance. Diabetes 64(11):3700–3712
Purcell C, Taylor SM (2019) Idiopathic facial lipoatrophy in a healthy middle-aged woman: a case report. J Otolaryngol 48(1):63
Qian S-W, Tang Y, Li X, Liu Y, Zhang Y-Y, Huang H-Y et al (2013) BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci 110(9):E798–E807
Rabiee A (2020) Beige fat maintenance; toward a sustained metabolic health. Front Endocrinol 11(634)
Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I et al (2014) Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157(6):1279–1291
Reinehr T (2010) Obesity and thyroid function. Mol Cell Endocrinol 316(2):165–171
Remie CME, Moonen MPB, Roumans KHM, Nascimento EBM, Gemmink A, Havekes B et al (2021) Metabolic responses to mild cold acclimation in type 2 diabetes patients. Nat Commun 12(1):1516
Riechman SE, Balasekaran G, Roth SM, Ferrell RE (2004) Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J Appl Physiol 97(6):2214–2219
Rinnov A, Yfanti C, Nielsen S, Åkerström TC, Peijs L, Zankari A et al (2014) Endurance training enhances skeletal muscle interleukin-15 in human male subjects. Endocrine 45(2):271–278
Roberts LD, Boström P, O’Sullivan JF, Schinzel RT, Lewis GD, Dejam A et al (2014) β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 19(1):96–108
Rockstroh D, Landgraf K, Wagner IV, Gesing J, Tauscher R, Lakowa N et al (2015) Direct evidence of brown adipocytes in different fat depots in children. PLoS One 10(2):e0117841
Rodgers A, Sferruzzi-Perri AN (2021) Developmental programming of offspring adipose tissue biology and obesity risk. Int J Obes (Lond) 45(6):1170–1192
Rodríguez A, Becerril S, Méndez-Giménez L, Ramírez B, Sáinz N, Catalán V et al (2015) Leptin administration activates irisin-induced myogenesis via nitric oxide-dependent mechanisms, but reduces its effect on subcutaneous fat browning in mice. Int J Obes (Lond) 39(3):397–407
Rodríguez A, Becerril S, Ezquerro S, Méndez-Giménez L, Frühbeck G (2017) Crosstalk between adipokines and myokines in fat browning. Acta Physiol 219(2):362–381
Romieu I, Dossus L, Barquera S, Blottière HM, Franks PW, Gunter M et al (2017) Energy balance and obesity: what are the main drivers? Cancer Causes Control 28(3):247–258
Rondinone CM (2006) Adipocyte-derived hormones, cytokines, and mediators. Endocrine 29(1):81–90
Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS et al (1999) PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4(4):611–617. https://doi.org/10.1016/S1097-2765(00)80211-7
Rowland LA, Bal NC, Kozak LP, Periasamy M (2015) Uncoupling protein 1 and sarcolipin are required to maintain optimal thermogenesis, and loss of both systems compromises survival of mice under cold stress. J Biol Chem 290(19):12282–12289
Ruan H-B, Dietrich MO, Liu Z-W, Zimmer MR, Li M-D, Singh JP et al (2014) O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell 159(2):306–317
Ruge T, Hodson L, Cheeseman J, Dennis AL, Fielding BA, Humphreys SM et al (2009) Fasted to fed trafficking of fatty acids in human adipose tissue reveals a novel regulatory step for enhanced fat storage. J Clin Endocrinol Metabol 94(5):1781–1788
Rui L, Yuan M, Frantz D, Shoelson S, White MF (2002) SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem 277(44):42394–42398
Ruigrok SR, Stöberl N, Yam K-Y, de Lucia C, Lucassen PJ, Thuret S et al (2021) Modulation of the hypothalamic nutrient sensing pathways by sex and early-life stress. Front Neurosci 15(914)
Sahu B, Pani S, Swalsingh G, Bal NC (2019) Non and epigenetic mechanisms in regulation of adaptive thermogenesis in skeletal muscle. Front Endocrinol (Lausanne) 10:517
Sanchez-Gurmaches J, Tang Y, Jespersen NZ, Wallace M, Calejman CM, Gujja S et al (2018) Brown fat AKT2 is a cold-induced kinase that stimulates ChREBP-mediated de novo lipogenesis to optimize fuel storage and thermogenesis. Cell Metab 27(1):195–209.e6
Sánchez-Jiménez R, Alvarado-Vásquez N (2013) IL-15 that a regulator of TNF-α in patients with diabetes mellitus type 2. Med Hypotheses 80(6):776–777
Sandoval DA, D'Alessio DA (2015) Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev 95(2):513–548
Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K et al (2010) Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 59(7):1817–1824
Schlein C, Fischer AW, Sass F, Worthmann A, Tödter K, Jaeckstein MY et al (2021) Endogenous fatty acid synthesis drives brown adipose tissue involution. Cell Rep 34(2):108624
Schreiber R, Diwoky C, Schoiswohl G, Feiler U, Wongsiriroj N, Abdellatif M et al (2017) Cold-induced thermogenesis depends on ATGL-mediated lipolysis in cardiac muscle, but not Brown adipose tissue. Cell Metab 26(5):753–63.e7
Schwandt HJ, Heyduck B, Gunga HC, Röcker L (1991) Influence of prolonged physical exercise on the erythropoietin concentration in blood. Eur J Appl Physiol Occup Physiol 63(6):463–466
Shamsi F, Xue R, Huang TL, Lundh M, Liu Y, Leiria LO et al (2020) FGF6 and FGF9 regulate UCP1 expression independent of brown adipogenesis. Nat Commun 11(1):1–16
Shao M, Ishibashi J, Kusminski CM, Wang QA, Hepler C, Vishvanath L et al (2016a) Zfp423 maintains white adipocyte identity through suppression of the beige cell thermogenic gene program. Cell Metab 23(6):1167–1184. https://doi.org/10.1016/j.cmet.2016.04.023
Shao X, Yang W, Shao X, Qiu C, Wang X, Wang Y (2016b) The role of active brown adipose tissue (aBAT) in lipid metabolism in healthy Chinese adults. Lipids Health Dis 15(1):138
Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L et al (2012) Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 7(11):e49452
Shi H, Cave B, Inouye K, Bjørbæk C, Flier JS (2006) Overexpression of suppressor of cytokine signaling 3 in adipose tissue causes local but not systemic insulin resistance. Diabetes 55(3):699–707
Shijun L, Khan R, Raza SHA, Jieyun H, Chugang M, Kaster N et al (2020) Function and characterization of the promoter region of perilipin 1 (PLIN1): roles of E2F1, PLAG1, C/EBPβ, and SMAD3 in bovine adipocytes. Genomics 112(3):2400–2409
Sidossis LS, Porter C, Saraf MK, Børsheim E, Radhakrishnan RS, Chao T et al (2015) Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab 22(2):219–227
Singh RG, Yoon HD, Wu LM, Lu J, Plank LD, Petrov MS (2017) Ectopic fat accumulation in the pancreas and its clinical relevance: a systematic review, meta-analysis, and meta-regression. Metabolism 69:1–13
Singh AK, Aryal B, Chaube B, Rotllan N, Varela L, Horvath TL et al (2018) Brown adipose tissue derived ANGPTL4 controls glucose and lipid metabolism and regulates thermogenesis. Molecular Metabolism 11:59–69
Sleeman MW, Wortley KE, Lai K-MV, Gowen LC, Kintner J, Kline WO et al (2005) Absence of the lipid phosphatase SHIP2 confers resistance to dietary obesity. Nat Med 11(2):199–205
Smith FM, Holt LJ, Garfield AS, Charalambous M, Koumanov F, Perry M et al (2007) Mice with a disruption of the imprinted Grb10 gene exhibit altered body composition, glucose homeostasis, and insulin signaling during postnatal life. Mol Cell Biol 27(16):5871–5886
Smith RL, Soeters MR, Wüst RCI, Houtkooper RH (2018) Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr Rev 39(4):489–517
Solinas G, Borén J, Dulloo AG (2015) De novo lipogenesis in metabolic homeostasis: more friend than foe? Mol Metab 4(5):367–377
Stautemas J, Van Kuilenburg A, Stroomer L, Vaz F, Lefevere F, Blancquaert L et al (2018) The acute exercise metabolism of the so-called myokine BAIBA. 23rd annual congress of the European College of Sport Science (ECSS 2018): sport science at the cutting edge, European College of Sport Science
Stautemas J, Van Kuilenburg AB, Stroomer L, Vaz F, Blancquaert L, Lefevere FB et al (2019) Acute aerobic exercise leads to increased plasma levels of R-and S-β-aminoisobutyric acid in humans. Front Physiol 10:1240
Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK (2003) IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 285(2):E433–E4E7
Stengel A, Hofmann T, Goebel-Stengel M, Elbelt U, Kobelt P, Klapp BF (2013) Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity–correlation with body mass index. Peptides 39:125–130
Stephens MAC, Wand G (2012) Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res 34(4):468–483
Sun K, Asterholm IW, Kusminski CM, Bueno AC, Wang ZV, Pollard JW et al (2012) Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci 109(15):5874–5879
Tan HYA, Sim MFM, Tan S-X, Ng Y, Gan SY, Li H et al (2021) HOXC10 suppresses browning to maintain white adipocyte identity. Diabetes 70(8):1654–1663
Tang X, Powelka AM, Soriano NA, Czech MP, Guilherme A (2005) PTEN, but not SHIP2, suppresses insulin signaling through the phosphatidylinositol 3-kinase/Akt pathway in 3T3-L1 adipocytes. J Biol Chem 280(23):22523–22529. https://doi.org/10.1074/jbc.M501949200
Tapia P, Fernández-Galilea M, Robledo F, Mardones P, Galgani JE, Cortés VA (2018) Biology and pathological implications of brown adipose tissue: promises and caveats for the control of obesity and its associated complications. Biol Rev 93(2):1145–1164
Thyagarajan B, Foster MT (2017) Beiging of white adipose tissue as a therapeutic strategy for weight loss in humans. Horm Mol Biol Clin Invest 31(2)
Timper K, Brüning JC (2017) Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech 10(6):679–689
Townsend KL, Tseng Y-H (2014) Brown fat fuel utilization and thermogenesis. Trends Endocrinol Metab 25(4):168–177
Tran K-V, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A et al (2012) The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab 15(2):222–229
Tsuchiya Y, Ando D, Goto K, Kiuchi M, Yamakita M, Koyama K (2014) High-intensity exercise causes greater irisin response compared with low-intensity exercise under similar energy consumption. Tohoku J Exp Med 233(2):135–140
Ussar S, Lee KY, Dankel SN, Boucher J, Haering M-F, Kleinridders A et al (2014) ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci Transl Med 6(247):247ra103
Valgas P, da Silva C, Hernández-Saavedra D, White JD, Stanford KI (2019) Cold and exercise: therapeutic tools to activate brown adipose tissue and combat obesity. Biology 8(1):9
van der Zijl NJ, Goossens GH, Moors CC, van Raalte DH, Muskiet MH, Pouwels PJ et al (2011) Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on β-cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metabol 96(2):459–467
VandeKopple MJ, Wu J, Baer LA, Bal NC, Maurya SK, Kalyanasundaram A et al (2017) Stress-responsive HILPDA is necessary for thermoregulation during fasting. J Endocrinol 235(1):27–38
Villarroya F, Cereijo R, Villarroya J, Gavaldà-Navarro A, Giralt M (2018) Toward an understanding of how immune cells control brown and beige adipobiology. Cell Metab 27(5):954–961
Virtanen KA, Iozzo P, Hällsten K, Huupponen R, Parkkola R, Janatuinen T et al (2005) Increased fat mass compensates for insulin resistance in abdominal obesity and type 2 diabetes, a positron–emitting tomography study. Diabetes 54(9):2720–2726
Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J (2012) Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol 302(1):E19–E31
Wang W, Seale P (2016) Control of brown and beige fat development. Nat Rev Mol Cell Biol 17(11):691–702
Wang S, Yang X (2017) Inter-organ regulation of adipose tissue browning. Cell Mol Life Sci 74(10):1765–1776
Wang W, Kissig M, Rajakumari S, Huang L, Lim H-W, Won K-J et al (2014) Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci 111(40):14466–14471
Wang Y-L, Lin S-P, Hsieh P, Hung S-C (2016) Concomitant beige adipocyte differentiation upon induction of mesenchymal stem cells into brown adipocytes. Biochem Biophys Res Commun 478
Wang B, Li A, Li X, Ho PWL, Wu D, Wang X et al (2018) Activation of hypothalamic RIP-Cre neurons promotes beiging of WAT via sympathetic nervous system. EMBO Rep 19(4):e44977. https://doi.org/10.15252/embr.201744977
White PJ, McGarrah RW, Herman MA, Bain JR, Shah SH, Newgard CB (2021) Insulin action, type 2 diabetes, and branched-chain amino acids: a two-way street. Mol Metab:101261
Whittle Andrew J, Carobbio S, Martins L, Slawik M, Hondares E, Vázquez María J et al (2012) BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149(4):871–885. https://doi.org/10.1016/j.cell.2012.02.066
Worsch S, Heikenwalder M, Hauner H, Bader BL (2018) Dietary n-3 long-chain polyunsaturated fatty acids upregulate energy dissipating metabolic pathways conveying anti-obesogenic effects in mice. Nutr Metab 15(1):65
Wu J, Boström P, Sparks Lauren M, Ye L, Choi Jang H, Giang A-H et al (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150(2):366–376
Xue Y, Cao R, Nilsson D, Chen S, Westergren R, Hedlund E-M et al (2008) FOXC2 controls Ang-2 expression and modulates angiogenesis, vascular patterning, remodeling, and functions in adipose tissue. Proc Natl Acad Sci 105(29):10167–10172
Yang Y, Fu M, Li M-D, Zhang K, Zhang B, Wang S et al (2020) O-GlcNAc transferase inhibits visceral fat lipolysis and promotes diet-induced obesity. Nat Commun 11(1):181
Yasmeen R, Shen Q, Lee A, Leung JH, Kowdley D, DiSilvestro DJ et al (2018) Epiregulin induces leptin secretion and energy expenditure in high-fat diet-fed mice. J Endocrinol 239(3):377–388
Ye J (2013) Mechanisms of insulin resistance in obesity. Front Med 7(1):14–24
Yki-Järvinen H (2002) Ectopic fat accumulation: an important cause of insulin resistance in humans. J R Soc Med 95(Suppl 42):39–45
Yoneshiro T, Wang Q, Tajima K, Matsushita M, Maki H, Igarashi K et al (2019) BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572(7771):614–619
Yoneshiro T, Kataoka N, Walejko JM, Ikeda K, Brown Z, Yoneshiro M et al (2021) Metabolic flexibility via mitochondrial BCAA carrier SLC25A44 is required for optimal fever. Elife 10:e66865
Youngren JF (2007) Regulation of insulin receptor function. Cell Mol Life Sci 64(7):873
Yu Y, Yoon S-O, Poulogiannis G, Yang Q, Ma XM, Villén J et al (2011) Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 332(6035):1322–1326
Yu F, Wang Z, Zhang T, Chen X, Xu H, Wang F et al (2021) Deficiency of intestinal Bmal1 prevents obesity induced by high-fat feeding. Nat Commun 12(1):5323
Zhang J, Gao Z, Yin J, Quon MJ, Ye J (2008) S6K directly phosphorylates IRS-1 on Ser-270 to promote insulin resistance in response to TNF-α signaling through IKK2. J Biol Chem 283(51):35375–35382
Zhou H, Kaplan T, Li Y, Grubisic I, Zhang Z, Wang PJ et al (2013) Dual functions of TAF7L in adipocyte differentiation. Elife 2:e00170
Zhou H, Wan B, Grubisic I, Kaplan T, Tjian R (2014) TAF7L modulates brown adipose tissue formation. Elife 3:e02811
Zhu Y, Gao Y, Tao C, Shao M, Zhao S, Huang W et al (2016) Connexin 43 mediates White adipose tissue beiging by facilitating the propagation of sympathetic neuronal signals. Cell Metab 24(3):420–433
Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV (2018) Anatomical, physiological, and functional diversity of adipose tissue. Cell Metab 27(1):68–83
Acknowledgments
We thank Punyadhara Pani, Gourabmani Swalsingh, and Sunil Pani for constructive critical suggestions and discussions that helped us to shape this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Author Contributions
BS and NCB conceived the idea. BS, OT, and the US prepared the first draft and figures. All authors critically discussed and edited the manuscript.
Data Availability Statement
Data sharing does not apply to this article as no new data were created or analyzed in this study.
Funding
This work was supported by the Council of Scientific and Industrial Research (CSIR), India (file no.09/1035 (0011)/2017-EMR-I to BS for Junior Research Fellowship), the Science and Engineering Research Board (SERB), DST, India (Grant number ECR/2016/001247 to NCB), and DBT, India (Grant number BT/RLF/Re-entry/41/2014 and BT/PR28935/MED/30/2035/2018).
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sahu, B., Tikoo, O., Pati, B., Senapati, U., Bal, N.C. (2022). Role of Distinct Fat Depots in Metabolic Regulation and Pathological Implications. In: Pedersen, S.H.F. (eds) Reviews of Physiology, Biochemistry and Pharmacology. Reviews of Physiology, Biochemistry and Pharmacology, vol 186. Springer, Cham. https://doi.org/10.1007/112_2022_73
Download citation
DOI: https://doi.org/10.1007/112_2022_73
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-25627-1
Online ISBN: 978-3-031-25628-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)