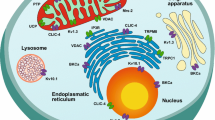
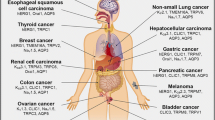
Abstract
The expression and function of many ion channels and transporters in cancer cells display major differences in comparison to those from healthy cells. These differences provide the cancer cells with advantages for tumor development. Accordingly, targeting ion channels and transporters have beneficial anticancer effects including inhibition of cancer cell proliferation, migration, invasion, metastasis, tumor vascularization, and chemotherapy resistance, as well as promoting apoptosis. Some of the molecular mechanisms associating ion channels and transporters with cancer include the participation of oxidative stress, immune response, metabolic pathways, drug synergism, as well as noncanonical functions of ion channels. This diversity of mechanisms offers an exciting possibility to suggest novel and more effective therapeutic approaches to fight cancer. Here, we review and discuss most of the current knowledge suggesting novel therapeutic approaches for cancer therapy targeting ion channels and transporters. The role and regulation of ion channels and transporters in cancer provide a plethora of exceptional opportunities in drug design, as well as novel and promising therapeutic approaches that may be used for the benefit of cancer patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cancer is one of the most devastating diseases; it generates profound emotional, financial, and physical stress both to the patients and family members. Besides, the cost of cancer treatment has a strong impact on the economy of any country. Current treatments include surgical resection, chemotherapy, radiation, and immunotherapy. However, many patients have a very poor response to current treatments and/or acquire resistance leading to cancer relapse. Medical research has been looking for more effective and specific drugs to improve the quality of life of cancer patients (Hoelder et al. 2012). One of the most novel approaches in cancer research is to study the role of ion channels and transporters as potential therapeutic targets for anticancer therapy. Since the recognition that ion channels and transporters play an important role in the carcinogenesis process, there has been great scientific interest in discovering new treatments using these genes and proteins as novel tools in oncology. Actually, several compounds targeting ion channels and transporters demonstrate promising potential to be used in cancer patients (Arcangeli and Becchetti 2010; Litan and Langhans 2015). The anticancer potential of these compounds is enhanced when different therapeutic approaches are considered, for instance, by its combination with antineoplastic drugs, immunotherapy, or other molecules targeting essential processes in cancer development including oxidative stress or metabolic pathways. Here, we review and discuss most of the current knowledge suggesting novel therapeutic approaches for cancer therapy by targeting ion channels and transporters. The expression and activity of ion channels and transporters in cancer have been reviewed in detail in several excellent articles of these series. Thus, before going into details of the topics of this review, first we will provide a general and brief panorama of ion channels and transporters in cancer.
1.1 Ion Channels and Transporters in Cancer
Potassium (K+) channels are some of the most studied and deregulated channels in malignancies. The voltage-gated K+ channels Kv10.1 (EAG1) and Kv11.1 (HERG) have been implicated in the pathogenesis of various cancers (Asher et al. 2010; Serrano-Novillo et al. 2019). Kv10.1 channel ectopic expression is associated with malignant transformation, tumor development, metastasis, and poor prognosis; channel overexpression has been observed in most of the human tumors (Pardo et al. 1999; Gavrilova-Ruch et al. 2002; Gessner and Heinemann 2003; Farias et al. 2004; Camacho 2006; Hemmerlein et al. 2006; Queiroz et al. 2006; Pardo and Stühmer 2008; Garcia-Becerra et al. 2010; Asher et al. 2011; Ortiz et al. 2011; Liu et al. 2015; Martinez et al. 2015; Serrano-Novillo et al. 2019). Inhibition of either its expression or activity decreases cancer cell proliferation both in vitro and in vivo (Pardo et al. 1999; Gomez-Varela et al. 2007; Garcia-Quiroz et al. 2014; Chavez-Lopez et al. 2015). Kv11.1 channel altered expression increases cell proliferation, angiogenesis, invasiveness, migration, and lymph node dissemination and decreases cell differentiation (He et al. 2020; Jehle et al. 2011). Overexpression of Kv11.1 channels has been observed in a variety of neoplastic tissues including endometrial, colorectal, esophageal, pancreatic, gastric, ovarian, breast, thyroid, and brain cancers, as well as leukemias (Jehle et al. 2011; Lastraioli et al. 2015a, b; Iorio et al. 2018; Lastraioli et al. 2019; Iorio et al. 2020; He et al. 2020). In gastric tumors, these channels participate in the PI3K/Akt-dependent pathway that induces hypoxia-inducible factors (HIF) and vascular endothelial growth factor (VEGF) to promote cancer progression (Crociani et al. 2014). Interestingly, Kv11.1 is also aberrantly expressed in human gastric dysplasia samples, representing a potential novel marker for progression toward gastric cancer (Lastraioli et al. 2019). In pancreatic ductal adenocarcinoma (PDAC) cells, Kv11.1 activity is essential to induce cell migration by modulating the f-actin organization (Manoli et al. 2019). In addition, Kv11.1 channels may serve as prognostic factors and potential targets for cancer treatment (He et al. 2020; Lastraioli et al. 2015b). Channel blockade reduces proliferation and migration and induces apoptosis in cancer cell lines and tissues (Roy et al. 2008; Jehle et al. 2011; Lastraioli et al. 2015). Interestingly, activation of Kv11.1 also promotes anticancer effects. In SKBr3 or MDA-MB-231 mammary gland adenocarcinoma cell lines, prolonged stimulation of Kv11.1 with the diphenylurea compound NS1643 triggered a senescence-like phenotype, arresting the cell cycle in the G0/G1 phase (Lansu and Gentile 2013). Besides, NS1643 treatment (6 mg/kg) of MDA-MB-231 cell-derived breast cancer xenografts generated significantly smaller tumors, expressed lower levels of Ki67, and showed increased expression of the senescence markers p21waf/cip and p16INK4A compared with untreated mice; these NS1643-treated animals did not show cardiac function alterations (Fukushiro-Lopes et al. 2018). Likewise, NS1643 treatment of the B-RAF-dependent melanoma cell line A375 (that expresses Kv11.3 channels but not Kv11.1), significantly reduced cell proliferation. This antiproliferative effect included lowering the expression of cell cycle promoters (cyclin E, cyclin D, and phosphorylated WEE1), as well as increasing senescence markers (p21waf and p16INK4A) and autophagy markers (phosphorylation of ULK1 and LC3-II), suggesting that activation of Kv11.3 generates tumor suppression (Perez-Neut et al. 2016).
Similarly, high expression of Kv1.3 channels is detected in a great number of human malignancies including breast, colon, and prostate cancer (Comes et al. 2013; Huang and Jan 2014), and blockade of these type of channels inhibits cancer cell proliferation by arresting the cell cycle in the G1 phase (Teisseyre et al. 2015). Likewise, the expression of ATP-sensitive K+ (KATP) channels has been observed in multiple malignancies, including bladder, gastric, and cervical cancer, as well as in glioma and hepatocellular carcinoma (Monen et al. 1998; Wondergem et al. 1998; Malhi et al. 2000; Qian et al. 2008; Huang et al. 2009; Núñez et al. 2013; Vazquez-Sanchez et al. 2018). Because K+ channels have a high potential to be targeted in cancer diagnosis and treatment, several patents have been filed concerning these channels as tools for diagnostic or therapeutic purposes in oncology (D’Amico et al. 2013).
Calcium ions participate as second messengers in cellular homeostasis like gene transcription, proliferation, migration, autophagy, and apoptosis (Bootman et al. 2001; Harr and Distelhorst 2010; Varghese et al. 2019). Some of the most studied calcium channels in cancer are those from the ORAI family and the TRP (transient receptor potential) Ca2+ channel superfamily. ORAI channels are located in the plasma membrane and interact with the stromal interaction molecules (STIMs) located in the endoplasmic reticulum (ER). ORAI1 and ORAI3 isoforms are overexpressed in breast cancer (Lis et al. 2007; Azimi et al. 2014); in prostate cancer, these isoforms confer apoptosis resistance (Dubois et al. 2014). The TRP family consists of seven subfamilies: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPP (polycystin), TRPA (ankyrin), TRPML (mucolipin), and TRPN (NOMPC-like); they are permeable to monovalent and divalent cations and are expressed in a variety of cell types, including sensory neurons (Clapham 2003). These channels are altered in various cancers favoring carcinogenesis mainly by dysfunction in Ca2+ signaling pathways (Miller and Zhang 2011; Nielsen et al. 2014). Changes in the expression of several TRP channels have been implicated in prostate, breast, and lung cancer progression, as well as in ovarian cancer differentiation (Zeng et al. 2013; Azimi et al. 2014; Deliot and Constantin 2015). The SERCA-ATPase pump responsible for reloading the sarco/endoplasmic reticulum with Ca2+ and regulating cytosolic free Ca2+ has been associated with different tumors including gastric, colon, prostate, lung, and breast cancers (Denmeade and Isaacs 2005; Korošec et al. 2006; Dang and Rao 2016; Izquierdo-Torres et al. 2017). SERCA3 is downregulated or absent in colon, gastric, breast, and lung cancers (Gélébart et al. 2002; Papp and Brouland 2011; Arbabian et al. 2013), whereas SERCA2 is overexpressed in colon cancer and correlates with metastasis and decreased survival in patients (Chung et al. 2006). Voltage-gated calcium (Cav) channels are also involved in the development and progression of diverse types of cancer (Wang et al. 2015a; Martinez-Delgado and Felix 2017). These channels are organized into three subfamilies: (1) L-type, (2) P/Q-, N- and R-type, and (3) T-type channels (Gao et al. 2000, 2001; Buchanan and McCloskey 2016). Several Cav’s channels are overexpressed in a variety of cancers including leukemia, sarcomas, brain, colorectal, gastric, lung, ovarian, pancreas, breast, uterus, and prostate cancer (Wang et al. 2015a; Taylor et al. 2008). Upon activation of L-type channels, gene regulation can be addressed through the activation of transcription factors such as cAMP-response-element-binding protein (CREB), nuclear factor of activated T cells (NFAT), and downstream of the regulatory element antagonist modulator (DREAM); these transcription factors favor cancer cell proliferation, invasion, and metastasis (Shankar et al. 2005; Barbado et al. 2009; Mancini and Toker 2009; Xiao et al. 2010). The blockade of T-type channel expression or activity reduces cancer cell proliferation and induces apoptosis (Bertolesi et al. 2002). Interestingly, Ca2+ channel blockers approved for the treatment of other conditions may be repurposed to treat some cancers (Buchanan and McCloskey 2016). Actually, the use of Ca2+ channel blockers for the treatment of hypertension, epilepsy, and other conditions may be inversely correlated with prostate cancer (Fitzpatrick et al. 2001; Debes et al. 2004). Lastly, voltage-gated sodium channels have been mainly associated to the metastatic potential of several cancers (Arcangeli and Becchetti 2010; Litan and Langhans 2015).
One of the major problems in cancer treatment is chemoresistance produced partly because of drug extrusion by ATP-binding cassette (ABC) transporters. Although the etiology of multidrug resistance (MDR) is multifactorial, the most common mechanism in the majority of resistant cell lines involves the overexpression of P-glycoprotein (Silva et al. 2015). Other transporters related to drug efflux are multidrug resistance-associated protein1 (MRP-1) and multixenobiotic resistance (MXR) (Xue and Liang 2012). Interestingly, some ion channels and transporters have been associated with therapy resistance by diverse mechanisms; in accordance, ion channel inhibitors restore chemotherapy sensitivity of different cancer cells (Kischel et al. 2019).
In summary, searching for high-efficacy therapies modulating the activity and/or expression of ion channels and transporters is a very active and promising field in cancer. Table 1 shows some examples of the potential therapeutic, diagnostic, and/or prognostic use of ion channels and transporters in cancer including some clinical trials in cancer patients.
A major opportunity for cancer treatment comes by taking advantage of the molecular mechanisms associating ion channels and transporters with cancer. Relevant cellular processes involved in cancer progression including oxidative stress, immune response, and mitochondrial activity, as well as chemoresistance, have been associated with the different roles of ion channels and transporters in tumor progression. Therefore, novel therapeutic approaches may be suggested by simultaneously targeting ion channels and transporters and the cell processes or molecular mechanisms involved. These approaches should provide better and potentiated effects of cancer therapies. Following, we will go into details of the novel therapeutic approaches suggested by a number of groups, based on the participation of ion channels and transporters in cancer.
2 Association of Oxidative Stress with Ion Channels and Transporters in Cancer: Friends and Foes
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) participate in the regulation of metabolism, gene transcription, protein posttranslational modifications, ion transport, cell differentiation, proliferation, and migration, among other processes (Birben et al. 2012; Tochhawng et al. 2013). When ROS/RNS rises beyond their physiological levels, oxidative stress is produced (Chio and Tuveson 2017) potentially leading to DNA mutations, gene transcription alterations, protein oxidation, lipid peroxidation, ion transport alterations, mutagenesis, and cell death (Rani et al. 2016; Poprac et al. 2017). Cancer cells display increased metabolic activity that leads to ROS/RNS overproduction, to counteract this oxidative stress; they have larger pools of antioxidants (Denicola et al. 2011; Harris et al. 2015; Beatty and Gladney 2015; Sullivan et al. 2016). In fact, the use of antioxidants in cancer is controversial, since they may either prevent tumor growth and genomic instability or favor tumor progression and migration (Bjelakovic et al. 2007; Klein et al. 2011; Porporato et al. 2014; Sayin et al. 2014; Le Gal et al. 2015; Harris et al. 2015; Prasad et al. 2017). For instance, in mouse models of B-RAF- and K-RAS-induced lung cancer, treatment with the antioxidants N-acetylcysteine (NAC) and vitamin E increased tumor cell proliferation and reduced survival by reducing ROS levels which leads to the reduction of p53 expression (Sayin et al. 2014). In melanoma, oxidative stress decreases metastasis in vivo; it is melanoma metastatic tumor cells overproduce glutathione and NADPH antioxidants, resisting the damage caused by oxidative stress and promoting metastasis (Piskounova et al. 2015). On the other hand, moderate to high levels of ROS/RNS in cancer cells promote initiation, proliferation, survival, and angiogenesis (Roderick and Cook 2008; Trachootham et al. 2009; Gorrini et al. 2013; Harris et al. 2015; Sullivan et al. 2016; Chio and Tuveson 2017).
Chemotherapy drugs like doxorubicin cause cell death by increasing the production of ROS/RNS, so, pro-oxidant drugs are also currently studied as anticancer options (Kong et al. 2000; Gorrini et al. 2013; Noh et al. 2015; Vilema-Enríquez et al. 2016). Actually, the strategy of delivering and augmenting the concentration of H2O2 in tumors has been proposed for lung cancer (Vilema-Enríquez et al. 2016). Although various therapeutic approaches targeting the redox status in cancer cells have been proposed, clinical results remain elusive (Tong et al. 2015). Ion channels/transporters can be oxidized by direct interaction with ROS/RNS, particularly by H2O2, via their sulfhydryl groups and cysteine residues, or indirectly by altering signaling pathways that are involved in their regulation, expression, or function (Ramírez et al. 2016). Oxidative stress can increase [Ca2+]i inducing protein phosphorylation and gene transcription, contributing to cancer cell survival (Roderick and Cook 2008). Depending on the duration, intensity, and type of oxidant, oxidative stress may cause either influx of Ca2+ into the cytosol via different channels and transporters in the plasma membrane or efflux of Ca2+ from the endoplasmic reticulum (ER), which in turn can cause Ca2+ overload that may lead to disruption of the mitochondrial metabolism and cell death (Ermak and Davies 2002). Thus, further research is needed to take advantage of the potential anticancer effects of oxidative stress and redox status. In this regard, the modulation of ion channels and transporters by ROS/RNS may bring a new therapeutic opportunity. Next, some potential candidates for this approach are discussed.
2.1 The SERCA-ATPase Pump and the Plasma Membrane Ca2+ ATPase
SERCA inhibitors have been proposed as an anticancer therapy since its blockage generates ER stress that leads to the activation of apoptotic pathways (Denmeade and Isaacs 2005). In breast cancer, the antioxidant and anti-inflammatory compound resveratrol induces the expression of SERCA3 decreasing cell viability (Izquierdo-Torres et al. 2017). Curcumin (a SERCA inhibitor) causes apoptosis by inducing ER stress in ovarian and thyroid cancer cells (Seo et al. 2016; Zhang et al. 2018). The blockage of SERCA with thapsigargin induces sustained elevation of [Ca2+]i also leading to apoptosis in cancer cells (Denmeade and Isaacs 2005). Since the SERCA pump is widely expressed, the more specific thapsigargin-based prodrug mipsagargin has been used in a hepatocarcinoma (HCC) phase II clinical trial; the prodrug altered the tumor vasculature reducing tumor blood flow in HCC sites (Mahalingam et al. 2019).
The plasma membrane Ca2+ ATPase (PMCA) is responsible for pumping Ca+2 to the extracellular space and maintain [Ca2+]i homeostasis. The platinum (II) complex [Pt(O,O′-acac)(γ-acac)(DMS)] decreased PMCA activity and induced higher levels of ROS by activating NADPH oxidase and mitochondrial ROS production in the chemotherapeutic-resistant breast cancer cell line MCF-7 (Muscella et al. 2011). Silencing of PMCA2 and PMCA4 combined with a Bcl-2 inhibitor (ABT-263) mediated cell death in MDA-MB-231 breast cancer cells (Curry et al. 2012, 2016).
2.2 ORAI Channels
Store-operated Ca2+ entry (SOCE) is the main mechanism for the entrance of Ca2+ in the cells; it is mediated by the STIMs Ca2+sensors in the ER and the ORAI channels in the plasma membrane, both interact to restore the depletion of Ca2+ from the ER (Xie et al. 2016). SOCE inhibitors have antitumor activity in vitro, and some compounds have been studied in clinical trials (Chen et al. 2019). In fact, SOCE is necessary to induce cytotoxicity of cisplatin in non-small cell lung cancer cells, and depletion of STIM1 reduces the oxidative stress promoted by cisplatin (Gualdani et al. 2019). ORAIs and STIMs have been correlated with proliferation, apoptosis resistance, migration, and metastasis of many tumors (Fiorio Pla et al. 2016). ROS target ORAI channels, modulating [Ca2+]i, and H2O2 blocks Orai1 and Orai2, but not Orai3 because it lacks a cysteine residue at position 195 (Bogeski et al. 2010). Immune and cancer cells have a different Orai1/Orai3 isoform ratio in the cell membrane; this may alter Ca2+ signaling in oxidative stress because Orai1 can be blocked by H2O2 (Frisch et al. 2019). Orai3 is overexpressed and correlated with chemotherapy resistance in breast cancer cells (Hasna et al. 2018), besides Orai1 interacts with Kv10.1 channels and the secretory pathway Ca2+ ATPase (SPCA2) mediating a store-independent calcium entry (SICE) necessary to promote cell survival; interestingly the three proteins are overexpressed in aggressive tumor tissues (Peretti et al. 2019). Furthermore, Orai1 and Orai3 can interact with TRPC6 causing translocation of Orai channels to the plasma membrane; reduction of TRPC6 expression significantly inhibited SOCE in MCF-7 and MDA-MB-231 breast cancer cells (Jardin et al. 2018). Treatment with the phenolic compound (−)-oleocanthal downregulates TRPC6 channel expression reducing cell viability and migration of MCF-7 and MDA-MB-231 cells (Diez-Bello et al. 2019).
2.3 Members of the TRP Channel Family
One of the most studied families of ion channels in oxidative stress is the TRP family, among them TRPC5, TRPV1, and TRPA1 channels are directly activated by ROS and/or RNS by modification on their cysteine residues (Takahashi and Mori 2011); TRPM2 and TRPM7 may be activated via ROS-signaling pathways (Simon et al. 2013), although TRPM2 can also be directly activated by H2O2 in some cell types including microglia and pancreatic ß cells (Kühn et al. 2005).
In most nonmalignant cells, TRPM2 channels participate in a variety of cellular processes including insulin release, inflammatory response, and cell migration; and they are considered as redox sensors that induce Ca2+ influx leading to cell death by intracellular Ca2+ overload (Lange et al. 2009; Sumoza-Toledo et al. 2011; Faouzi and Penner 2014). TRPM2 channels have also been found in the nucleus, but its role is unclear (Zeng et al. 2010; Hopkins et al. 2015; Zhao et al. 2016). H2O2 can mediate TRPM2 activation via mitochondrial ADPR release, which can bind directly to the NUDT9-H domain of the channel (Hara et al. 2002). However, in some cancers, activation of TRPM2 by moderate levels of ROS has been considered as a protective mechanism for the ongoing growth and survival (Chen et al. 2013; Blake et al. 2017). In vitro and in vivo studies demonstrate that TRPM2 supports cancer cell survival; for instance, in neuroblastoma cells the activation and expression of the full-length TRPM2 (TRPM2-L) channel protects cell viability by modulating the expression of the hypoxia-inducible factor (HIF)-1/2α; activation of Src, Pyk2, and CREB; and increasing the levels of forkhead box transcription factor 3a (FOXO3a) and superoxide dismutase 2 (Chen et al. 2013, 2014; Hirschler-Laszkiewicz et al. 2018). In xenografts of neuroblastoma cells, tumor growth was decreased by expressing the dominant-negative isoform TRPM2-S that inhibits the functional TRPM2-L (Chen et al. 2014; Bao et al. 2016). In gastric cancer cells, expression of TRPM2 is necessary to induce migration and invasion through the PTEN/Akt signaling pathway (Almasi et al. 2019a), and PTEN downregulation is correlated with advanced stages of gastric cancer (Zhu et al. 2013). Silencing TRPM2 in lung cancer cells (A549 and H1299) increases ROS/RNS levels, induces G2/M arrest, activates JNK signaling pathway, and in SCID mice xenografts reduces cell migration and tumor growth (Almasi et al. 2019b). In prostate cancer, melanoma, and lung cancer, overexpression of the long noncoding TRPM2-AS (an antisense transcript for TRPM2 channel) has been correlated with increased proliferation and poor prognosis in patients (Orfanelli et al. 2008, 2014; Huang et al. 2017). Interestingly, inhibition of TRPM2 increases ROS, causes mitochondria dysfunction, impairs autophagy, and promotes sensitivity to chemotherapy in some cancer cells (Chen et al. 2014; Koh et al. 2015; Bao et al. 2016; Almasi et al. 2018). Thus, combining chemotherapeutic agents with TRPM2 inhibitors is a promising therapeutic approach, although possible side effects need further analysis because these channels participate in important physiological processes including protection against cardiac ischemia-reperfusion (Miller et al. 2014), activation of the immune response (Yamamoto et al. 2008), and insulin secretion from pancreatic B cells (Togashi et al. 2006).
In the case of TRPC5 channels, overexpression generates Ca2+ signals that activate NFATC3 (nuclear factor of activated T cells 3) which upregulates the synthesis of P-glycoprotein inducing chemotherapeutic drug efflux in adriamycin-resistant breast cancer cells (Ma et al. 2012). Extracellular vesicles released from breast cancer cells increase ROS which in turn activates autophagy and stimulates the release of growth-promoting factors in human mammary epithelial cells (HMECs) (Dutta et al. 2014). Interestingly, extracellular vesicles containing TRPC5 have been found in peripheral blood of breast cancer patients that underwent chemotherapy, suggesting a manner to transfer TRPC5 channels to other cells (Ma et al. 2014).
TRPA1 channels are activated by ROS by targeting cysteine residues in the intracellular site and are upregulated by NRF2, a transcription factor involved in protection against oxidative stress (Mukhopadhyay et al. 2011; Schaefer et al. 2013; Takahashi et al. 2018). Activation of TRPA1 generates Ca2+ influx stimulating proliferation pathways like RAS-ERK, PI3K/AKT, and mTOR, as well as triggering anti-apoptotic pathways. In xenograft tumor models, TRPA1 induces resistance to carboplatin (which induces ROS), and the inhibition of TRPA1 reduces tumor growth and increases chemotherapy sensitivity (Takahashi et al. 2018). In another context, TRPA1 is expressed in C-fiber nerves, and activation of the channel by chemotherapy drugs induces peripheral neuropathy; short-term treatments with antagonists have been suggested as a strategy for preventing peripheral neuropathy induced by chemotherapy (Trevisan et al. 2013). Furthermore, mice treated with doxorubicin and HC-030031 (a TRPA1 inhibitor) generated protection against doxorubicin cardiac injury (Wang et al. 2018b).
TRPV1 is also modulated by oxidizing agents potentiating its activity in neuronal tissues (Susankova et al. 2006; Özdemir et al. 2016). Combinations of antioxidants with chemotherapeutics (for instance, melatonin with doxorubicin or selenium with cisplatinum) in MCF-7 breast cancer cells promoted ROS production and apoptosis; this mechanism was due in part by inhibiting TRPV1 (Koşar et al. 2016; Sakallı et al. 2017). In contrast, a combination of the antioxidant alpha-lipoic acid (ALA) and cisplatinum increased TRPV1 activation resulting in increased ROS production, depolarization of the mitochondrial membrane, and apoptosis (Nur et al. 2017).
2.4 Chloride Intracellular Channel Protein 1 (CLIC1)
CLIC1 is considered a sensor and effector of oxidative stress; it is expressed in the nucleus and cytosol, but upon oxidation, a disulfide bond in cysteine residues of the CLIC1 monomer is formed, and it translocates to the plasma membrane as an active chloride channel (Littler et al. 2004). CLIC1 is overexpressed in various tumors including gastric, colon, and lung cancers, contributing in cell cycle progression, proliferation, migration, and invasion (Chen et al. 2007; Petrova et al. 2008; Averaimo et al. 2010; Wang et al. 2011). In the highly metastatic colon cancer LOVO cells and the SGC-7901 human gastric cancer cell line treated in hypoxic and reoxygenating conditions, CLIC1 channel expression is increased; inhibition of CLIC1 decreases ROS production and p-p38 MAPK/p-ERK levels, as well as reduces MMP-2 and MMP-9 protein levels which inhibits cell migration and invasion (Wang et al. 2014a; Zhao et al. 2015). CLIC1 silencing promotes apoptosis and decreases proliferation in human gallbladder cancer (He et al. 2018). Metformin inhibits CLIC1 reducing glioblastoma stem cell proliferation and invasiveness, compared to normal mesenchymal stem cells (Gritti et al. 2014). CLIC1 is a promising pharmacological target in stress-related diseases, including cancer, where CLIC1 increases tumorigenic and metastatic potential (Peretti et al. 2015).
2.5 Amino Acid Transporter SLC7A11
Metabolic reprogramming occurs in cancer cells to acquire the necessary nutrients to sustain their biosynthetic and bioenergetic processes, which also increases oxidative stress. The cystine/glutamate antiporter solute carrier family 7 member 11 (SLC7A11, also called xCT) imports a cysteine molecule coupled with the efflux of one glutamate molecule (Koppula et al. 2017). SLC7A11 regulates intracellular redox balance by maintaining intracellular levels of glutathione and inhibiting ferroptosis, protecting the cells from oxidative stress-induced cell death (Lewerenz et al. 2013; Zheng et al. 2019). SLC7A11 promotes cancer growth and drug resistance (Lewerenz et al. 2013), and in response to oxidative stress, the proto-oncogene K-Ras stimulates SLC7A11 transcription upregulating glutathione levels in the tumor cells (Lim et al. 2019). Inhibitors of this transporter have antitumor effects by altering the entrance of cysteine necessary for glutathione (GSH) synthesis (Robe et al. 2009; Takeuchi et al. 2014; Shitara et al. 2017). Sulfasalazine, a nonselective blocker of SLC7A11, has been studied as an anticancer drug alone or in combination with other anticancer therapies in animal models and clinical trials, but more selective inhibitors are needed to reduce high adverse effects in humans (Guan et al. 2009; Lo et al. 2010; Takeuchi et al. 2014; Peretti et al. 2015; Sehm et al. 2016; Shitara et al. 2017).
Thus, the diverse association between oxidative stress and ion channel and transporters represents a very important opportunity for cancer therapy. However, the specific channel and transporter inhibitors, as well as the particular anticancer drugs concomitantly used, should be carefully considered. High levels of ROS are also produced in the mitochondria, and ion channels and transporters of this organelle have been also associated with cancer and proposed as targets for therapy.
3 Mitochondrial Ion Channels and Transporters in Novel Potential Therapies for Cancer
The mitochondria play many important cellular functions including ATP and ROS production, apoptosis, as well as Ca2+ homeostasis (Sharma et al. 2019). Dysfunction of this organelle has been correlated with several diseases including cancer, where the involvement of several ion channels and transporters has been studied (Bachmann et al. 2018; Leanza et al. 2018).
3.1 Voltage-Dependent Anion Channels
The voltage-dependent anion channel (VDAC) transports several ions (K+, Na+, and Ca2+), organic anions, ATP, ADP, Pi, and some metabolites depending on the state of the channel across the outer mitochondrial membrane (OMM) (Camara et al. 2017). These channels interact with members of the Bcl-2 family and with hexokinase, regulating apoptosis and with IP3R for the passage of Ca2+ from the endoplasmic reticulum (Mazure 2017; Leanza et al. 2018; Sharma et al. 2019). VDACs are overexpressed in different types of cancers where their expression is related to abnormal proliferation (Shoshan-Barmatz and Ben-Hail 2012). The interaction of hexokinase with VDAC favors cellular glycolysis which is of great relevance for cancer cells; methyl jasmonate (MJ) is an inhibitor of hexokinase-2 that prevents the interaction of hexokinase with VDAC on the mitochondrial membrane and has anticancer effects. The research into new analogs of MJ should help to find new agents against different types of cancer (Sucu et al. 2019). Furthermore, erastin leads to VDAC opening and induces mitochondria dysfunction, increases ROS, inhibits GSH synthesis, decreases glycolysis, and also induces non-apoptotic cell death by ferroptosis in some types of cancers (Yagoda et al. 2007; Dixon et al. 2012; Maldonado et al. 2013).
3.2 Mitochondrial Permeability Transition Pore
Mitochondrial permeability transition pore (mPTP) is a nonspecific channel located on the inner membrane of the mitochondria (IMM). Its prolonged activation depolarizes the mitochondrial membrane and generates ROS, leading to cell death. Thus, drugs that induce mPTP activation in tumor cells have gained great interest (Zoratti and Szabò 1995; Bernardi et al. 2015). Icaritin is an active natural ingredient of the Chinese plant Epimedium that decreases the mitochondrial membrane potential by opening mPTP, leading to necrosis and decreasing proliferation in colorectal cancer (CRC) cells. In accordance, mPTP blockers such as sanglifehrin A, cyclosporin A, and bongkrekic acid, as well as siRNA targeting mPTP decreased the cytotoxic effect of icaritin on CRC cells (Zhou et al. 2016). Similarly, the gold (III)-dithiocarbamate AUL12 contributes to mPTP opening and tumor cell death and shows very low systemic toxicity in vivo (Rasola and Bernardi 2014). Interestingly, various compounds that target the mitochondrial machinery are currently being studied in clinical trials (Suh et al. 2013).
3.3 Mitochondrial Calcium Uniporter
High levels of mitochondrial Ca2+ lead to the activation of the mitochondrial Ca2+ uniporter (MCU) triggering apoptosis (Mammucari et al. 2017). MCU also participates in the proliferation, invasion, and redox signaling in some types of cancers (Vultur et al. 2018). For instance, in triple-negative breast cancer cells, MCU silencing reduces the production of mitochondrial ROS and HIF1-α, impairing cell motility (Tosatto et al. 2016). Actually, MCU overexpression has been linked to lymph node migration, poor prognosis, and breast tumor size (Tang et al. 2015; Yu et al. 2017). Moreover, hepatocellular carcinoma progression and metastasis are associated with overexpression of the MCU-regulator 1 (MCUR1) protein (Jin et al. 2019a), and the anticancer properties of minocycline and doxycycline have been suggested to be related to their inhibitory effect on MCU (Cui et al. 2019). Finally, the thiourea derivative KB-R7943 inhibits MCU reducing Ca2+ release in HeLa cervical cancer cells (Santo-Domingo et al. 2007).
3.4 Uncoupling Protein 2
The uncoupling protein (UCP) is a proton (H+) transporter located on the IMM (Berry et al. 2018). It has been suggested that UCP2 participates in the regulation of cell survival by reducing ROS and mitigating oxidative stress (Cannon et al. 2006; Baffy 2010). UCP2 is upregulated in different tumors, including hepatocellular carcinoma, colorectal, pancreatic, and thyroid cancer (Baffy 2010). UCP2 protects the cells from oxidative stress and prevents the apoptotic effects of different drugs (Derdak et al. 2008). The UCP2 inhibitor genipin reduces cell proliferation, enhances the response to chemotherapy, reverses chemotherapy resistance in some cancer cell lines, and reduces tumor growth in vivo (Mailloux et al. 2010; Dalla Pozza et al. 2012; Pons et al. 2015; Shanmugam et al. 2018). On the contrary, UCP2 expression in melanoma is associated with T-cell tumor infiltration, higher antitumor response, and prolonged survival (Cheng et al. 2019). Also, induced overexpression of UCP2 in melanoma cells generates an immunostimulatory microenvironment by producing chemokines and cytokines, enhancing CD8+ T-cell infiltration in the tumor microenvironment, and suppressing tumor progression. Furthermore, the expression of UCP2 sensitizes melanoma cells against anti-programmed cell death 1 (PD-1) treatment (Cheng et al. 2019). Immune checkpoint-block therapy (like anti-PD-1) is a novel way to fight cancer, and targeting UCP2 expression may convert this immune therapy more efficient for some cancers.
Therefore, targeting different mitochondrial ion channels and transporters should be considered to design novel anticancer therapies (Leanza et al. 2018). Regarding the immune system, ion channels and transporters are becoming an attractive field in onco-immunology.
4 Ion Channels and Transporters in Cancer Immunotherapy
The participation of the immune system is fundamental for the recognition and elimination of tumor cells. Different immune cells infiltrate the tumor microenvironment activating the immune response, for instance, by CD4+T or CD8+T cells. These cells bind directly to the MHC class I molecules presented by the tumor cells inducing the release of cytokines and cytotoxic granules, killing tumor cells (Ostroumov et al. 2018). However, cancer cells evade the immune response by several mechanisms that include defective antigen presentation, repression of T-cell activation, and production of immune-suppressive cytokines (Vinay et al. 2015; Liubomirski et al. 2019). In accordance, several types of immunotherapies are used in clinical practice including immune checkpoint inhibitors, immune system modulators, monoclonal antibodies, vaccines, and CAR T-cell therapy (Khalil et al. 2016).
4.1 Ion Channels and Leucocytes at a Glance
Some ion channels are implicated in the activation, differentiation, proliferation, chemotaxis, and migration of leucocytes (Feske et al. 2015). Lymphocyte function depends on ion channel-mediated Ca2+ signaling induced by antigen recognition. Briefly, activation of lymphocytes by binding of the antigen to the TRC (T cell) or BCR (B cells) receptor activates PLCγ1 in T cells and PLCγ2 in B cells increasing the formation of IP3. Then, the IP3 receptor (IP3R) is activated releasing Ca2+ from the endoplasmic reticulum. The depletion of Ca2+ from the ER activates either STIM1 or STIM2 subunits to oligomerize with IP3R in the ER and interacting with the ORAI channels in the plasma membrane forming functional CRAC channels. These channels allow the entrance of Ca2+ from the extracellular space into the lymphocyte. To maintain the electrical driving force for Ca2+ influx, activation of KCa3.1 channels by Ca2+ and activation of KV1.3 channels by membrane depolarization are required. Following calcium influx, the calcineurin-NFAT pathway is activated increasing the transcription of genes associated with proliferation, cytokine production, and cytotoxicity (Panyi et al. 2014; Feske et al. 2015; Chiang et al. 2017). The blockage of these potassium channels has been proposed as a therapeutic strategy for immunosuppression in a variety of conditions including chronic inflammation, autoimmune diseases, and immunologic-derived cancers (Lam and Wulff 2011). Kv1.3 is also expressed in the IMM where it participates in apoptosis by interacting with Bax and inhibiting channel activity with the subsequent elevation of ROS and release of cytochrome C (Szabó et al. 2008). Two new inhibitors of mitoKv1.3 (PCARBTP and PAPTP) induce ROS production, promote cell death in chemoresistant cells, and reduce tumor growth in melanoma and pancreatic adenocarcinoma in vivo while preserving immune cells and healthy tissues. The authors propose that the selectivity to cancer cells may be partially due to the higher expression of mitoKv1.3 in cancer cells, which hyperpolarizes the IMM and alters the redox status (Leanza et al. 2017).
4.2 Cancer Immunotherapy Targeting Ion Channels
Several approaches targeting ion channels in cells from the immune system have been used. B cells from patients with chronic lymphocytic leukemia (B-CLL) have altered redox state and overexpress Kv1.3 channels in the plasma membrane and mitochondria compared with B cells from healthy subjects. Clofazimine induced cell death by blocking Kv1.3 channels in the mitochondria and activating the intrinsic apoptotic pathway in B-CLL cells. Furthermore, healthy B/T cells or B-CLL treated with the antioxidant enzymes catalase and superoxide dismutase was resistant to apoptosis induced by clofazimine, indicating a synergic action between inhibition of Kv1.3 and ROS production (Leanza et al. 2013). Kv1.3 channels with incomplete inactivation are overexpressed in Daudi B cells. Treatment of Daudi B cells with the antihuman CD20 antibody rituximab (used in patients with non-Hodgkin’s lymphoma) downregulates Kv1.3 channels by activation of the FcγRIIB receptor, contributing to the induction of apoptosis (Wang et al. 2012). Also, in primary malignant T cells isolated from patients with Sézary syndrome, blockage of Kv1.3 inhibited activation and cell proliferation (Hu et al. 2019). Likewise, KCa3.1 is overexpressed in several cancers promoting cell proliferation, metastasis, and therapy resistance (Mohr et al. 2019). Treatment of CLL cells with clotrimazole or TRAM-34 (KCa3.1 channel blockers) decreases Ki67 expression and cell viability (Grössinger et al. 2014). Natural killer (NK) cells also express Kv1.3 and KCa3.1 in the plasma membrane. TRAM-34 increased the proliferation and degranulation levels of adherent NK cells in the presence of the leukemia cell line K562, and mice bearing K562 tumors treated with adherent NK cells and TRAM-34 formed smaller tumors (Koshy et al. 2013). Recently, it was described that radiation to the glioblastoma cell line GL-15 and primary cell cultures from tumors of patients with glioblastoma induced migration, and invasion mediated by KCa3.1channels. The blockage of KCa3.1 channels with TRAM-34 abolished the invasive phenotype of these cells (D’Alessandro et al. 2019). Besides, in the tumor microenvironment, high amounts of adenosine (ADO) are released from tumor cells in hypoxic conditions and regulatory T cells, as well as high amounts of ATP secreted from immune, stromal, apoptotic, and necrotic cells. ATP can be converted to ADO by the ectonucleotidases CD39 and CD75. In solid tumors, the excessive accumulation of ADO generates immunosuppression and failure of effector T cells to eliminate cancer cells, which is associated with tumor growth, metastasis, poor prognosis, and resistance to therapy (Allard et al. 2016). The function of KCa3.1 is inhibited by ADO in human T cells via A2A receptors, reducing T-cell migration and cytokine release (Chimote et al. 2013). In addition, ADO inhibits chemotaxis of CD8+ T cells from head and neck squamous cell carcinoma (HNSCC) patients via its A2A receptor, reducing KCa3.1 channel activity and their ability to infiltrate the solid tumor. Enhancing KCa3.1 channel activity with the agonist 1-EBIO recovers the chemotaxis ability of CD8+ T cells of HNSCC even in the presence of ADO (Chimote et al. 2018).
Adoptive T-cell transfer (ACT) therapy may be also used to target specific ion channels and transporters in cancer. ACT therapy options include tumor-infiltrating lymphocytes (TILs), T-cell receptor (TCR), and chimeric antigen receptor (CAR) therapies (June et al. 2018). KCa1.1 potassium channels (encoded by the KCNMA1 gene) have been associated with glioma, breast, prostate, and cervical cancer and express multiple splice variants (Liu et al. 2002; Bloch et al. 2007; Khaitan et al. 2009; Ge et al. 2012; Ramírez et al. 2018). Alternative splicing leads to the production of multiple mRNAs from a single gene, thus, encoding a diversity of proteins (Liu and Cheng 2013; Wang et al. 2015a). Some pathways deregulated in cancer frequently promote aberrant splicing, which in turn contributes to many aspects of tumor biology, including metabolism, apoptosis, cell cycle control, invasion, metastasis, and angiogenesis (David and Manley 2010; Wang et al. 2015a). Alternative splicing in ion channels modify their pharmacological profile, surface expression, intracellular localization, or electrophysiological properties; actually, in some instances, the splice variants lack conductive properties acting as dominant-negative subunits (Ramos Gomes et al. 2015). The gBK splice variant of KCNMA1 channels is strongly expressed in glioma cell lines and tumor tissues (Liu et al. 2002). This variant has two epitopes for T cells, namely, gBK1 and gBK2, that bind to the human leukocyte antigen HLA-A*0201 on the surface of dendritic cells (DCs). DC cells previously pulsed with gBK1 or gBK2 peptides induce cytotoxic T lymphocyte (CTL) response and cell death in glioma, gastric, lung, and breast cancer cell lines (Ge et al. 2012). Similar results were obtained with small cell lung cancer (SCLC) cell lines, where gBK-specific CTL-killing inhibits growth and stimulates IFN-γ, proposing gBK as a target for immunotherapy and vaccination in some types of cancer (Hoa et al. 2014). Since tumor cells expressed higher levels of gBK than noncancerous cells, targeting this splice variant may be a more selective therapy.
Another immunotherapy alternative is using antibodies against specific ion channels or transporters involved in cancer. The development of specific antibodies for cancer therapy has been studied for Kv10.1, Kv11.1, nfP2X7, α2δ1 subunit (isoform 5 of voltage-gated Ca2+ channels), and MRP1 (Binyamin et al. 2004; Sette et al. 2013; Zhao et al. 2013; Hartung and Pardo 2016; Gilbert et al. 2017) among other proteins. Kv10.1 is abnormally expressed in approximately 70% of all types of cancers (Hemmerlein et al. 2006). The scFv62-TRAIL antibody targeting the pore of Kv10.1 (scFc62) and linked to the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) sensitized MDA-MB435S breast cancer cells to antineoplastic drugs commonly used in the clinic including paclitaxel and doxorubicin. The combination of the antibody with doxorubicin showed also significant inhibitory effects in vivo experiments, and in prostate cancer cells, the antibody induced apoptosis only in those expressing Kv10.1 channels (Hartung et al. 2011; Hartung and Pardo 2016). Thus, this antibody-based approach provides a very selective anticancer therapy. A monoclonal antibody against Kv11.1 conjugated with TiO2 nanoparticles (Kv11.1-Mab-PEG-TiO2 NPs) was designed and tested in the pancreatic ductal adenocarcinoma cell lines MIAPaCa-2 and Panc-1. Although that treatment with the Kv11.1-Mab-PEG-TiO2 NPs did not change cell viability, other options can be considered to generate cytotoxicity like testing the photocatalytic properties of TiO2 which induce ROS production (Sette et al. 2013), or other chemotherapeutic agents linked to Kv11.1 channels. The ion channel and transporter splice variants can also be used for cancer immunotherapy, and P2X7 is an ATP-gated Ca2+ channel overexpressed in various cancers, promoting cell proliferation and invasiveness; a phase I clinical trial studying the topical administration of an antibody against the non-pore functional P2X7 (nfP2X7) variant reported reduced lesions in basal cell carcinoma, a very common skin cancer (Gilbert et al. 2017). In this way, the development of specific antibodies against malignant splice variants is emerging as a possible therapeutic approach to treat cancer. Despite that further investigation in cancer patients is needed; ion channels and transporters represent a promising alternative in cancer immunotherapy.
5 Splice Variants and Noncanonical Functions of Ion Channels in Cancer Therapy
5.1 Splice Variants
Targeting channel isoforms that are tumor-specific can provide more selectivity for drug development. In this direction, the pyrimido-indole compound CD-160130 is more effective in blocking the Kv11.1 isoform B (IC50 = 1.8 ± 0.26 mM) compared to Kv11.1 isoform A (IC50 = 13.4 ± 3.0 mM). Interestingly, leukemia cells mainly express Kv11.1 isoform B. Accordingly, CD-160130 induced apoptosis in vitro and prolonged survival in an acute myeloid leukemia mouse model at a dose of 10 mg/kg; it is worth mentioning that CD-160130 did not induce significant QT prolongation in mice and guinea pigs (Gasparoli et al. 2015).
Overexpression of different G proteins activated inwardly rectifying K+ channel 1 (GIRK1) splice variants exerts opposite actions in breast cancer cells. While GIRK1a and GIRK1c overexpression reinforces parameters associated with malignancy; overexpression of GIRK1d has the contrary effect. A segment comprising aminoacids 235–402 present in GIRK1a and GIRK1c but not in GIRK1d seems to be the responsible component for the carcinogenic effect of these channels (Rezania et al. 2016). Overexpression of GIRK1 in the primary tumor is associated with lymph node metastasis and poor prognosis (Stringer et al. 2001). In addition, in breast cancer cells, the overexpression of GIRK1 affects wound healing, invasion, cellular velocities/motilities, and angiogenesis suggesting a pathophysiological role in breast cancer (Wagner et al. 2010; Rezania et al. 2016). Alternative transcripts have been also identified for Kv10.1 channels, namely, Kv10.1a and Kv10.1b. Two shorter splice variants, E65 and E70, isolated from the human brain and cancer cell lines lack the transmembrane segments. These variants produce cytoplasmic proteins without conducting properties but reduce the current of the full-length channels when co-expressed. E65 triggers the activation of cyclin-dependent kinases in Xenopus laevis oocytes, suggesting a role in cell cycle control (Gomes et al. 2015; Ouadid-Ahidouch et al. 2016). TRPC channel splice variants play an important role in human ovarian cancer development. The nonselective TRPC channel blockers 2APB and SKF-96365 significantly inhibited the cell proliferation, while the increase of TRPC channel activity promoted the cell proliferation (Zeng et al. 2013). Some voltage-gated sodium (Nav) channels are expressed in the colon, small intestine, stomach, prostate, bladder, and breast, but the higher expression is found in the brain, as well as in skeletal and cardiac muscle. Interestingly, the neonatal splice variant of the Nav α-subunit subtype Nav1.5 (nNav1.5) displays a restricted expression pattern among tissues but is upregulated in human breast cancer. The high-level expression of this splice variant is associated with the estrogen receptor (ER) status. Thus, the nNav1.5 splice variant may be exploited both as a novel biomarker and a potential specific target for some common types of breast cancer (Yamaci et al. 2017).
5.2 Noncanonical Functions
Several splice variants may form non-conducting ion channels strongly suggesting that noncanonical functions of ion channels are also involved in carcinogenesis. For instance, Downie and colleagues developed a mutant Kv10.1 channel eliminating ion permeation and studied its oncogenic potential. This mutant fails to completely abolish xenograft tumor formation by transfected cells, strongly suggesting that the oncogenic mechanism of Kv10.1 comprises other molecular mechanisms independently of its primary function as an ion channel (Downie et al. 2008). Noncanonical functions of Cav channels are also associated with cancer. Proteolytic cleavage of the C-terminus of L-type Ca2+ channels α1C and α1D subunits (Cav1.2 and Cav1.3, respectively) produces a fragment that is translocated to the nucleus regulating the transcription of genes involved in tumor progression (Buchanan and McCloskey 2016). C-terminus cleavage of Cav1.2 channel generates the transcription factor Ca2+ channel-associated transcriptional regulator (CCAT), which regulates the expression of connexin CX31.1 and NR3, but also provides negative feedback regulating Cav1.2 channel expression (Gomez-Ospina et al. 2013). Alternatively, CCAT may result from the alternative splicing of the Cav1.2 gene (Barbado et al. 2009). The overexpression of this fragment affects also the expression of other ion channels including TRPV4 and KCa2.3, potentially leading to a cancer phenotype (Buchanan and McCloskey 2016). Electromagnetic field therapy, like tumor treating fields (TTFields), delivers a low-intensity, intermediate frequency, alternating electric field through noninvasive transducer arrays to tumor regions. This FDA-approved treatment for glioblastoma multiforme therapy disrupts mitosis and cytokinesis, stimulates calcium entry mediated by Cav1.2, and arrests the cells in the S and G1 phase of the cell cycle in glioblastoma cell lines (Neuhaus et al. 2019). Thus, the participation of ion channel splice variants in cancer and the diverse molecular mechanisms associating channels with cancer including noncanonical functions offer additional drug design and therapeutic opportunities to fight cancer. One of these opportunities arises by using current drugs prescribed for conditions different from cancer but affecting ion channels and transporters involved in cancer.
6 Repurposing Existing Drugs Targeting Ion Channels and Transporters for Cancer Therapy
Drug repurposing is an attractive strategy to reduce the cost and developing times of new antineoplastic agents. The safety, pharmacokinetics, and pharmacodynamic profile of currently used drugs are well-known; thus, these drugs may be quickly translated into phase II and III clinical studies (Oprea et al. 2011; Gupta et al. 2013; Pantziarka et al. 2014). In silico chemical genomic approaches have been used to predict drug repositioning candidates for cancer therapy based on large-scale drug-induced transcriptional signatures (Lee et al. 2016). Because several drugs used for different indications target ion channels and transporters involved in cancer, repurposing of these drugs is a very attractive and low-cost alternative to fight cancer.
6.1 Antihistamines
Histamine is involved in cell proliferation and tumor growth; thus, several antihistamines have been strongly suggested for repurposing as antineoplastic agents (Faustino-Rocha et al. 2017). Astemizole is a long-acting, non-sedating second-generation antihistamine indicated in the treatment of allergies. This drug is an antagonist of H1-histamine receptors which are present in the gastrointestinal tract, uterus, blood vessels, and bronchial muscle, among other tissues (Garcia-Quiroz and Camacho 2011). Astemizole also targets several molecules involved in cancer development including ABC transporters (P-glycoprotein) and the potassium channels Kv10.1 and Kv11.1 (Pardo et al. 1999; Ishikawa et al. 2000; Garcia-Ferreiro et al. 2004; Camacho 2006; Garcia-Quiroz and Camacho 2011). This antihistamine has antiproliferative effects in cancer cell lines from breast such as MCF-7, SUM-229PE, T-47D, and BT-474, as well as in invasive ductal breast cancer primary cultures (Ouadid-Ahidouch et al. 2001; Roy et al. 2008; Garcia-Quiroz et al. 2012, 2019). It also inhibits proliferation and increases apoptosis in several cell lines from cervical, liver, prostate, and lung cancer (Chavez-Lopez et al. 2014, 2015, 2017; Bernal-Ramos et al. 2017;), as well as in cells from leukemia (Ishikawa et al. 2000) and in keratinocytes transfected with a human papillomavirus oncogene (Diaz et al. 2009). Moreover, astemizole inhibits the Kv10.1 mRNA expression both in vitro and in vivo in breast cancer and hepatocellular carcinoma, decreasing tumor development (Garcia-Quiroz et al. 2012, 2014; Chavez-Lopez et al. 2015). The antitumor activity of astemizole has been observed in several studies in animal tumor models. The oral administration of astemizole (50 mg/kg/day) reduced the growth rate of xenografts tumors induced by implantation of Kv10.1-transfected cells or MDA-MB435S breast cancer cells (Downie et al. 2008). In a rat model, astemizole was capable to prevent hepatocellular carcinoma (HCC) development induced by the carcinogen diethylnitrosamine (Chavez-Lopez et al. 2015). The daily administration of astemizole (50 mg/kg) in drinking water inhibited tumor growth in an in vivo preclinical model using athymic mice xenografted with two different human breast cancer cell lines: T-47D and a ductal infiltrating carcinoma breast cancer-derived primary cell culture (MBCDF) (Garcia-Quiroz et al. 2014). The dose of 50 mg/kg of astemizole was sufficient to inhibit tumor growth in mice without producing noticeable adverse effects (loss of body weight, diarrhea, or alterations in physical activity) (Downie et al. 2008; Garcia-Quiroz et al. 2014). In contrast, 30 mg/kg of astemizole induced ventricular contractions in dogs and torsade de pointes in one animal (Izumi-Nakaseko et al. 2016). Astemizole was withdrawn from the US market in 1999 due to its pro-arrhythmic potential; it soon became evident that most cases of toxicity involved either overdosing, drug interaction, or subjects with predisposed cardiac disease (Paakkari 2002). At the defined daily dose of prescribed astemizole (10 mg/day), the spontaneous cardiac adverse drug reaction reported in a lapse of 10 years were 110 cases per million of doses sold (Garcia-Quiroz and Camacho 2011). These side effects are mainly attributed to the blockade of the Kv11.1 cardiac potassium channels (IC50 of 48.4 ± 3.8 nM) (Suessbrich et al. 1996; Zhou et al. 1999). It is important to mention that not all Kv11.1 channel blockers produce torsade de points, for instance, verapamil and sertindole (D’Amico et al. 2013; Gentile et al. 2016), but several anticancer drugs have a pharmacological effect on Kv11.1 (Gentile et al. 2016). Therefore, the use of Kv11.1 blockers that do not induce cardiac side effect has been suggested for cancer treatment. One of the alternative proposed approaches is using drugs that bind to a specific state of the channel, like R-roscovitine that interacts with the channel in its open state, which is longer in tumors than in cardiac cells (D’Amico et al. 2013).
A very interesting property of astemizole is that its concomitant use with other antineoplastic agents has synergistic effects. Astemizole potentiates the growth-inhibitory activity of doxorubicin in doxorubicin-resistant human leukemia cells K562/DXR by inhibiting the P-glycoprotein (Ishikawa et al. 2000). The antihistamine also synergizes the calcitriol antiproliferative effects by downregulating CYP24A1 (which inactivates calcitriol), upregulating the vitamin D receptor (VDR), and targeting Kv10.1 (Garcia-Quiroz et al. 2012). The co-administration of astemizole and calcitriol to mice xenografted with human breast cancer cells inhibited tumor growth more efficiently than each drug alone (Garcia-Quiroz et al. 2014). Likewise, in a HCC model, astemizole increased VDR expression both in vitro and in vivo, enhanced vitamin D-induced decrease in cell viability and proliferation, increased apoptosis, decreased cell migration and invasion in vitro, as well as reduced the amount and mass of tumors (Xu et al. 2018b). Furthermore, in lung cancer cells, astemizole potentiated the inhibitory effect of vinorelbine on the colony formation of NCI-H1299 and cisplatin on the colony formation of NCI-H661 cells (Ellegaard et al. 2016). The combined effect of astemizole with the epidermal growth factor receptor type 1 (EGFR) inhibitor gefitinib further repressed the proliferation, survival, and Kv10.1 expression and increased the apoptosis more than the monotherapy in the lung cancer cell lines A549 and NCI-H1974 (Chavez-Lopez et al. 2017). In the same manner, astemizole and gefitinib synergistically inhibited the proliferation of breast cancer cells expressing the targets Kv10.1 and EGFR (Garcia-Quiroz et al. 2019). In addition, astemizole acts synergistically with radiation to increase the death of prostate cancer cells through a mechanism involving autophagy (Oprea et al. 2011).
Terfenadine, a second-generation H1 receptor antagonist targets other molecules involved in cancer such as Kv11.1 (Suessbrich et al. 1996). This antihistamine induces apoptosis and inhibits tumor growth in murine models (Blaya et al. 2010). In human refractory prostate cancer cells, terfenadine upregulates and activates Bak and the cleavage of Mcl-1, leading to the loss of mitochondrial membrane potential and activation of caspase cascade resulting in DNA damage response and apoptosis (Wang et al. 2014b). Breast cancer cells resistant to HER-2/neu targeted therapy express high levels of H1 receptors and are more sensitive to terfenadine. This drug leads to Sub-G0 cell accumulation, suppresses proliferation, promotes cell motility, and triggers the activation of extracellular signal-regulated kinase (ERK), initiating the mitochondrial apoptotic pathway in basal breast cancer. Moreover, in vivo experiments showed that terfenadine (10 mg/kg) therapy reduced the tumor growth of basal and trastuzumab-resistant breast cancer cells (Fernández-Nogueira et al. 2018). The combined treatment of terfenadine with epirubicin synergistically inhibits the growth and metastatic process of chemotherapy-resistant non-small cell lung cancer (NSCLC) cells both in vitro and in vivo (An et al. 2017), and ketoconazole potentiates terfenadine-induced apoptosis in human HepG2 cells through inhibition of p450 3A4 activity (Wang et al. 2002). Terfenadine was withdrawn from the market due to the induction of prolonged QT interval in cases of overdose, inappropriate co-medications or in subjects with predisposed cardiac disease. The FDA recommended terfenadine to be replaced by its active and nontoxic metabolite fexofenadine (Berul and Morad 1995; Paakkari 2002). Another antihistamine with important antineoplastic effects is loratadine, which is associated with significantly reduced all-cause mortality among patients with non-localized non-small cell lung cancer (NSCLC) or any non-localized cancer. Astemizole showed a similar significant association with reduced mortality in patients with non-localized cancer, and ebastine shows a similar tendency. Interestingly, submicromolar concentrations of these antihistamines sensitized NSCLC cells to chemotherapy and reverted multidrug resistance in NSCLC, breast, and prostate cancer cells (Ellegaard et al. 2016). Similar results with antihistamines were observed in ovarian cancer patients (Verdoodt et al. 2019).
6.2 Imipramine
Imipramine is a tricyclic antidepressant indicated for symptom relief of depression and other conditions including panic and obsessive-compulsive disorders, bulimia, and nocturnal enuresis; it acts by blocking the sodium-dependent serotonin and norepinephrine transporters reducing reuptake and increasing their concentration in the synaptic cleft (Gillman 2007). In addition, imipramine inhibits the current through Kv10.1 channels in a voltage-dependent manner and reduces the proliferation of cancer cells (Gavrilova-Ruch et al. 2002; Garcia-Ferreiro et al. 2004; Gomez-Varela et al. 2006). This drug also promotes apoptosis in the ovarian cancer cells SK-OV-3 (Asher et al. 2011). In brain cancer patients, the effect of imipramine is associated with the channel abundance; thus, the antidepressant improves the survival rate better in patients with moderate Kv10.1 expression (Martinez et al. 2015). These findings suggest that personalized therapy with this tricyclic antidepressant based on the expression of Kv10.1 channels may be used for brain malignancies. Besides, in 2013, Jahchan and colleagues used bioinformatic tools to identify potential candidate drugs for the treatment of small cell lung cancer from FDA-approved drugs and identified imipramine as a potential candidate. Imipramine at 20 μM decreased survival in H82, H69, and H187 human small cell lung cancer (SCLC) cells and Kp1, Kp2, and Kp3 mouse SCLC cells. In vivo, imipramine (25 mg/kg) inhibited the growth of SCLC allografts (mouse SCLC cell line Kp1), xenografts (human SCLC cell line H187), and one primary patient-derived xenograft (human SCLC tumor NJH29). This drug was effective also in cisplatin-resistant SCLC cells, suggesting that imipramine may be used as second-line therapy for SCLC patients who become refractory to cisplatin/etoposide (Jahchan et al. 2013; Kale et al. 2015). Imipramine also has cardiovascular side effects including orthostatic hypotension, atrioventricular conduction delay, reduced heart rate variability in response to exercise, tachycardia, syncope, and arrhythmias particularly observed in patients with concurrent cardiovascular disease or at high doses of treatment. This may be explained because imipramine blocks several neuronal and cardiac K+, Na+, and Ca2+ channels whit IC50 values ranging from 1 to 30 μM; its IC50 in cloned Kv11.1 channels is 3.4 ± 0.4 μM, and the complete blockage is achieved with 30 μM (Teschemacher et al. 1999; Garcia-Ferreiro et al. 2004).
To increase the antineoplastic effects, imipramine has been co-administered with other compounds. The combination of imipramine with doxorubicin enhanced the anti-invasive effect, whereas a combination with ticlopidine suppressed ATG7, a member of the autophagy survival signaling, resulting in cell death (Abdelaleem et al. 2019). The combined treatment of imipramine and radiotherapy in prostate cancer did not enhance the radiosensitivity of DU145 cells; unexpectedly, the treatment of imipramine alone was more effective (Barlaz Us et al. 2019). Several studies have evaluated the effect of imipramine blue, which is an organic triphenylmethane dye synthesized from imipramine and 4,4′-diethylaminobenzophenone. This compound was suggested because gentian violet (another triphenylmethane dye) also exhibits anticancer properties. This imipramine analog inhibits the invasion of glioma cells both in vitro and in vivo and enhances the efficacy of doxorubicin (Munson et al. 2012). In addition, imipramine blue inhibits breast cancer growth, progression, and metastasis (Rajamanickam et al. 2016); moreover, it has antineoplastic effects on head and neck cancer (Yang et al. 2016), Burkitt lymphoma (Klingenberg et al. 2014), as well as on acute (Metts et al. 2017) and chronic myeloid leukemia (Laidlaw et al. 2016).
6.3 Calcitriol
The endogenous synthesis of calcitriol begins in the skin by the action of ultraviolet radiation from sunlight but takes place mainly in the kidney and has been reported in other tissues such as skin, prostate, intestine, pancreatic islets, lymph nodes, brain, colon, and the mammary gland, where local calcitriol synthesis takes place (Deeb et al. 2007; Glowka et al. 2019). The coupling of calcitriol with the VDR allows dimerization with the retinoid receptor X (RXR); this heterodimer translocates to the nucleus and binds to VDR response elements (VDREs) in the promoter of target genes inducing gene expression.
Calcitriol acting via VDR promotes cytodifferentiation and apoptosis, modulates oncogene expression, and inhibits cell proliferation and migration, reducing or preventing cancer progression. Another potential antiproliferative mechanism of this secosteroid is its ability to downregulate Kv10.1 expression in cell lines (SUM-229PE and MCF-7) and primary cultures from breast cancer (Garcia-Becerra et al. 2010; Garcia-Quiroz et al. 2012), as well as in cervical (SiHa, HeLa) and prostate (PC-3) cancer cells and in syncytiotrophoblasts from normal human placenta (Avila et al. 2010). Kv10.1 repression by calcitriol in cervical cancer cells occurs at the transcriptional level and involves a functional nVDRE (negative-VDRE) in the Kv10.1 promoter (Cazares-Ordonez et al. 2015). Calcitriol also decreases Kv10.1 expression and tumor growth in vivo of the xenografted breast cancer cell lines T-47D and HCC-1806 and the MBCDF breast cancer primary culture (Garcia-Quiroz et al. 2014, 2016). The antineoplastic effect of calcitriol has also been observed in melanoma, pancreatic, prostate, and colorectal cancer, as well as in hepatocellular carcinoma. In fact, a large number of epidemiological studies have demonstrated an association between low circulating levels of the calcitriol precursor calcidiol, with higher risk to develop colorectal and breast cancer and hepatocellular carcinoma (Diaz et al. 2015).
In addition, the antineoplastic effects of calcitriol are potentiated in breast cancer in vitro and in vivo, by combining it with other antineoplastic agents including the natural compounds curcumin and resveratrol (García-Quiroz et al. 2019). Besides, the combination of calcitriol with the receptor tyrosine kinase inhibitors gefitinib, lapatinib, and neratinib is more effective to inhibit the growth of breast cancer cell lines in comparison with each compound alone (Segovia-Mendoza et al. 2015, 2017). Furthermore, the combinations of calcitriol or its analogs with chemotherapeutic agents such as antimetabolites, platinum compounds, or taxanes improve the antineoplastic effects in different types of cancer (Abu El Maaty and Wölfl 2017). Thus, calcitriol is an endogenous natural anticancer factor targeting ion channels and promising antineoplastic agent.
6.4 Clarithromycin
Clarithromycin is a macrolide antibiotic drug having a broad spectrum of antimicrobial activity for gram-positive and gram-negative organisms, atypical pathogens, and some anaerobes (Peters and Clissold 1992). Interestingly, in colorectal cancer, this macrolide modulates the PI3K/Akt pathway by targeting Kv11.1, modulating autophagic flux, and triggering apoptosis. This drug preferentially binds to Kv11.1 channels in their closed state and inhibits the formation of a macromolecular complex between the channel and the p85 subunit of PI3K, impairing this signaling pathway (Petroni et al. 2020). Additionally, clarithromycin targets the P-glycoprotein, which is overexpressed in different kinds of tumors and confers resistance to chemotherapy (Vermeer et al. 2016). This drug also enhances the cytotoxic effect of 5-fluorouracil both in vitro and in vivo (Petroni et al. 2020).
6.5 Fluoxetine
Fluoxetine is a selective serotonin reuptake inhibitor, initially intended for the treatment of depression; however, nowadays it is also prescribed to treat other conditions like obsessive-compulsive disorders (Wong et al. 1995). Interestingly, fluoxetine is also a non-torsadogenic Kv11.1 inhibitor successfully used in glioblastoma therapy without obvious cardiotoxicity and the added benefit of treating depression (Pointer et al. 2017). Kv11.1 channel blockers reduced glioblastoma cell proliferation and improved survival in patients who received one or more Kv11.1 blockers but only if their tumors exhibited high Kv11.1 expression levels (Pointer et al. 2017), which represents another example of the potential use of channel expression levels for personalized therapy.
6.6 Glibenclamide
Glibenclamide is a second-generation sulphonylurea, used for the treatment of non-insulin-dependent diabetes mellitus; this drug binds to the sulphonylurea receptor (SUR1) expressed in pancreatic B cells and blocks KATP channels, leading to insulin release (Payen et al. 2001). KATP channels are composed of at least two types of subunits, an inwardly rectifying K+ channel (Kir6.x) and a regulatory subunit SUR. SUR1 belongs to the ATP-binding cassette (ABC) protein superfamily. Glibenclamide inhibits the activity of various ABC transporters and multidrug resistance proteins (MRPs). In the human lung cancer cells GLC4/Sb30 that overexpress MRP1 and are resistant to the anticancer drugs doxorubicin and vincristine, glibenclamide (0.39–100 μM) inhibited MRP1 activity in a dose-dependent manner reverting drug resistance (Payen et al. 2001). This drug (0.5–200 μM) also decreased cell viability and induced apoptosis in the gastric cancer cell line MGC-803 by activating mitochondrial death pathways related to ROS generation, activation of JNK, and inhibition of Akt (Qian et al. 2008). Whereas in the breast cancer cell line MDA-MB-231, the sulphonylurea (10–50 μM) inhibited cell growth and induced G0/G1 arrest (Núñez et al. 2013). Glibenclamide (150 μM) also decreased the proliferation of several cervical cancer cell lines; the higher the expression of Kir6.2 subunit in the cervical cancer cells, the higher the inhibitory effect of the drug. The overexpression of the Kir6.2 subunit was also observed in cervical tumor tissues; therefore, glibenclamide is a potential therapy for this type of cancer (Vazquez-Sanchez et al. 2018). Interestingly, the combined treatment of glibenclamide with CoCl2 decreased the expression of metalloproteinase-9 (MMP-9) and inhibited the growth in highly metastatic breast cancer cells (Rong et al. 2013). The antitumor effect of glibenclamide has been also observed in preclinical studies in melanoma (Suzuki et al. 2012), bladder carcinoma (Wondergem et al. 1998), prostate (Abdul and Hoosein 2002), and liver cancer (Malhi et al. 2000). The antineoplastic effects of glibenclamide may be explained by its ability to block KATP channels, ABC transporters, and MRPs and decrease the expression of MMP-9 (Payen et al. 2001; Rong et al. 2013).
6.7 Verapamil
Verapamil is an L-type Ca2+ channel blocker classified as a class IV antiarrhythmic agent that also blocks Kv11.1 currents (Zhang et al. 1999). This drug also exhibits anticancer effects attributed to its combined inhibitory activity against potassium and Ca2+ channels (Kale et al. 2015). Verapamil has antiproliferative effects on the breast cancer cells HT-39 both in vitro (IC50 = 10 μM) and in vivo (3.5 mg/day) (Taylor and Simpson 1992), as well as in prostate cancer (Rybalchenko et al. 2001), melanoma (Huber et al. 1989), and neuroblastoma (Schmidt et al. 1988) and in a nude mouse model of meningiomas (Jensen and Wurster 2001). Interestingly, verapamil overcomes the vincristine resistance both in vitro and in vivo in P388 leukemia cells (Yusa and Tsuruo 1989), doxorubicin-resistant myeloma (Durie and Dalton 1988), and vinblastine-resistant pediatric tumors (Cairo et al. 1989). In a prospective study in 99 patients with anthracycline-resistant metastatic breast carcinoma, verapamil given in conjunction with chemotherapy increased survival (Belpomme et al. 2000). In a randomized trial of 72 patients with advanced non-small cell lung cancer (NSCLC), verapamil plus chemotherapy (vindesine/ifosfamide) improved patient outcome (Millward et al. 1993). The reversal mechanism of MDR by verapamil is because the antiarrhythmic drug interacts with specific binding sites on the P-glycoprotein (Yusa and Tsuruo 1989); however, the clinical use of this agent has been hampered because of the unacceptable toxicity and side effects at the doses required to modulate the P-glycoprotein (Arora et al. 2005). Thus, the synthesis of new analogs of verapamil deserves further investigation.
6.8 Nifedipine and Mibefradil
Nifedipine is a potent L-type Ca2+ channel blocker indicated as an antihypertensive drug from several years ago and has an acceptable safety profile. In vitro studies showed that nifedipine reduces the mitogenic effect of endothelin-1 by blocking Ca2+ channels in lung cancer cells (Kale et al. 2015). In endometrial carcinoma cells, nifedipine induced autophagy through Beclin1 and the mTor pathway (Bao et al. 2012). In addition, the Ca2+ channel blockers nifedipine, mibefradil, and tetrandrine modulated the androgen receptor-mediated gene expression and induced cytotoxicity in LNCaP, LAPAC-4, and C4-2 androgen receptor-positive prostate cancer cells (Loughlin 2014). The antitumor effect of cisplatin was enhanced by nifedipine in cisplatin-sensitive human glioblastoma U-87MG cells and cisplatin-resistant U87-MG-CR cells both in vitro and in vivo (Kondo et al. 1995), as well as in lung carcinoma cells (Onoda et al. 1988). However, the potential use of nifedipine as antineoplastic is controversial because it cannot be used in hypotensive cancer patients. Nevertheless, the alternate dosing systems like the continuous release system developed by Bayer may help to control the blood Ca2+ levels and avoid rapid hypotension (Kale et al. 2015). Mibefradil, a T-type channel blocker was approved as an antihypertensive drug by the FDA in 1997 but voluntarily withdrawn from the market by Roche Laboratories in 1998 after reports of dangerous and even fatal interactions with at least other 25 drugs, including antibiotics, antihistamines, and anticancer drugs (SoRelle 1998). This drug has important antineoplastic effects in glioblastoma (Keir et al. 2013), breast cancer, and retinoblastoma (Bertolesi et al. 2002). Holdhoff and colleagues designed a phase I study to determine the safety and the maximum tolerated dose of mibefradil when given sequentially with temozolomide in recurrent high-grade gliomas. The study enrolled 27 patients; mibefradil followed by temozolomide was well tolerated; and the lack of toxicity and response in some patients warrants further investigation (Holdhoff et al. 2017). Besides, mibefradil regulates the gating of Kv10.1 channels inducing an apparent inactivation, probably by binding to the voltage sensor domain (Gómez-Lagunas et al. 2017), which adds a new potential mechanism of the anticancer effects of this drug.
6.9 Celecoxib
Celecoxib has been used as an anti-inflammatory, analgesic, and antipyretic drug, but it also has antineoplastic properties. The mechanism of action of celecoxib as an antineoplastic agent has been not sufficiently investigated (Toloczko-Iwaniuk et al. 2019). This drug decreases the proliferation of rat pheochromocytoma PC12 cells in a dose-dependent manner by blocking Cav-mediated currents (Zhang et al. 2007). The clinical efficacy and safety of celecoxib have been evaluated in combination with chemotherapy in metastatic or postoperative recurrent gastric patients, which offers more clinical benefits (Guo et al. 2019). A case report described that HCC practically disappeared in a patient after 8 months of treatment with celecoxib and pentoxifylline (Jimenez-Luevano et al. 2018). The combination of celecoxib with antineoplastic agents as capecitabine could be a good option for patients with thymic carcinoma (Wood et al. 2018). In addition, the combination of the anti-inflammatory drug with erlotinib may be efficacious for patients with advanced non-small cell lung carcinoma and wild-type EGFR (Jin et al. 2019b). Preclinical and clinical studies have demonstrated promising results of the role of celecoxib in the treatment and prevention of some cancers such as colon, breast, prostate, and head and neck (Toloczko-Iwaniuk et al. 2019). Whether calcium channels are involved in all these effects remains elusive.
6.10 Bromocriptine
Bromocriptine is an ergot and dopamine D2 receptor agonist used to treat Parkinson’s disease, acromegaly, hyperprolactinemia, galactorrhea, and diabetes mellitus. The drug is active also against prolactinomas and growth hormone-producing adenomas. This drug reduces tumor mass in 80–90% of patients with microadenomas and in 70% of patients with macroadenomas (Seo et al. 2018). Prolactin constitutes a growth factor for breast cancer cells, is associated with poor prognosis, and reduced efficacy of antitumor therapies in metastatic breast carcinoma. A clinical study evaluated the effect of taxotere versus taxotere plus bromocriptine in metastatic breast cancer patients pretreated with anthracyclines. The results suggested that the inhibition of prolactin secretion by antiprolactinemic drugs such as bromocriptine might enhance the efficacy of chemotherapy for metastatic breast cancer (Lissoni et al. 2002). More recently, bromocriptine (0.001–100 μM) was proved to inhibit drug-resistant tumor cells in a hormone-independent manner. The combination of bromocriptine with either doxorubicin or paclitaxel resulted in a synergic effect in the MDR P-glycoprotein overexpressing CEM/ADR5000 leukemic cells (Seo et al. 2018).
Thus, several approved drugs originally prescribed for other indications may be repurposed for cancer therapy because of their antineoplastic properties acting on ion channels or transporters. This approach should accelerate the development of clinical trials, especially for poor prognosis cancers. Toxins targeting ion channels and transporters represent an additional alternative to fight cancer.
7 Therapeutic Potential of Animal Venoms Against Channels and Transporters in Cancer
Over ten million of active peptides and proteins are estimated to be present in animal venoms; in many cases small amounts of the venom are sufficient to kill either preys or predators and microbial invaders (Wulff et al. 2019). Several venom components (salts, nucleotides, biogenic amines, enzymes such as phospholipase, hyaluronidase, L-amino acid oxidase, metalloproteinase, serine protease, mucoproteins, peptides, and proteins) possess antineoplastic effects via regulating the expression or activity of ion channels (Ding et al. 2014; Chen et al. 2018; Wulff et al. 2019).
7.1 Scorpion and Spider Venom Peptides as Antineoplastic Agents
Chlorotoxin is one of the most abundant peptides from the Leiurus quinquestriatus hebraeus deathstalker scorpion venom and exhibits great specificity for gliomas and tumors of neuroectodermal origin blocking glioma-specific chloride ion channels with high affinity and MMP-2, decreasing cells invasion (Lyons et al. 2002; McFerrin and Sontheimer 2006). Chloride channels are either absent or in low abundance in healthy tissues and in tumors of non-glial origin; however, their expression increases as gliomas progress and are crucial in tumor cell invasion and migration; chlorotoxin potently blocks these channels (Dardevet et al. 2015). Another target of chlorotoxin on the surface of glioma cells is MMP-2, which is upregulated in gliomas and related cancers but is not expressed in the normal brain. Chlorotoxin binds to MMP-2, inhibits its catalytic activity in a dose-dependent manner, and reduces its surface expression by inducing its internalization (Deshane et al. 2003). This toxin also targets other cancer cells including those from melanoma, small cell lung carcinoma, neuroblastoma, medulloblastoma, Ewing’s sarcoma, and pheochromocytoma (Dardevet et al. 2015). A chlorotoxin:CY5.5 bioconjugate that emits near-IR fluorescent signal was developed as a contrast agent with the potential to improve intraoperative detection and resection of malignancies. This bioconjugate demonstrated preferential accumulation in a wide variety of tumors, including prostate and intestinal cancer, and sarcoma (Veiseh et al. 2007). In addition, a chlorotoxin-modified doxorubicin-loaded liposome delivery system for targeting gliomas was developed to improve chemotherapeutic efficacy. The liposomes enhanced the cellular uptake by the murine (C6) and human glioma (U87MG and 251MG) cell lines and mice brain microvascular endothelial cells (BMECs), which increased drug cytotoxicity. The encapsulated doxorubicin enhanced the targeting efficiency to subcutaneous and intracranial gliomas improving the antitumor efficacy and lowering blood toxicity (Xiang et al. 2011). Furthermore, a platinum (IV) complex was conjugated to chlorotoxin in order to deliver cisplatin to cancer cells (Graf et al. 2012). In vivo assays showed that the iodine 125- and 131-labeled chlorotoxin specifically bind to brain tumor cells, making this peptide a promising candidate in radiotherapy of the postsurgical brain tumors (Srairi-Abid et al. 2019). Several chlorotoxin-based clinical trials for cancer therapy and diagnoses have been developed (Xiang et al. 2011; Pennington et al. 2018). The synthetic version of chlorotoxin reached phase III clinical trials under the name of TM-601 (Srairi-Abid et al. 2019). The FDA approved the iodine-131 radioconjugate of synthetic chlorotoxin (131I-TM-601) for glioma therapy and diagnostics (Dardevet et al. 2015).
KAaH1 is a peptide from Androctonus australis scorpion blocking Kv1.1 and Kv1.3 channels with an IC50 of 5 and 50 nM, respectively (Srairi-Abid et al. 2005). Both channels are expressed in U87 (glioblastoma), MDA-MB-231 (breast cancer), and LS174 (colon adenocarcinoma) cell lines. KAaH1 inhibited the migration of the three cell lines. KAaH2 is slightly active only on Kv1.1 channels and inhibits U87 cell proliferation probably via either the EGFR signaling pathway or other K+ channels (Aissaoui et al. 2018). Kv10.1 and Kv11.1 channels could be potential candidates for the KAaH2 effect since EGFR forms a multimeric complex with the Kv11.1 channel (Aissaoui et al. 2018), and EGFR regulates Kv10.1 currents (Wu et al. 2012). Margatoxin is a toxin from Centruroides margaritatus scorpion venom that inhibits human lung adenocarcinoma A549 cell proliferation by selective inhibition of Kv1.3 channels. This toxin increases the expression of P21Waf1/Cip1 and decreases that of Cdk4 and cyclin D3; in vivo, the toxin reduced the tumor volume when injected into the tumor (Jang et al. 2011). Moreover, margatoxin also inhibits the proliferation of the weakly metastatic rat prostate cancer cell line AT2, but not that of the strongly metastatic prostate cancer cell line Mat-LyLu (Fraser et al. 2000). Although different studies were focused mainly on the implication of Kv1.3 in regulating cell proliferation, several reports suggested that the mechanism could be also extrapolated for other K+ channels subtypes (Srairi-Abid et al. 2019). Iberiotoxin is a peptide from Mesobuthus tumulus scorpion venom that inhibits Ca2+-activated K+ channels and decreases the human malignant glioma cell number in a dose- and time-dependent manner. The toxin arrested glioma cells in the S phase of the cell cycle, which eventually led to cell death. The expression of these Ca2+-activated K+ channels is upregulated in human glioma, and the expression levels increase with the grade of the tumor (Weaver et al. 2004). The antiproliferative effects of iberiotoxin have been also observed in 1321N1 astrocytomas (Basrai et al. 2002) and PC-3 prostate cancer cell lines (Bloch et al. 2007). Charybdotoxin is another scorpion toxin inhibiting Ca2+-activated K+ channels that slow the migration of melanoma cells in a dose-dependent manner (Schwab et al. 1999). The scorpion peptide κ-hefutoxin 1 inhibits the Kv10.1 channel in a dose-dependent manner (IC50~26 μM) (Moreels et al. 2017b). Other toxins targeting Kv11.1 channels are ErgoTx from the Centruroides noxious scorpion venom, BeKm-1 from the scorpion Buthus eupeus, and BmTx3 from the scorpion Butus martensi. The analgesic-antitumor peptide (AGAP) from scorpion venom is an inhibitor of voltage-gated sodium channels and decreases proliferation and migration of glioma cells (Zhao et al. 2011; Chen et al. 2018).
Psalmotoxin is a specific acid-sensing ion channel (ASIC1) blocker from the Psalmopoeus cambridgei tarantula venom. This toxin inhibits cation currents in malignant astroglioma and glioblastoma multiforme cells, arresting the cell cycle in the G0/G1 phase and upregulating p21 and p27 protein expression due to a reduction of the phosphorylation of ERK1/2 (Wu et al. 2019). Aa1a and Ap1a spider venom peptides are gating modifiers of the Kv10.1 channel. Ap1a peptide is more selective (>30-fold) for Kv10.1 than for Kv11.1 (Ma et al. 2018). Several peptides from the Chilean tarantula Grammostla rosea venom blocks Kv11.1 channels currents transiently expressed in CHO cells in a reversible manner (Wanke and Restano-Cassulini 2007).
7.2 Blarina brevicauda Saliva Peptides, Snake Venoms, and Anemone Toxins with Antineoplastic Effects
Soricidin is a novel paralytic peptide found in the saliva of the northern short-tailed shrew Blarina brevicauda that modulates TRPV6 channels, which are highly expressed in ovarian, breast, prostate, colon, and thyroid cancers, as well as certain leukemias and lymphomas. Actually, the overexpression of these channels is associated with tumor development and progression, and its inhibition decreases cancer cell proliferation and promotes apoptosis (Pennington et al. 2018; Wulff et al. 2019). SOR-C13 and SORC-27 are two shorter peptides derived from the C-terminus of soricidin and bind to TRPV6 channels with high affinity targeting tumor sites in mice bearing ovarian or prostate tumors; thus, these peptides may be used as either drug carriers or diagnostic agents in TRPV6-enriched tumors (Bowen et al. 2013). In an ovarian cancer xenograft mouse model, daily i.p. injection of SORC-13 and SORC-27 inhibited tumor growth (Xue et al. 2018). A phase I study of SORC-13 in patients with advanced tumors of epithelial origin showed stable disease in 54.5% of the patients (ranging from 2.8 to 12.5 months) without drug-related serious adverse events. The best response in this study was a 27% reduction in a pancreatic tumor with a 55% reduction in the levels of the tumor marker CA19-9 (Fu et al. 2017a, b).
Snake venom components inhibit cell proliferation and promote cell death; the mechanisms of action include increasing Ca2+ influx, cytochrome C release, and modified protein expression. These venom toxins also prevent metastasis, promote toxicity and free-radical generation, inhibit nucleic acid synthesis, decrease the expression and activity of matrix metalloproteinase, and inhibit integrins preventing migration and invasion of cancer cells, as well as angiogenesis (Chen et al. 2018). APETx1 and APETx4 toxins from the sea anemone Anthopleura elegantissima block Kv11.1 (IC50 = 34 nM) and Kv10.1 (IC50 = 1.1 μM) channels. APETx1 modifies the voltage dependence of Kv11.1, while APETx4 seems to keep Kv10.1 channels in a closed state (Diochot et al. 2003; Moreels et al. 2017a). APETx4 induces a concentration-dependent cytotoxic and proapoptotic effect in various cancer and noncancer cell lines (Moreels et al. 2017a). The large diversity of venom components and their multiple antineoplastic effects makes them excellent candidates to establish new therapeutic and specific strategies with fewer side effects in cancer treatment. Finally, we will discuss how ion channels and transporter-based nanomedicine can be used to fight cancer.
8 Ion Channel and Transporter-Based Nanomedicine in Cancer Therapy
The medical applications of nanotechnology led to the emergence of nanomedicine with the idea of inserting nanorobots into patients to treat several diseases, including cancer (Freitas 2005). Then some nanomaterials including liposomes, nanofibers, polymeric micelles, magnetic, inorganic, and polymeric nanoparticles have been used to decrease the side effects of chemotherapy by increasing its specificity, direct targeting, easy absorption, and sustained release and reducing drug degradation (Xie et al. 2019; Yang et al. 2019). Additional advantages of drug carrier systems over traditional chemotherapy include better solubility of hydrophobic drugs, higher stability, and improved blood half-life of the therapeutic agent. Nevertheless, drug delivery is complicated due to multiple physical barriers that limit diffusion and tumor penetration of the released drugs (Sun et al. 2008b). However, nanoparticles do not necessarily have to penetrate into tumor cells because the antineoplastic efficacy could be improved if the delivered drugs target cell membrane proteins involved in cancer like ion channels and transporters. For instance, nanoprobes composed of polyethylene glycol (PEG)-coated iron oxide nanoparticles were functionalized with chlorotoxin and the fluorescent molecule Cy5.5 targeting glioma tumors with high affinity, high resolution, and good therapeutic effect (Veiseh et al. 2005, 2009; Sun et al. 2008a). Chlorotoxin was also conjugated in nanoparticles with the chemotherapeutic agent methotrexate, demonstrating preferential accumulation and increased cytotoxicity in tumor cells, as well as prolonged nanoparticle retention within tumors (Sun et al. 2008b). A cancer cell-specific magnetic nanovector functionalized with siRNA and chlorotoxin was developed with the aims of efficient siRNA delivery and noninvasive monitoring by magnetic resonance imaging (MRI). The nanovector demonstrated both increased siRNA internalization by tumor cells and intracellular trafficking toward enhanced knockdown of targeted gene expression. In addition, this nanovector enhanced MRI contrast in vitro, potentially enabling monitoring of the treatment in vivo. The elevated specificity and potency of this nanovector system make it a potential gene therapy approach for malignant tumors (Veiseh et al. 2010).
Drug resistance has been also addressed by nanomedicine. Functionalized nanoparticles deliver and concentrate drugs at the plasma membrane where ABC transporters are located and are saturated with either the antineoplastic drug, ABC transporter blockers, or inhibitors (Xue and Liang 2012). Mesoporous silica nanoparticles were used to deliver doxorubicin and P-glycoprotein siRNA to the drug-resistant cancer cell line KB-V1 (derived from HeLa cervical cancer cells). The dual delivery of doxorubicin and siRNA in KB-V1cells increased the intracellular and intranuclear drug concentration to levels exceeding free doxorubicin or the drug being delivered by mesoporous in the absence of siRNA co-delivery (Meng et al. 2010). Wang and colleagues observed similar results; the co-delivery of doxorubicin and MDR1-siRNA by mesoporous silica nanoparticles-polymerpolyethylenimine improved oral squamous carcinoma treatment in vitro and in vivo (Wang et al. 2018a). In breast cancer, the dual delivery system of doxorubicin and siRNA against P-glycoprotein resulted in synergistic inhibition of tumor growth (Meng et al. 2013). Similar results were observed using doxorubicin/P-glycoprotein siRNA-loaded nanomicelles (Suo et al. 2016) and RGD peptide (arginine-glycine-aspartic acid)-modified liposomes containing also doxorubicin and P-glycoprotein siRNA (Xue and Liang 2012).
Photothermal therapy (PTT) converts near-infrared light (NIR) stimulation into local mild heat that can stimulate a photosensitizer to control biological processes in a remote and noninvasive manner. Photothermal transducers of graphene oxide linked to a calmodulin-binding peptide (rGO-P) have been designed to activate or inactivate potassium channels based on their binding to Ca2+-calmodulin (Chai et al. 2018). Kv10.1 channel activity is inhibited by [Ca2+]i through the binding of Ca2+-calmodulin (Lörinczi et al. 2016). HEK-293 cells transfected with Kv10.1, treated with rGO-P, and stimulated with NIR irradiation displayed an open state of Kv10.1 channels because the channel cannot be closed via binding of Ca2+-calmodulin, suggesting a new strategy to regulate ion channels involved in cancer like Kv10.1 (Chai et al. 2018). The application of nanomaterials to PTT is emerging as a new strategy for cancer therapy and showing encouraging results in vivo (Zou et al. 2016).
A number of studies suggest that targeting ion channels and transporters is a feasible approach to achieve therapeutic efficacy and tumor selectivity in cancer treatment. Table 2 summarizes some of these findings, and Fig. 1 shows the diversity of the potential anticancer therapeutic options based on ion channels and transporters.
Novel therapeutic opportunities targeting ion channels or transporters in cancer. (a) Inducing higher ROS production in cancer cells by blocking ion channels or transporters may convert cancer cells more sensitive to chemotherapy. (b) Immunotherapy against channels overexpressed in cancer cells offers a more selective approach. (c) Toxins targeting ion channels and transporters may also help to improve selectivity and the antitumor response. (d) Identifying and investigating new uses of approved drugs hold potential and many advantages for anticancer therapy. (e) New technologies like nanoparticles and phototherapy targeting ion channels and transporters are potential more selective anticancer therapies
9 Conclusions
Cancer is a multifactorial disease; therefore, several issues should be considered to find the most promising anticancer therapy for each patient. Specific inhibitors of ion channels and transporters including drugs, antibodies, or toxins, concomitantly used with regulators of the redox status and enhancers of the immune response, should improve the therapeutic outcome. Moreover, repurposing drugs targeting ion channels and transporters combined with currently used anticancer treatments should enhance the antitumor response. This drug repurposing approach adds several anticancer mechanisms to the treatment, may decrease the side effects by lowering the administrated doses, and should decrease treatment costs, as well as accelerating the development of clinical trials. The specificity of many toxins targeting ion channels and transporters should allow the directed and specific treatment of tumors. Exploiting ion channel and transporter-based approaches to fight cancer and the use of nanotechnology and nanomedicine should improve the current therapies for the benefit of cancer patients.
References
Abdelaleem M et al (2019) Prospects for repurposing CNS drugs for cancer treatment. Oncol Rev. https://doi.org/10.4081/oncol.2019.411
Abdul M, Hoosein N (2002) Expression and activity of potassium ion channels in human prostate cancer. Cancer Lett. https://doi.org/10.1016/s0304-3835(02)00348-8
Abu El Maaty MA, Wölfl S (2017) Effects of 1, 25(OH)2D3 on cancer cells and potential applications in combination with established and putative anti-cancer agents’. Nutrients. https://doi.org/10.3390/nu9010087
Aissaoui D et al (2018) Functional role of Kv1.1 and Kv1.3 channels in the neoplastic progression steps of three cancer cell lines, elucidated by scorpion peptides. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2018.01.144
Allard B et al (2016) Immunosuppressive activities of adenosine in cancer. Curr Opin Pharmacol. https://doi.org/10.1016/j.coph.2016.04.001
Almasi S et al (2018) TRPM2 channel–mediated regulation of autophagy maintains mitochondrial function and promotes gastric cancer cell survival via the JNK-signaling pathway. J Biol Chem. https://doi.org/10.1074/jbc.M117.817635
Almasi S, Sterea AM et al (2019a) TRPM2 ion channel promotes gastric cancer migration, invasion and tumor growth through the AKT signaling pathway. Sci Rep. https://doi.org/10.1038/s41598-019-40330-1
Almasi S, Long CY et al (2019b) TRPM2 silencing causes G2/M arrest and apoptosis in lung cancer cells via increasing intracellular ROS and RNS levels and activating the JNK pathway. Cell Physiol Biochem. https://doi.org/10.33594/000000052
An L et al (2017) Terfenadine combined with epirubicin impedes the chemo-resistant human non-small cell lung cancer both in vitro and in vivo through EMT and Notch reversal. Pharmacol Res. https://doi.org/10.1016/j.phrs.2017.07.021
Arbabian A et al (2013) Modulation of endoplasmic reticulum calcium pump expression during lung cancer cell differentiation. FEBS J. https://doi.org/10.1111/febs.12064
Arcangeli A, Becchetti A (2010) New trends in cancer therapy: targeting ion channels and transporters. Pharmaceuticals. https://doi.org/10.3390/ph3041202
Arora A et al (2005) Modulation of P-glycoprotein-mediated multidrug resistance in K562 leukemic cells by indole-3-carbinol. Toxicol Appl Pharmacol. https://doi.org/10.1016/j.taap.2004.06.017
Asher V et al (2010) Eag and HERG potassium channels as novel therapeutic targets in cancer. World J Surg Oncol. https://doi.org/10.1186/1477-7819-8-113
Asher V et al (2011) The role of Eag and HERG channels in cell proliferation and apoptotic cell death in SK-OV-3 ovarian cancer cell line. Cancer Cell Int. https://doi.org/10.1186/1475-2867-11-6
Averaimo S et al (2010) Chloride intracellular channel 1 (CLIC1): sensor and effector during oxidative stress. FEBS Lett. https://doi.org/10.1016/j.febslet.2010.02.073
Avila E et al (2010) Calcitriol down-regulates human ether a go-go 1 potassium channel expression in cervical cancer cells. Anticancer Res. https://doi.org/10.1016/j.yexcr.2009.11.008
Azimi I, Roberts-Thomson SJ, Monteith GR (2014) Calcium influx pathways in breast cancer: opportunities for pharmacological intervention. Br J Pharmacol. https://doi.org/10.1111/bph.12486
Azimi I et al (2019) ORAI1 and ORAI3 in breast cancer molecular subtypes and the identification of ORAI3 as a hypoxia sensitive gene and a regulator of hypoxia responses. Cancer. https://doi.org/10.3390/cancers11020208
Bachmann M et al (2018) Targeting mitochondrial ion channels to fight cancer. Int J Mol Sci. https://doi.org/10.3390/ijms19072060
Baffy G (2010) Uncoupling protein-2 and cancer. Mitochondrion. https://doi.org/10.1016/j.mito.2009.12.143
Bao XX et al (2012) Nifedipine induced autophagy through Beclin1 and mTOR pathway in endometrial carcinoma cells. Chin Med J (Engl). https://doi.org/10.3760/cma.j.issn.0366-6999.2012.17.028
Bao L et al (2016) Depletion of the human ion channel TRPM2 in neuroblastoma demonstrates its key role in cell survival through modulation of mitochondrial reactive oxygen species and bioenergetics. J Biol Chem. https://doi.org/10.1074/jbc.M116.747147
Barbado M et al (2009) Gene regulation by voltage-dependent calcium channels. Biochim Biophys Acta. https://doi.org/10.1016/j.bbamcr.2009.02.004
Barlaz Us S et al (2019) Effect of imipramine on radiosensitivity of prostate cancer: an in vitro study. Cancer Invest. https://doi.org/10.1080/07357907.2019.1662434
Basrai D et al (2002) BK channel blockers inhibit potassium-induced proliferation of human astrocytoma cells. Neuroreport. https://doi.org/10.1097/00001756-200203250-00008
Beatty GL, Gladney WL (2015) Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-14-1860
Belpomme D et al (2000) Verapamil increases the survival of patients with anthracycline-resistant metastatic breast carcinoma. Ann Oncol. https://doi.org/10.1023/a:1026556119020
Benzerdjeb, N. et al. (2016) ‘Orai3 is a predictive marker of metastasis and survival in resectable lung adenocarcinoma’, Oncotarget. doi: https://doi.org/10.18632/oncotarget.13149
Bernal-Ramos G et al (2017) Astemizole inhibits cell proliferation in human prostate tumorigenic cells expressing ether a-go-go-1 potassium channels. Cell Mol Biol (Noisy-le-Grand). https://doi.org/10.14715/cmb/2017.63.12.4
Bernardi P et al (2015) The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev. https://doi.org/10.1152/physrev.00001.2015
Berry BJ et al (2018) Use the protonmotive force: mitochondrial uncoupling and reactive oxygen species. J Mol Biol. https://doi.org/10.1016/j.jmb.2018.03.025
Bertolesi GE et al (2002) The Ca(2+) channel antagonists mibefradil and pimozide inhibit cell growth via different cytotoxic mechanisms. Mol Pharmacol. https://doi.org/10.1124/mol.62.2.210
Berul CI, Morad M (1995) Regulation of potassium channels by nonsedating antihistamines. Circulation. https://doi.org/10.1161/01.CIR.91.8.2220
Binyamin L et al (2004) Targeting an extracellular epitope of the human multidrug resistance protein 1 (MRP1) in malignant cells with a novel recombinant single chain Fv antibody. Int J Cancer. https://doi.org/10.1002/ijc.20177
Birben E et al (2012) Oxidative stress and antioxidant defense. World Allergy Organ J. https://doi.org/10.1097/WOX.0b013e3182439613
Bjelakovic G et al (2007) Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. https://doi.org/10.1001/jama.297.8.842
Blake SD et al (2017) Transient receptor potential, Melastatin-2 (TRPM2) blockade: perspectives on potential novel clinical utility in cancer. Transl Cancer Res. https://doi.org/10.21037/tcr.2017.03.11
Blaya B et al (2010) Histamine and histamine receptor antagonists in cancer biology. Inflamm Allergy Drug Targets. https://doi.org/10.2174/187152810792231869
Bloch M et al (2007) KCNMA1 gene amplification promotes tumor cell proliferation in human prostate cancer. Oncogene. https://doi.org/10.1038/sj.onc.1210036
Bogeski I et al (2010) Differential redox regulation of ORAI ion channels: a mechanism to tune cellular calcium signaling. Sci Signal. https://doi.org/10.1126/scisignal.2000672
Bootman MD et al (2001) Calcium signalling – an overview. Semin Cell Dev Biol. https://doi.org/10.1006/scdb.2000.0211
Bowen CV et al (2013) In vivo detection of human TRPV6-rich tumors with anti-cancer peptides derived from soricidin. PLoS One. https://doi.org/10.1371/journal.pone.0058866
Buchanan PJ, McCloskey KD (2016) CaV channels and cancer: canonical functions indicate benefits of repurposed drugs as cancer therapeutics. Eur Biophys J. https://doi.org/10.1007/s00249-016-1144-z
Cairo MS et al (1989) Clinical trial of continuous infusion verapamil, bolus vinblastine, and continuous infusion VP-16 in drug-resistant pediatric tumors. Cancer Res 49(4):1063–1066
Camacho J (2006) Ether a go-go potassium channels and cancer. Cancer Lett. https://doi.org/10.1016/j.canlet.2005.02.016
Camara AKS et al (2017) Mitochondrial VDAC1: a key gatekeeper as potential therapeutic target. Front Physiol. https://doi.org/10.3389/fphys.2017.00460
Cannon B et al (2006) Uncoupling proteins: a role in protection against reactive oxygen species-or not? Biochim Biophys Acta Bioenerg. https://doi.org/10.1016/j.bbabio.2006.05.016
Cazares-Ordonez V et al (2015) A cis-acting element in the promoter of human ether a go-go 1 potassium channel gene mediates repression by calcitriol in human cervical cancer cells. Biochem Cell Biol. https://doi.org/10.1139/bcb-2014-0073
Chai R et al (2018) Remote-controlling potassium channels in living cells through photothermal inactivation of calmodulin. Adv Healthc Mater. https://doi.org/10.1002/adhm.201800674
Chavez-Lopez MG et al (2014) Antiproliferative and proapoptotic effects of astemizole on cervical cancer cells. Int J Gynecol Cancer. https://doi.org/10.1097/IGC.0000000000000151
Chavez-Lopez MG et al (2015) Astemizole-based anticancer therapy for hepatocellular carcinoma (HCC), and Eag1 channels as potential early-stage markers of HCC. Tumour Biol. https://doi.org/10.1007/s13277-015-3299-0
Chavez-Lopez MG et al (2017) The combination astemizole-gefitinib as a potential therapy for human lung cancer. Onco Targets Ther. https://doi.org/10.2147/OTT.S144506
Chen CD et al (2007) Overexpression of CLIC1 in human gastric carcinoma and its clinicopathological significance. Proteomics. https://doi.org/10.1002/pmic.200600663
Chen SJ et al (2013) Role of TRPM2 in cell proliferation and susceptibility to oxidative stress. Am J Physiol Cell Physiol. https://doi.org/10.1152/ajpcell.00069.2012
Chen SJ et al (2014) A splice variant of the human ion channel TRPM2 modulates neuroblastoma tumor growth through hypoxia-inducible factor (HIF)-1/2α. J Biol Chem. https://doi.org/10.1074/jbc.M114.620922
Chen N et al (2018) Animal protein toxins: origins and therapeutic applications. Biophys Rep. https://doi.org/10.1007/s41048-018-0067-x
Chen YF et al (2019) Store-operated Ca2+ entry in tumor progression: From molecular mechanisms to clinical implications. Cancer. https://doi.org/10.3390/cancers11070899
Cheng WC et al (2019) Uncoupling protein 2 reprograms the tumor microenvironment to support the anti-tumor immune cycle. Nat Immunol. https://doi.org/10.1038/s41590-018-0290-0
Chiang EY et al (2017) Potassium channels Kv1.3 and KCa3.1 cooperatively and compensatorily regulate antigen-specific memory T cell functions. Nat Commun. https://doi.org/10.1038/ncomms14644
Chimote AA et al (2013) Selective inhibition of KCa3.1 channels mediates adenosine regulation of the motility of human T cells. J Immunol. https://doi.org/10.4049/jimmunol.1300702
Chimote AA et al (2018) A defect in KCa3.1 channel activity limits the ability of CD8+ T cells from cancer patients to infiltrate an adenosine-rich microenvironment. Sci Signal. https://doi.org/10.1126/scisignal.aaq1616
Chio IIC, Tuveson DA (2017) ROS in cancer: the burning question. Trends Mol Med. https://doi.org/10.1016/j.molmed.2017.03.004
Chioni AM et al (2005) A novel polyclonal antibody specific for the Nav1.5 voltage-gated Na+ channel “neonatal” splice form. J Neurosci Methods. https://doi.org/10.1016/j.jneumeth.2005.03.010
Chung FY et al (2006) Sarco/endoplasmic reticulum calcium-ATPase 2 expression as a tumor marker in colorectal cancer. Am J Surg Pathol. https://doi.org/10.1097/00000478-200608000-00006
Clapham DE (2003) TRP channels as cellular sensors. Nature. https://doi.org/10.1038/nature02196
Comes N et al (2013) The voltage-dependent K(+) channels Kv1.3 and Kv1.5 in human cancer. Front Physiol. https://doi.org/10.3389/fphys.2013.00283
Crociani O et al (2014) hERG1 channels regulate VEGF-A secretion in human gastric cancer: clinicopathological correlations and therapeutical implications. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-13-2633
Cui C et al (2019) Progress in understanding mitochondrial calcium uniporter complex-mediated calcium signalling: a potential target for cancer treatment. Br J Pharmacol. https://doi.org/10.1111/bph.14632
Curry MC et al (2012) Distinct regulation of cytoplasmic calcium signals and cell death pathways by different plasma membrane calcium ATPase isoforms in MDA-MB-231 breast cancer cells. J Biol Chem. https://doi.org/10.1074/jbc.M112.364737
Curry M, Roberts-Thomson SJ, Monteith GR (2016) PMCA2 silencing potentiates MDA-MB-231 breast cancer cell death initiated with the Bcl-2 inhibitor ABT-263. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2016.09.030
D’Alessandro G et al (2019) Radiation increases functional KCa3.1 expression and invasiveness in glioblastoma. Cancer. https://doi.org/10.3390/cancers11030279
D’Amico M, Gasparoli L, Arcangeli A (2013) Potassium channels: novel emerging biomarkers and targets for therapy in cancer. Recent Pat Anticancer Drug Discov 8(1):53–65
Dalla Pozza E et al (2012) Role of mitochondrial uncoupling protein 2 in cancer cell resistance to gemcitabine. Biochim Biophys Acta Mol Cell Res. https://doi.org/10.1016/j.bbamcr.2012.06.007
Dang D, Rao R (2016) Calcium-ATPases: gene disorders and dysregulation in cancer. Biochim Biophys Acta Mol Cell Res. https://doi.org/10.1016/j.bbamcr.2015.11.016
Dardevet L et al (2015) Chlorotoxin: a helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins (Basel). https://doi.org/10.3390/toxins7041079
David CJ, Manley JL (2010) Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged. Genes Dev. https://doi.org/10.1101/gad.1973010
Debes JD et al (2004) Inverse association between prostate cancer and the use of calcium channel blockers. Cancer Epidemiol Biomarkers. https://doi.org/10.1158/1055-9965.epi-03-0093
Deeb KK, Trump DL, Johnson CS (2007) Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. https://doi.org/10.1038/nrc2196
Deliot N, Constantin B (2015) Plasma membrane calcium channels in cancer: alterations and consequences for cell proliferation and migration. Biochim Biophys Acta. https://doi.org/10.1016/j.bbamem.2015.06.009
Denicola GM et al (2011) Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. https://doi.org/10.1038/nature10189
Denmeade SR, Isaacs JT (2005) The SERCA pump as a therapeutic target: making a “smart bomb” for prostate cancer. Cancer Biol Ther. https://doi.org/10.4161/cbt.4.1.1505
Denmeade SR et al (2012) Engineering a prostate-specific membrane antigen-activated tumor endothelial cell prodrug for cancer therapy. Sci Transl Med. https://doi.org/10.1126/scitranslmed.3003886
Derdak Z et al (2008) The mitochondrial uncoupling protein-2 promotes chemoresistance in cancer cells. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-08-0053
Deshane J, Garner CC, Sontheimer H (2003) Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. https://doi.org/10.1074/jbc.M205662200
Diaz L et al (2009) Estrogens and human papilloma virus oncogenes regulate human ether-a-go-go-1 potassium channel expression. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-08-2036
Diaz L et al (2015) Mechanistic effects of calcitriol in cancer biology. Nutrients. https://doi.org/10.3390/nu7065020
Diez-Bello R et al (2019) (−)-Oleocanthal inhibits proliferation and migration by modulating Ca2+ entry through TRPC6 in breast cancer cells. Biochim Biophys Acta Mol Cell Res. https://doi.org/10.1016/j.bbamcr.2018.10.010
Ding XW et al (2008) Overexpression of hERG1 in resected esophageal squamous cell carcinomas: a marker for poor prognosis. J Surg Oncol. https://doi.org/10.1002/jso.20891
Ding J et al (2014) Scorpion venoms as a potential source of novel cancer therapeutic compounds. Exp Biol Med (Maywood). https://doi.org/10.1177/1535370213513991
Diochot S et al (2003) APETx1, a new toxin from the sea anemone Anthopleura elegantissima, blocks voltage-gated human ether-a-go-go-related gene potassium channels. https://doi.org/10.1124/mol.64.1.59
Dixon SJ et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. https://doi.org/10.1016/j.cell.2012.03.042
Downie BR et al (2008) Eag1 expression interferes with hypoxia homeostasis and induces angiogenesis in tumors. J Biol Chem. https://doi.org/10.1074/jbc.M801830200
Dubois C et al (2014) Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cell. https://doi.org/10.1016/j.ccr.2014.04.025
Durie BG, Dalton WS (1988) Reversal of drug-resistance in multiple myeloma with verapamil. Br J Haematol. https://doi.org/10.1111/j.1365-2141.1988.tb06190.x
Dutta S et al (2014) Interactions between exosomes from breast cancer cells and primary mammary epithelial cells leads to generation of reactive oxygen species which induce DNA damage response, stabilization of p53 and autophagy in epithelial cells. PLoS One. https://doi.org/10.1371/journal.pone.0097580
Ellegaard AM et al (2016) Repurposing cationic amphiphilic antihistamines for cancer treatment. EBioMedicine. https://doi.org/10.1016/j.ebiom.2016.06.013
Enfissi A et al (2004) The blocking of capacitative calcium entry by 2-aminoethyl diphenylborate (2-APB) and carboxyamidotriazole (CAI) inhibits proliferation in Hep G2 and Huh-7 human hepatoma cells. Cell Calcium. https://doi.org/10.1016/j.ceca.2004.04.004
Ermak G, Davies KJA (2002) Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol. https://doi.org/10.1016/S0161-5890(01)00108-0
Faouzi M, Penner R (2014) TRPM2. Handb Exp Pharmacol. https://doi.org/10.1007/978-3-642-54215-2_16
Farias LM et al (2004) Ether a go-go potassium channels as human cervical cancer markers. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-04-1204
Faustino-Rocha AI et al (2017) Antihistamines as promising drugs in cancer therapy. Life Sci. https://doi.org/10.1016/j.lfs.2016.12.008
Fernández-Nogueira P et al (2018) Histamine receptor 1 inhibition enhances antitumor therapeutic responses through extracellular signal-regulated kinase (ERK) activation in breast cancer. Cancer Lett. https://doi.org/10.1016/j.canlet.2018.03.014
Feske S, Wulff H, Skolnik EY (2015) Ion channels in innate and adaptive immunity. Annu Rev Immunol. https://doi.org/10.1146/annurev-immunol-032414-112212
Fiorio Pla A, Kondratska K, Prevarskaya N (2016) STIM and ORAI proteins: crucial roles in hallmarks of cancer. Am J Physiol Cell Physiol. https://doi.org/10.1152/ajpcell.00364.2015
Fitzpatrick AL et al (2001) Hypertension, heart rate, use of antihypertensives, and incident prostate cancer. Ann Epidemiol. https://doi.org/10.1016/s1047-2797(01)00246-0
Fraser SP, Grimes JA, Djamgoz MBA (2000) Effects of voltage-gated ion channel modulators on rat prostatic cancer cell proliferation: comparison of strongly and weakly metastatic cell lines. Prostate. https://doi.org/10.1002/1097-0045(20000615)44:1<61::aid-pros9>3.0.co;2-3
Freitas RA Jr (2005) What is nanomedicine? Nanomedicine. https://doi.org/10.1016/j.nano.2004.11.003
Frisch J et al (2019) STIM-Orai channels and reactive oxygen species in the tumor microenvironment. Cancer. https://doi.org/10.3390/cancers11040457
Fu S et al (2017a) Erratum to: first-in-human phase I study of SOR-C13, a TRPV6 calcium channel inhibitor, in patients with advanced solid tumors. Invest New Drugs. https://doi.org/10.1007/s10637-017-0455-y
Fu S et al (2017b) First-in-human phase I study of SOR-C13, a TRPV6 calcium channel inhibitor, in patients with advanced solid tumors. Invest New Drugs. https://doi.org/10.1007/s10637-017-0438-z
Fukushiro-Lopes DF et al (2018) Preclinical study of a Kv11.1 potassium channel activator as antineoplastic approach for breast cancer. Oncotarget. https://doi.org/10.18632/oncotarget.22925
Gao T et al (2000) Role of the C terminus of the alpha 1C (CaV1.2) subunit in membrane targeting of cardiac L-type calcium channels. J Biol Chem. https://doi.org/10.1074/jbc.M003465200
Gao T et al (2001) C-terminal fragments of the alpha 1C (CaV1.2) subunit associate with and regulate L-type calcium channels containing C-terminal-truncated alpha 1C subunits. J Biol Chem. https://doi.org/10.1074/jbc.M008000200
Garcia-Becerra R et al (2010) Calcitriol inhibits ether-a go-go potassium channel expression and cell proliferation in human breast cancer cells. Exp Cell Res. https://doi.org/10.1016/j.yexcr.2009.11.008
Garcia-Ferreiro RE et al (2004) Mechanism of block of hEag1 K+ channels by imipramine and astemizole. J Gen Physiol. https://doi.org/10.1085/jgp.200409041
Garcia-Quiroz J, Camacho J (2011) Astemizole: an old anti-histamine as a new promising anti-cancer drug. Anticancer Agents Med Chem. https://doi.org/10.2174/187152011795347513
Garcia-Quiroz J et al (2012) Astemizole synergizes calcitriol antiproliferative activity by inhibiting CYP24A1 and upregulating VDR: a novel approach for breast cancer therapy. PLoS One. https://doi.org/10.1371/journal.pone.0045063
Garcia-Quiroz J et al (2014) In vivo dual targeting of the oncogenic ether-a-go-go-1 potassium channel by calcitriol and astemizole results in enhanced antineoplastic effects in breast tumors. BMC Cancer. https://doi.org/10.1186/1471-2407-14-745
Garcia-Quiroz J et al (2016) Calcitriol stimulates gene expression of cathelicidin antimicrobial peptide in breast cancer cells with different phenotype. J Biomed Sci. https://doi.org/10.1186/s12929-016-0298-4
Garcia-Quiroz J et al (2019) Astemizole, an inhibitor of ether-a-go-go-1 potassium channel, increases the activity of the tyrosine kinase inhibitor gefitinib in breast cancer cells. Rev Invest Clin. https://doi.org/10.24875/RIC.18002840
García-Quiroz J et al (2019) Synergistic antitumorigenic activity of calcitriol with curcumin or resveratrol is mediated by angiogenesis inhibition in triple negative breast cancer xenografts. Cancer. https://doi.org/10.3390/cancers11111739
Gasparoli L et al (2015) New pyrimido-indole compound CD-160130 preferentially inhibits the KV11.1B isoform and produces antileukemic effects without cardiotoxicity. Mol Pharmacol. https://doi.org/10.1124/mol.114.094920
Gavrilova-Ruch O et al (2002) Effects of imipramine on ion channels and proliferation of IGR1 melanoma cells. J Membr Biol. https://doi.org/10.1007/s00232-001-0181-3
Ge L et al (2012) Glioma big potassium channel expression in human cancers and possible T cell epitopes for their immunotherapy. J Immunol. https://doi.org/10.4049/jimmunol.1102965
Gélébart P et al (2002) Expression of endomembrane calcium pumps in colon and gastric cancer cells. Induction of SERCA3 expression during differentiation. J Biol Chem. https://doi.org/10.1074/jbc.M201747200
Gentile S et al (2016) hERG1 potassium channel in cancer cells: a tool to reprogram immortality. Eur Biophys J. https://doi.org/10.1007/s00249-016-1169-3
Gessner G, Heinemann SH (2003) Inhibition of hEAG1 and hERG1 potassium channels by clofilium and its tertiary analogue LY97241. Br J Pharmacol 138(1):161–171. https://doi.org/10.1038/sj.bjp.0705025
Gilbert SM et al (2017) A phase I clinical trial demonstrates that nfP2X7-targeted antibodies provide a novel, safe and tolerable topical therapy for basal cell carcinoma. Br J Dermatol. https://doi.org/10.1111/bjd.15364
Gillman PK (2007) Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. https://doi.org/10.1038/sj.bjp.0707253
Glowka E, Stasiak J, Lulek J (2019) Drug delivery systems for vitamin D supplementation and therapy. Pharmaceutics. https://doi.org/10.3390/pharmaceutics11070347
Gomes FR et al (2015) Alternatively spliced isoforms of KV10.1 potassium channels modulate channel properties and can activate cyclin-dependent kinase in Xenopus oocytes. J Biol Chem. https://doi.org/10.1074/jbc.M115.668749
Gómez-Lagunas F et al (2017) Gating modulation of the tumor-related Kv10.1 channel by mibefradil. J Cell Physiol. https://doi.org/10.1002/jcp.25448
Gomez-Ospina N et al (2013) A promoter in the coding region of the calcium channel gene CACNA1C generates the transcription factor CCAT. PLoS One. https://doi.org/10.1371/journal.pone.0060526
Gomez-Varela D et al (2006) Different relevance of inactivation and F468 residue in the mechanisms of hEag1 channel blockage by astemizole, imipramine and dofetilide. FEBS Lett. https://doi.org/10.1016/j.febslet.2006.08.030
Gomez-Varela D et al (2007) Monoclonal antibody blockade of the human Eag1 potassium channel function exerts antitumor activity. Cancer. https://doi.org/10.1158/0008-5472.CAN-07-0107
Gorrini C, Harris IS, Mak TW (2013) Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. https://doi.org/10.1038/nrd4002
Graf N et al (2012) Platinum(IV)-chlorotoxin (CTX) conjugates for targeting cancer cells. J Inorg Biochem. https://doi.org/10.1016/j.jinorgbio.2012.02.012
Gritti M et al (2014) Metformin repositioning as antitumoral agent: Selective antiproliferative effects in human glioblastoma stem cells, via inhibition of CLIC1-mediated ion current. Oncotarget. https://doi.org/10.18632/oncotarget.2617
Grössinger EM et al (2014) Targeting proliferation of chronic lymphocytic leukemia (CLL) cells through KCa3.1 blockade. Leukemia. https://doi.org/10.1038/leu.2014.37
Gualdani R et al (2019) Store-operated calcium entry contributes to cisplatin-induced cell death in non-small cell lung carcinoma. Cancer. https://doi.org/10.3390/cancers11030430
Guan J et al (2009) The xc- cystine/glutamate antiporter as a potential therapeutic target for small-cell lung cancer: use of sulfasalazine. Cancer Chemother Pharmacol. https://doi.org/10.1007/s00280-008-0894-4
Guo Q et al (2019) A comprehensive evaluation of clinical efficacy and safety of celecoxib in combination with chemotherapy in metastatic or postoperative recurrent gastric cancer patients: a preliminary, three-center, clinical trial study. Medicine (Baltimore). https://doi.org/10.1097/MD.0000000000016234
Gupta SC et al (2013) Cancer drug discovery by repurposing: teaching new tricks to old dogs. Trends Pharmacol Sci. https://doi.org/10.1016/j.tips.2013.06.005
Hara Y et al (2002) LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. https://doi.org/10.1016/S1097-2765(01)00438-5
Harr MW, Distelhorst CW (2010) Apoptosis and autophagy: decoding calcium signals that mediate life or death. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a005579
Harris IS et al (2015) Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. https://doi.org/10.1016/j.ccell.2014.11.019
Hartung F, Pardo LA (2016) Guiding TRAIL to cancer cells through Kv10.1 potassium channel overcomes resistance to doxorubicin. Eur Biophys J. https://doi.org/10.1007/s00249-016-1149-7
Hartung F, Stühmer W, Pardo LA (2011) Tumor cell-selective apoptosis induction through targeting of KV10.1 via bifunctional TRAIL antibody. Mol Cancer. https://doi.org/10.1186/1476-4598-10-109
Hasna J et al (2018) Orai3 calcium channel and resistance to chemotherapy in breast cancer cells: the p53 connection. Cell Death Differ. https://doi.org/10.1038/s41418-017-0007-1
He YM et al (2018) Effect of CLIC1 gene silencing on proliferation, migration, invasion and apoptosis of human gallbladder cancer cells. J Cell Mol Med. https://doi.org/10.1111/jcmm.13499
He S et al (2020) HERG channel and cancer: a mechanistic review of carcinogenic processes and therapeutic potential. Biochim Biophys Acta Rev Cancer. https://doi.org/10.1016/j.bbcan.2020.188355
Hemmerlein B et al (2006) Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer. https://doi.org/10.1186/1476-4598-5-41
Hirschler-Laszkiewicz I et al (2018) The human ion channel TRPM2 modulates neuroblastoma cell survival and mitochondrial function through Pyk2, CREB, and MCU activation. Am J Physiol Cell Physiol. https://doi.org/10.1152/ajpcell.00098.2018
Hoa NT et al (2014) Small cell lung cancer cells express the late stage gBK tumor antigen: a possible immunotarget for the terminal disease. Am J Transl Res. https://doi.org/10.1158/1538-7445.am2014-2895
Hoelder S, Clarke PA, Workman P (2012) Discovery of small molecule cancer drugs: successes, challenges and opportunities. Mol Oncol. https://doi.org/10.1016/j.molonc.2012.02.004
Holdhoff M et al (2017) Timed sequential therapy of the selective T-type calcium channel blocker mibefradil and temozolomide in patients with recurrent high-grade gliomas. Neuro Oncol. https://doi.org/10.1093/neuonc/nox020
Hopkins MM et al (2015) Inhibition of the transient receptor potential melastatin-2 channel causes increased DNA damage and decreased proliferation in breast adenocarcinoma cells. Int J Oncol. https://doi.org/10.3892/ijo.2015.2919
Hu T et al (2019) Expression and function of Kv1.3 channel in malignant T cells in Sézary syndrome. Oncotarget. https://doi.org/10.18632/oncotarget.27122
Huang X, Jan LY (2014) Targeting potassium channels in cancer. J Cell Biol. https://doi.org/10.1083/jcb.201404136
Huang L et al (2009) ATP-sensitive potassium channels control glioma cells proliferation by regulating ERK activity. Carcinogenesis. https://doi.org/10.1093/carcin/bgp034
Huang C et al (2017) Downregulation of a novel long noncoding RNA TRPM2-AS promotes apoptosis in non–small cell lung cancer. Tumor Biol. https://doi.org/10.1177/1010428317691191
Huber KR et al (1989) Effect of verapamil on cell cycle transit and c-myc gene expression in normal and malignant murine cells. Br J Cancer. https://doi.org/10.1038/bjc.1989.150
Iorio J et al (2018) hERG1 channel expression associates with molecular subtypes and prognosis in breast cancer. Cancer Cell Int. https://doi.org/10.1186/s12935-018-0592-1
Iorio J et al (2020) hERG1 and HIF-2α behave as biomarkers of positive response to bevacizumab in metastatic colorectal cancer patients. Transl Oncol. https://doi.org/10.1016/j.tranon.2020.01.001
Ishikawa M et al (2000) Reversal of acquired resistance to doxorubicin in K562 human leukemia cells by astemizole. Biol Pharm Bull. https://doi.org/10.1248/bpb.23.112
Izquierdo-Torres E et al (2017) ATP2A3 gene as an important player for resveratrol anticancer activity in breast cancer cells. Mol Carcinog. https://doi.org/10.1002/mc.22625
Izumi-Nakaseko H et al (2016) Possibility as an anti-cancer drug of astemizole: evaluation of arrhythmogenicity by the chronic atrioventricular block canine model. J Pharmacol Sci. https://doi.org/10.1016/j.jphs.2016.04.024
Jahchan NS et al (2013) A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-13-0183
Jang SH et al (2011) Anti-proliferative effect of Kv1.3 blockers in A549 human lung adenocarcinoma in vitro and in vivo. Eur J Pharmacol. https://doi.org/10.1016/j.ejphar.2010.10.066
Jardin I et al (2018) Trpc6 channels are required for proliferation, migration and invasion of breast cancer cell lines by modulation of orai1 and orai3 surface exposure. Cancer. https://doi.org/10.3390/cancers10090331
Jehle J et al (2011) Novel roles for hERG K(+) channels in cell proliferation and apoptosis. Cell Death Dis. https://doi.org/10.1038/cddis.2011.77
Jensen RL, Wurster RD (2001) Calcium channel antagonists inhibit growth of subcutaneous xenograft meningiomas in nude mice. Surg Neurol. https://doi.org/10.1016/s0090-3019(01)00444-x
Jimenez-Luevano MA et al (2018) Treatment of hepatocarcinoma with celecoxib and pentoxifylline: a case report. Rev Med Inst Mex Seguro Soc
Jin M et al (2019a) MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. https://doi.org/10.1186/s13046-019-1135-x
Jin YH et al (2019b) Efficacy of erlotinib and celecoxib for patients with advanced non-small cell lung cancer: a retrospective study. Medicine. https://doi.org/10.1097/MD.0000000000014785
June CH et al (2018) CAR T cell immunotherapy for human cancer. Science. https://doi.org/10.1126/science.aar6711
Kale VP, Amin SG, Pandey MK (2015) Targeting ion channels for cancer therapy by repurposing the approved drugs. Biochim Biophys Acta. https://doi.org/10.1016/j.bbamem.2015.03.034
Keir ST et al (2013) Mibefradil, a novel therapy for glioblastoma multiforme: cell cycle synchronization and interlaced therapy in a murine model. J Neurooncol. https://doi.org/10.1007/s11060-012-0995-0
Khaitan D et al (2009) Role of KCNMA1 gene in breast cancer invasion and metastasis to brain. BMC Cancer. https://doi.org/10.1186/1471-2407-9-258
Khalil DN et al (2016) The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. https://doi.org/10.1038/nrclinonc.2016.25
Kischel P et al (2019) Ion channels: new actors playing in chemotherapeutic resistance. Cancer. https://doi.org/10.3390/cancers11030376
Klein EA et al (2011) Vitamin E and the risk of prostate cancer: the selenium and vitamin E cancer prevention trial (SELECT). JAMA. https://doi.org/10.1001/jama.2011.1437
Klingenberg M et al (2014) The NADPH oxidase inhibitor imipramine-blue in the treatment of Burkitt lymphoma. Mol Cancer Ther. https://doi.org/10.1158/1535-7163.MCT-13-0688
Koh DW et al (2015) Enhanced cytotoxicity in triple-negative and estrogen receptor-positive breast adenocarcinoma cells due to inhibition of the transient receptor potential melastatin-2 channel. Oncol Rep. https://doi.org/10.3892/or.2015.4131
Kondo S et al (1995) Combination therapy with cisplatin and nifedipine induces apoptosis in cisplatin-sensitive and cisplatin-resistant human glioblastoma cells. Br J Cancer. https://doi.org/10.1038/bjc.1995.57
Kong Q, Beel JA, Lillehei KO (2000) A threshold concept for cancer therapy. Med Hypotheses. https://doi.org/10.1054/mehy.1999.0982
Koppula P et al (2017) The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J Biol Chem. https://doi.org/10.1074/jbc.M117.798405
Korošec B et al (2006) Alterations in the ATP2A2 gene in correlation with colon and lung cancer. Cancer Genet Cytogenet. https://doi.org/10.1016/j.cancergencyto.2006.06.016
Koşar PA et al (2016) Synergic effects of doxorubicin and melatonin on apoptosis and mitochondrial oxidative stress in MCF-7 breast cancer cells: involvement of TRPV1 channels. J Membr Biol. https://doi.org/10.1007/s00232-015-9855-0
Koshy S et al (2013) Blocking KCa3.1 channels increases tumor cell killing by a subpopulation of human natural killer lymphocytes. PLoS One. https://doi.org/10.1371/journal.pone.0076740
Kühn FJP, Heiner I, Lückhoff A (2005) TRPM2: a calcium influx pathway regulated by oxidative stress and the novel second messenger ADP-ribose. Pflugers Arch Eur J Physiol. https://doi.org/10.1007/s00424-005-1446-y
Laidlaw KM et al (2016) Cooperation of imipramine blue and tyrosine kinase blockade demonstrates activity against chronic myeloid leukemia. Oncotarget. https://doi.org/10.18632/oncotarget.10541
Lam J, Wulff H (2011) The lymphocyte potassium channels Kv1.3 and KCa3.1 as targets for immunosuppression. Drug Dev Res. https://doi.org/10.1002/ddr.20467
Lange I et al (2009) TRPM2 functions as a lysosomal Ca2+-release channel in cells. Sci Signal. https://doi.org/10.1126/scisignal.2000278
Lansu K, Gentile S (2013) Potassium channel activation inhibits proliferation of breast cancer cells by activating a senescence program. Cell Death Dis. https://doi.org/10.1038/cddis.2013.174
Lastraioli E et al (2015a) hERG1 potassium channels: novel biomarkers in human solid cancers. Biomed Res Int. https://doi.org/10.1155/2015/896432
Lastraioli E et al (2015b) hERG1 channels drive tumour malignancy and may serve as prognostic factor in pancreatic ductal adenocarcinoma. Br J Cancer. https://doi.org/10.1038/bjc.2015.28
Lastraioli E et al (2019) The hERG1 potassium channel behaves as prognostic factor in gastric dysplasia endoscopic samples. Onco Targets Ther. https://doi.org/10.2147/OTT.S226257
Le Gal K et al (2015) Antioxidants can increase melanoma metastasis in mice. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aad3740
Leanza L et al (2013) Clofazimine, Psora-4 and PAP-1, inhibitors of the potassium channel Kv1.3, as a new and selective therapeutic strategy in chronic lymphocytic leukemia. Leukemia. https://doi.org/10.1038/leu.2013.56
Leanza L et al (2017) Direct pharmacological targeting of a mitochondrial ion channel selectively kills tumor cells in vivo. Cancer Cell. https://doi.org/10.1016/j.ccell.2017.03.003
Leanza L et al (2018) Pharmacological modulation of mitochondrial ion channels. Br J Pharmacol. https://doi.org/10.1111/bph.14544
Lee H, Kang S, Kim W (2016) Drug repositioning for cancer therapy based on large-scale drug-induced transcriptional signatures. PLoS One. https://doi.org/10.1371/journal.pone.0150460
Lee JR et al (2019) The inhibition of chloride intracellular channel 1 enhances Ca2+ and reactive oxygen species signaling in A549 human lung cancer cells. Exp Mol Med. https://doi.org/10.1038/s12276-019-0279-2
Lewerenz J et al (2013) The cystine/glutamate antiporter system xc- in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal. https://doi.org/10.1089/ars.2011.4391
Lim JKM et al (2019) Cystine/glutamate antiporter xCT (SLC7A11) facilitates oncogenic RAS transformation by preserving intracellular redox balance. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1821323116
Lis A et al (2007) CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol. https://doi.org/10.1016/j.cub.2007.03.065
Lissoni P et al (2002) A clinical study of taxotere versus taxotere plus the antiprolactinemic agent bromocriptine in metastatic breast cancer pretreated with anthracyclines. Anticancer Res 22(2B):1131–1134
Litan A, Langhans SA (2015) Cancer as a channelopathy: ion channels and pumps in tumor development and progression. Front Cell Neurosci. https://doi.org/10.3389/fncel.2015.00086
Littler DR et al (2004) The intracellular chloride ion channel protein CLIC1 undergoes a redox-controlled structural transition. J Biol Chem. https://doi.org/10.1074/jbc.M308444200
Liu S, Cheng C (2013) Alternative RNA splicing and cancer. Wiley Interdiscip Rev RNA. https://doi.org/10.1002/wrna.1178
Liu X et al (2002) Cloning and characterization of glioma BK, a novel BK channel isoform highly expressed in human glioma cells. J Neurosci. https://doi.org/10.1523/jneurosci.22-05-01840.2002
Liu GX et al (2015) Expression of eag1 channel associated with the aggressive clinicopathological features and subtype of breast cancer. Int J Clin Exp Pathol 8(11):15093–15099
Liubomirski Y et al (2019) Tumor-stroma-inflammation networks promote pro-metastatic chemokines and aggressiveness characteristics in triple-negative breast cancer. Front Immunol. https://doi.org/10.3389/fimmu.2019.00757
Lo M et al (2010) Potential use of the anti-inflammatory drug, sulfasalazine, for targeted therapy of pancreatic cancer. Curr Oncol. https://doi.org/10.3747/co.v17i3.485
Lörinczi E et al (2016) Calmodulin regulates human ether à Go-Go 1 (hEAG1) potassium channels through interactions of the Eag domain with the cyclic nucleotide binding homology domain. J Biol Chem. https://doi.org/10.1074/jbc.M116.733576
Loughlin KR (2014) Calcium channel blockers and prostate cancer. Urol Oncol. https://doi.org/10.1016/j.urolonc.2013.08.001
Luzzi KJ et al (1998) Inhibition of angiogenesis in liver metastases by carboxyamidotriazole (CAI). Angiogenesis. https://doi.org/10.1023/A:1009259521092
Lyons SA, O’Neal J, Sontheimer H (2002) Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia. https://doi.org/10.1002/glia.10083
Ma X et al (2012) Transient receptor potential channel TRPC5 is essential for P-glycoprotein induction in drug-resistant cancer cells. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1202989109
Ma X et al (2014) Essential role for TrpC5-containing extracellular vesicles in breast cancer with chemotherapeutic resistance. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1400272111
Ma L et al (2018) Novel venom-derived inhibitors of the human EAG channel, a putative antiepileptic drug target. Biochem Pharmacol. https://doi.org/10.1016/j.bcp.2018.08.038
Mahalingam D et al (2019) A phase II, multicenter, single-arm study of mipsagargin (G-202) as a second-line therapy following sorafenib for adult patients with progressive advanced hepatocellular carcinoma. Cancer. https://doi.org/10.3390/cancers11060833
Mailloux RJ, Adjeitey CNK, Harper ME (2010) Genipin-induced inhibition of uncoupling protein-2 sensitizes drug-resistant cancer cells to cytotoxic agents. PLoS One. https://doi.org/10.1371/journal.pone.0013289
Maldonado EN et al (2013) Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells: regulation by free tubulin and erastin. J Biol Chem. https://doi.org/10.1074/jbc.M112.433847
Malhi H et al (2000) KATP channels regulate mitogenically induced proliferation in primary rat hepatocytes and human liver cell lines. Implications for liver growth control and potential therapeutic targeting. J Biol Chem. https://doi.org/10.1074/jbc.M001576200
Mammucari C, Gherardi G, Rizzuto R (2017) Structure, activity regulation, and role of the mitochondrial calcium uniporter in health and disease. Front Oncol. https://doi.org/10.3389/fonc.2017.00139
Mancini M, Toker A (2009) NFAT proteins: emerging roles in cancer progression. Nat Rev Cancer 9(11):810–820. https://doi.org/10.1038/nrc2735
Manoli S et al (2019) The activity of Kv11.1 potassium channel modulates F-actin organization during cell migration of pancreatic ductal adenocarcinoma cells. Cancer 11(2). pii: E135. https://doi.org/10.3390/cancers11020135
Martinez R et al (2015) Analysis of the expression of Kv10.1 potassium channel in patients with brain metastases and glioblastoma multiforme: impact on survival. BMC Cancer. https://doi.org/10.1186/s12885-015-1848-y
Martinez-Delgado G, Felix R (2017) Emerging role of CaV1.2 channels in proliferation and migration in distinct cancer cell lines. Oncology. https://doi.org/10.1159/000464293
Mazure NM (2017) VDAC in cancer. Biochim Biophys Acta Bioenerg. https://doi.org/10.1016/j.bbabio.2017.03.002
McFerrin MB, Sontheimer H (2006) A role for ion channels in glioma cell invasion. Neuron Glia Biol. https://doi.org/10.1017/S17440925X06000044
Meng H et al (2010) Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano. https://doi.org/10.1021/nn100690m
Meng H et al (2013) Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano. https://doi.org/10.1021/nn3044066
Metts J et al (2017) Imipramine blue sensitively and selectively targets FLT3-ITD positive acute myeloid leukemia cells. Sci Rep. https://doi.org/10.1038/s41598-017-04796-1
Miller BA, Zhang W (2011) TRP channels as mediators of oxidative stress. Adv Exp Med Biol 704:531–544. https://doi.org/10.1007/978-94-007-0265-3_29
Miller BA et al (2014) TRPM2 channels protect against cardiac ischemia-reperfusion injury: role of mitochondria. J Biol Chem. https://doi.org/10.1074/jbc.M113.533851
Millward MJ et al (1993) Oral verapamil with chemotherapy for advanced non-small cell lung cancer: a randomised study. Br J Cancer. https://doi.org/10.1038/bjc.1993.189
Mohr CJ et al (2019) Cancer-associated intermediate conductance Ca 2+-activated K + channel K Ca 3.1. Cancer. https://doi.org/10.3390/cancers11010109
Monen SH, Schmidt PH, Wondergem R (1998) Membrane potassium channels and human bladder tumor cells. I. Electrical properties. J Membr Biol. https://doi.org/10.1007/s002329900331
Moreels L, Peigneur S, Galan DT et al (2017a) APETx4, a novel sea anemone toxin and a modulator of the cancer-relevant potassium channel KV10.1. Mar Drugs. https://doi.org/10.3390/md15090287
Moreels L, Peigneur S, Yamaguchi Y et al (2017b) Expanding the pharmacological profile of kappa-hefutoxin 1 and analogues: a focus on the inhibitory effect on the oncogenic channel Kv10.1. Peptides. https://doi.org/10.1016/j.peptides.2016.08.008
Mukhopadhyay I et al (2011) Expression of functional TRPA1 receptor on human lung fibroblast and epithelial cells. J Recept Signal Transduct Res. https://doi.org/10.3109/10799893.2011.602413
Munson JM et al (2012) Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma. Sci Transl Med. https://doi.org/10.1126/scitranslmed.3003016
Muscella A et al (2011) The platinum (II) complex [Pt(O,O’-acac)(γ-acac)(DMS)] alters the intracellular calcium homeostasis in MCF-7 breast cancer cells. Biochem Pharmacol 81(1):91–103. https://doi.org/10.1016/j.bcp.2010.09.012
Neuhaus E et al (2019) Alternating electric fields (TTFields) activate Cav1.2 channels in human glioblastoma cells. Cancers (Basel) 11(1). https://doi.org/10.3390/cancers11010110
Nielsen N, Lindemann O, Schwab A (2014) TRP channels and STIM/ORAI proteins: sensors and effectors of cancer and stroma cell migration. Br J Pharmacol. https://doi.org/10.1111/bph.12721
Noh J et al (2015) Amplification of oxidative stress by a dual stimuli-responsive hybrid drug enhances cancer cell death. Nat Commun. https://doi.org/10.1038/ncomms7907
Núñez M et al (2013) Glibenclamide inhibits cell growth by inducing G0/G1 arrest in the human breast cancer cell line MDA-MB-231. BMC Pharmacol Toxicol. https://doi.org/10.1186/2050-6511-14-6
Nur G, Nazıroğlu M, Deveci HA (2017) Synergic prooxidant, apoptotic and TRPV1 channel activator effects of alpha-lipoic acid and cisplatin in MCF-7 breast cancer cells. J Recept Signal Transduct Res. https://doi.org/10.1080/10799893.2017.1369121
Omuro A et al (2018) Multicenter phase IB trial of carboxyamidotriazole orotate and temozolomide for recurrent and newly diagnosed glioblastoma and other anaplastic gliomas. J Clin Oncol 36(17):1702–1709. https://doi.org/10.1200/JCO.2017.76.9992
Onoda JM et al (1988) Cisplatin and nifedipine: synergistic antitumor effects against an inherently cisplatin-resistant tumor. Cancer Lett. https://doi.org/10.1016/0304-3835(88)90260-1
Oprea TI et al (2011) Drug repurposing from an academic perspective. Drug Discov Today Ther Strateg 8(3–4):61–69. https://doi.org/10.1016/j.ddstr.2011.10.002
Orfanelli U et al (2008) Identification of novel sense and antisense transcription at the TRPM2 locus in cancer. Cell Res. https://doi.org/10.1038/cr.2008.296
Orfanelli U et al (2014) Antisense transcription at the TRPM2 locus as a novel prognostic marker and therapeutic target in prostate cancer. Oncogene. https://doi.org/10.1038/onc.2014.144
Ortiz CS et al (2011) Eag1 potassium channels as markers of cervical dysplasia. Oncol Rep. https://doi.org/10.3892/or.2011.1441
Ostroumov D et al (2018) CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci. https://doi.org/10.1007/s00018-017-2686-7
Ouadid-Ahidouch H et al (2001) Changes in the K+ current-density of MCF-7 cells during progression through the cell cycle: possible involvement of a h-ether.a-gogo K+ channel. Receptors Channels
Ouadid-Ahidouch H, Ahidouch A, Pardo LA (2016) Kv10.1 K+ channel: from physiology to cancer. Pflugers Arch 468(5):751–762. https://doi.org/10.1007/s00424-015-1784-3
Özdemir ÜS et al (2016) Hypericum perforatum attenuates spinal cord injury-induced oxidative stress and apoptosis in the dorsal root ganglion of rats: involvement of TRPM2 and TRPV1 channels. Mol Neurobiol 53(6):3540–3551. https://doi.org/10.1007/s12035-015-9292-1
Paakkari I (2002) Cardiotoxicity of new antihistamines and cisapride. Toxicol Lett 127(1–3):279–284. https://doi.org/10.1016/s0378-4274(01)00510-0
Pantziarka P et al (2014) The repurposing drugs in oncology (ReDO) project. Ecancermedicalscience 8:442. https://doi.org/10.3332/ecancer.2014.442
Panyi G, Beeton C, Felipe A (2014) Ion channels and anti-cancer immunity. Philos Trans R Soc Lond B Biol Sci. https://doi.org/10.1098/rstb.2013.0106
Papp B, Brouland JP (2011) Altered endoplasmic reticulum calcium pump expression during breast tumorigenesis. Breast Cancer: Basic Clin Res. https://doi.org/10.4137/BCBCR.S7481
Pardo LA, Stühmer W (2008) Eag1: an emerging oncological target. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-07-5710
Pardo LA et al (1999) Oncogenic potential of EAG K(+) channels. EMBO J. https://doi.org/10.1093/emboj/18.20.5540
Payen L et al (2001) The sulphonylurea glibenclamide inhibits multidrug resistance protein (MRP1) activity in human lung cancer cells. Br J Pharmacol. https://doi.org/10.1038/sj.bjp.0703863
Pennington MW, Czerwinski A, Norton RS (2018) Peptide therapeutics from venom: current status and potential. Bioorg Med Chem. https://doi.org/10.1016/j.bmc.2017.09.029
Peretti M et al (2015) Chloride channels in cancer: focus on chloride intracellular channel 1 and 4 (CLIC1 AND CLIC4) proteins in tumor development and as novel therapeutic targets. Biochim Biophys Acta. https://doi.org/10.1016/j.bbamem.2014.12.012
Peretti M et al (2019) Original association of ion transporters mediates the ECM-induced breast cancer cell survival: Kv10.1-Orai1-SPCA2 partnership. Sci Rep. https://doi.org/10.1038/s41598-018-37602-7
Perez-Neut M et al (2016) Activation of hERG3 channel stimulates autophagy and promotes cellular senescence in melanoma. Oncotarget 7(16):21991–22004. https://doi.org/10.18632/oncotarget.7831
Peters DH, Clissold SP (1992) Chlarythromicin. a review of its antimicrobial, pharmacokinetic properties and therapeutic potential. Drugs. https://doi.org/10.2165/00003495-199244010-00009
Petroni G et al (2020) Clarithromycin inhibits autophagy in colorectal cancer by regulating the hERG1 potassium channel interaction with PI3K. Cell Death Dis. https://doi.org/10.1038/s41419-020-2349-8
Petrova DT et al (2008) Expression of chloride intracellular channel protein 1 (CLIC1) and tumor protein D52 (TPD52) as potential biomarkers for colorectal cancer. Clin Biochem. https://doi.org/10.1016/j.clinbiochem.2008.07.012
Piskounova E et al (2015) Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527(7577):186–191. https://doi.org/10.1038/nature15726
Pointer KB et al (2017) Administration of non-torsadogenic human ether-a-go-go-related gene inhibitors is associated with better survival for high hERG-expressing glioblastoma patients. Clin Cancer Res 23(1):73–80. https://doi.org/10.1158/1078-0432.CCR-15-3169
Pons DG et al (2015) UCP2 inhibition sensitizes breast cancer cells to therapeutic agents by increasing oxidative stress. Free Radic Biol Med 86:67–77. https://doi.org/10.1016/j.freeradbiomed.2015.04.032
Poprac P et al (2017) Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci 38(7):592–607. https://doi.org/10.1016/j.tips.2017.04.005
Porporato PE et al (2014) A mitochondrial switch promotes tumor metastasis. Cell Rep 8(3):754–766. https://doi.org/10.1016/j.celrep.2014.06.043
Prasad S, Gupta SC, Tyagi AK (2017) Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Cancer Lett 387:95–105. https://doi.org/10.1016/j.canlet.2016.03.042
Qian X et al (2008) Glibenclamide exerts an antitumor activity through reactive oxygen species-c-jun NH2-terminal kinase pathway in human gastric cancer cell line MGC-803. Biochem Pharmacol. https://doi.org/10.1016/j.bcp.2008.09.009
Queiroz FM et al (2006) Ether a go-go potassium channel expression in soft tissue sarcoma patients. Mol Cancer. https://doi.org/10.1186/1476-4598-5-42
Rajamanickam S et al (2016) Inhibition of FoxM1-mediated DNA repair by imipramine blue suppresses breast cancer growth and metastasis. Clin Cancer Res 22(14):3524–3536. https://doi.org/10.1158/1078-0432.CCR-15-2535
Ramírez A et al (2016) Ion channels and oxidative stress as a potential link for the diagnosis or treatment of liver diseases. Oxid Med Cell Longev 2016:3928714. https://doi.org/10.1155/2016/3928714
Ramírez A et al (2018) Calcium-activated potassium channels as potential early markers of human cervical cancer. Oncol Lett. https://doi.org/10.3892/ol.2018.8187
Ramos Gomes F et al (2015) Alternatively spliced isoforms of KV10.1 potassium channels modulate channel properties and can activate cyclin-dependent kinase in Xenopus oocytes. J Biol Chem. https://doi.org/10.1074/jbc.M115.668749
Rani V et al (2016) Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci. https://doi.org/10.1016/j.lfs.2016.02.002
Rasola A, Bernardi P (2014) The mitochondrial permeability transition pore and its adaptive responses in tumor cells. Cell Calcium 56(6):437–445. https://doi.org/10.1016/j.ceca.2014.10.003
Rezania S et al (2016) Overexpression of KCNJ3 gene splice variants affects vital parameters of the malignant breast cancer cell line MCF-7 in an opposing manner. BMC Cancer. https://doi.org/10.1186/s12885-016-2664-8
Robe PA et al (2009) Early termination of ISRCTN45828668, a phase 1/2 prospective, randomized study of sulfasalazine for the treatment of progressing malignant gliomas in adults. BMC Cancer. https://doi.org/10.1186/1471-2407-9-372
Roderick HL, Cook SJ (2008) Ca2+ signalling checkpoints in cancer: remodelling Ca 2+ for cancer cell proliferation and survival. Nat Rev Cancer. https://doi.org/10.1038/nrc2374
Rong Z et al (2013) Combined treatment of glibenclamide and CoCl2 decreases MMP9 expression and inhibits growth in highly metastatic breast cancer. J Exp Clin Cancer Res. https://doi.org/10.1186/1756-9966-32-32
Roy J et al (2008) Pharmacological separation of hEAG and hERG K+ channel function in the human mammary carcinoma cell line MCF-7. Oncol Rep. https://doi.org/10.3892/or.19.6.1511
Rybalchenko V et al (2001) Verapamil inhibits proliferation of LNCaP human prostate cancer cells influencing K+ channel gating. Mol Pharmacol. https://doi.org/10.1124/mol.59.6.1376
Sakallı ÇE et al (2017) Selenium potentiates the anticancer effect of cisplatin against oxidative stress and calcium ion signaling-induced intracellular toxicity in MCF-7 breast cancer cells: involvement of the TRPV1 channel. J Recept Signal Transduct Res. https://doi.org/10.3109/10799893.2016.1160931
Santo-Domingo J et al (2007) The plasma membrane Na +/Ca 2+ exchange inhibitor KB-R7943 is also a potent inhibitor of the mitochondrial Ca2+ uniporter. Br J Pharmacol. https://doi.org/10.1038/sj.bjp.0707260
Sayin VI et al (2014) Cancer: antioxidants accelerate lung cancer progression in mice. Sci Transl Med. https://doi.org/10.1126/scitranslmed.3007653
Schaefer EAM et al (2013) Stimulation of the chemosensory TRPA1 cation channel by volatile toxic substances promotes cell survival of small cell lung cancer cells. Biochem Pharmacol. https://doi.org/10.1016/j.bcp.2012.11.019
Schmidt WF et al (1988) Antiproliferative effect of verapamil alone on brain tumor cells in vitro. Cancer Res 48(13):3617–3621
Schwab A et al (1999) K(+) channel-dependent migration of fibroblasts and human melanoma cells. Cell Physiol Biochem. https://doi.org/10.1159/000016309
Segovia-Mendoza M et al (2015) Calcitriol and its analogues enhance the antiproliferative activity of gefitinib in breast cancer cells. J Steroid Biochem Mol Biol. https://doi.org/10.1016/j.jsbmb.2014.12.006
Segovia-Mendoza M et al (2017) The addition of calcitriol or its synthetic analog EB1089 to lapatinib and neratinib treatment inhibits cell growth and promotes apoptosis in breast cancer cells. Am J Cancer Res 7(7):1486–1500
Sehgal P et al (2017) Inhibition of the sarco/endoplasmic reticulum (ER) Ca2+-ATPase by thapsigargin analogs induces cell death via ER Ca2+ depletion and the unfolded protein response. J Biol Chem. https://doi.org/10.1074/jbc.M117.796920
Sehm T et al (2016) Temozolomide toxicity operates in a xCT/SLC7a11 dependent manner and is fostered by ferroptosis. Oncotarget. https://doi.org/10.18632/oncotarget.11858
Seo J et al (2016) Curcumin induces apoptosis by inhibiting sarco/endoplasmic reticulum Ca2+ ATPase activity in ovarian cancer cells. Cancer Lett. https://doi.org/10.1016/j.canlet.2015.11.021
Seo EJ et al (2018) Repurposing of bromocriptine for cancer therapy. Front Pharmacol. https://doi.org/10.3389/fphar.2018.01030
Serrano-Novillo C et al (2019) Implication of voltage-gated potassium channels in neoplastic cell proliferation. Cancers (Basel). https://doi.org/10.3390/cancers11030287
Sette A et al (2013) Development of novel anti-Kv 11.1 antibody-conjugated PEG-TiO2 nanoparticles for targeting pancreatic ductal adenocarcinoma cells. J Nanopart Res. https://doi.org/10.1007/s11051-013-2111-6
Shankar DB et al (2005) The role of CREB as a proto-oncogene in hematopoiesis and in acute myeloid leukemia. Cancer Cell. https://doi.org/10.1016/j.ccr.2005.02.018
Shanmugam MK et al (2018) Potential role of genipin in cancer therapy. Pharmacol Res. https://doi.org/10.1016/j.phrs.2018.05.007
Sharma A et al (2019) Causal roles of mitochondrial dynamics in longevity and healthy aging. EMBO Rep. https://doi.org/10.15252/embr.201948395
Shitara K et al (2017) Dose-escalation study for the targeting of CD44v+ cancer stem cells by sulfasalazine in patients with advanced gastric cancer (EPOC1205). Gastric Cancer. https://doi.org/10.1007/s10120-016-0610-8
Shoshan-Barmatz V, Ben-Hail D (2012) VDAC, a multi-functional mitochondrial protein as a pharmacological target. Mitochondrion. https://doi.org/10.1016/j.mito.2011.04.001
Silva R et al (2015) Modulation of P-glycoprotein efflux pump: induction and activation as a therapeutic strategy. Pharmacol Ther. https://doi.org/10.1016/j.pharmthera.2014.11.013
Simon F, Varela D, Cabello-Verrugio C (2013) Oxidative stress-modulated TRPM ion channels in cell dysfunction and pathological conditions in humans. Cell Signal. https://doi.org/10.1016/j.cellsig.2013.03.023
SoRelle R (1998) Withdrawal of Posicor from market. Circulation. https://doi.org/10.1161/01.cir.98.9.831
Srairi-Abid N et al (2005) A new type of scorpion Na+-channel-toxin-like polypeptide active on K+ channels. Biochem J. https://doi.org/10.1042/BJ20041407
Srairi-Abid N et al (2019) Anti-tumoral effect of scorpion peptides: emerging new cellular targets and signaling pathways. Cell Calcium. https://doi.org/10.1016/j.ceca.2019.05.003
Stringer BK, Cooper AG, Shepard SB (2001) Overexpression of the G-protein inwardly rectifying potassium channel 1 (GIRK1) in primary breast carcinomas correlates with axillary lymph node metastasis. Cancer Res
Sucu BO et al (2019) Synthesis of novel methyl jasmonate derivatives and evaluation of their biological activity in various cancer cell lines. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2019.103146
Suessbrich H et al (1996) Blockade of HERG channels expressed in Xenopus oocytes by the histamine receptor antagonists terfenadine and astemizole. FEBS Lett. https://doi.org/10.1016/0014-5793(96)00355-9
Suh DH et al (2013) Mitochondrial permeability transition pore as a selective target for anti-cancer therapy. Front Oncol. https://doi.org/10.3389/fonc.2013.00041
Sullivan LB, Gui DY, Van Der Heiden MG (2016) Altered metabolite levels in cancer: Implications for tumour biology and cancer therapy. Nat Rev Cancer. https://doi.org/10.1038/nrc.2016.85
Sumoza-Toledo A et al (2011) Dendritic cell maturation and chemotaxis is regulated by TRPM2-mediated lysosomal Ca 2+ release. FASEB J. https://doi.org/10.1096/fj.10-178483
Sun C, Veiseh O et al (2008a) In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small. https://doi.org/10.1002/smll.200700784
Sun C, Fang C et al (2008b) Tumor-targeted drug delivery and MRI contrast enhancement by chlorotoxin-conjugated iron oxide nanoparticles. Nanomedicine (Lond). https://doi.org/10.2217/17435889.3.4.495
Suo A et al (2016) Comb-like amphiphilic polypeptide-based copolymer nanomicelles for co-delivery of doxorubicin and P-gp siRNA into MCF-7 cells. Korean J Couns Psychother. https://doi.org/10.1016/j.msec.2016.02.007
Susankova K et al (2006) Reducing and oxidizing agents sensitize heat-activated vanilloid receptor (TRPV1) current. Mol Pharmacol. https://doi.org/10.1124/mol.106.023069
Suzuki Y et al (2012) Depolarization potentiates TRAIL-induced apoptosis in human melanoma cells: role for ATP-sensitive K+ channels and endoplasmic reticulum stress. Int J Oncol. https://doi.org/10.3892/ijo.2012.1483
Szabó I et al (2008) Mitochondrial potassium channel Kv1.3 mediates Bax-induced apoptosis in lymphocytes. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.0804236105
Takahashi N, Mori Y (2011) TRP channels as sensors and signal integrators of redox status changes. Front Pharmacol. https://doi.org/10.3389/fphar.2011.00058
Takahashi N et al (2018) Cancer cells co-opt the neuronal redox-sensing channel TRPA1 to promote oxidative-stress tolerance. Cancer Cell. https://doi.org/10.1016/j.ccell.2018.05.001
Takeuchi S et al (2014) Sulfasalazine and temozolomide with radiation therapy for newly diagnosed glioblastoma. Neurol India. https://doi.org/10.4103/0028-3886.128280
Tang S et al (2015) Mitochondrial Ca 2+ uniporter is critical for store-operated Ca 2+ entry-dependent breast cancer cell migration. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2015.01.092
Taylor JM, Simpson RU (1992) Inhibition of cancer cell growth by calcium channel antagonists in the athymic mouse. Cancer Res 52(9)
Taylor JT et al (2008) Calcium signaling and T-type calcium channels in cancer cell cycling. World J Gastroenterol. https://doi.org/10.3748/wjg.14.4984
Teisseyre A, Gasiorowska J, Michalak K (2015) Voltage-gated potassium channels Kv1.3--potentially new molecular target in cancer diagnostics and therapy. Adv Clin Exp Med. https://doi.org/10.17219/acem/22339
Teschemacher AG et al (1999) Inhibition of the current of heterologously expressed HERG potassium channels by imipramine and amitriptyline. Br J Pharmacol. https://doi.org/10.1038/sj.bjp.0702800
Tochhawng L et al (2013) Redox regulation of cancer cell migration and invasion. Mitochondrion. https://doi.org/10.1016/j.mito.2012.08.002
Togashi K et al (2006) TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. https://doi.org/10.1038/sj.emboj.7601083
Toloczko-Iwaniuk N et al (2019) Celecoxib in cancer therapy and prevention – review. Curr Drug Targets. https://doi.org/10.2174/1389450119666180803121737
Tong L et al (2015) Reactive oxygen species in redox cancer therapy. Cancer Lett. https://doi.org/10.1016/j.canlet.2015.07.008
Tosatto A et al (2016) The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1α. EMBO Mol Med. https://doi.org/10.15252/emmm.201606255
Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. https://doi.org/10.1038/nrd2803
Trevisan G et al (2013) Novel therapeutic strategy to prevent chemotherapy-induced persistent sensory neuropathy by TRPA1 blockade. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-12-4370
Varghese E et al (2019) Anti-cancer agents in proliferation and cell death: the calcium connection. Int J Mol Sci. https://doi.org/10.3390/ijms20123017
Vazquez-Sanchez AY et al (2018) Expression of KATP channels in human cervical cancer: potential tools for diagnosis and therapy. Oncol Lett. https://doi.org/10.3892/ol.2018.8165
Veiseh O et al (2005) Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett. https://doi.org/10.1021/nl0502569
Veiseh M et al (2007) Tumor paint: a chlorotoxin: Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-06-3948
Veiseh O et al (2009) Inhibition of tumor-cell invasion with chlorotoxin-bound superparamagnetic nanoparticles. Small. https://doi.org/10.1002/smll.200800646
Veiseh O et al (2010) Chlorotoxin bound magnetic nanovector tailored for cancer cell targeting, imaging, and siRNA delivery. Biomaterials. https://doi.org/10.1016/j.biomaterials.2010.07.016
Verdoodt F et al (2019) Antihistamines and ovarian cancer survival: nationwide cohort study and in vitro cell viability assay. J Natl Cancer Inst. pii: djz217. https://doi.org/10.1093/jnci/djz217
Vermeer LM et al (2016) Evaluation of ketoconazole and its alternative clinical CYP3A4/5 inhibitors as inhibitors of drug transporters: the in vitro effects of ketoconazole, ritonavir, clarithromycin, and itraconazole on 13 clinically-relevant drug transporters. Drug Metab Dispos. https://doi.org/10.1124/dmd.115.067744
Vilema-Enríquez G et al (2016) Molecular and cellular effects of hydrogen peroxide on human lung cancer cells: potential therapeutic implications. Oxid Med Cell Longev. https://doi.org/10.1155/2016/1908164
Vinay DS et al (2015) Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2015.03.004
Vultur A et al (2018) The role of the mitochondrial calcium uniporter (MCU) complex in cancer. Pflugers Arch Eur J Physiol. https://doi.org/10.1007/s00424-018-2162-8
Wagner V et al (2010) Cloning and characterisation of GIRK1 variants resulting from alternative RNA editing of the KCNJ3 gene transcript in a human breast cancer cell line. J Cell Biochem. https://doi.org/10.1002/jcb.22564
Wang YJ et al (2002) Ketoconazole potentiates terfenadine-induced apoptosis in human Hep G2 cells through inhibition of cytochrome p450 3A4 activity. J Cell Biochem. https://doi.org/10.1002/jcb.10282
Wang W et al (2011) The expression and clinical significance of CLIC1 and HSP27 in lung adenocarcinoma. Tumour Biol. https://doi.org/10.1007/s13277-011-0223-0
Wang LH et al (2012) Rituximab inhibits Kv1.3 channels in human B lymphoma cells via activation of FcγRIIB receptors. Biochim Biophys Acta Mol Cell Res. https://doi.org/10.1016/j.bbamcr.2011.11.012
Wang P et al (2014a) Chloride intracellular channel 1 regulates colon cancer cell migration and invasion through ROS/ERK pathway. World J Gastroenterol. https://doi.org/10.3748/wjg.v20.i8.2071
Wang WT et al (2014b) Terfenadine induces anti-proliferative and apoptotic activities in human hormone-refractory prostate cancer through histamine receptor-independent Mcl-1 cleavage and Bak up-regulation. Naunyn Schmiedebergs Arch Pharmacol. https://doi.org/10.1007/s00210-013-0912-x
Wang CY et al (2015a) Meta-analysis of public microarray datasets reveals voltage-gated calcium gene signatures in clinical cancer patients. PLoS One. https://doi.org/10.1371/journal.pone.0125766
Wang T et al (2015b) Inhibition of transient receptor potential channel 5 reverses 5-fluorouracil resistance in human colorectal cancer cells. J Biol Chem. https://doi.org/10.1074/jbc.M114.590364
Wang D et al (2018a) Codelivery of doxorubicin and MDR1-siRNA by mesoporous silica nanoparticles-polymerpolyethylenimine to improve oral squamous carcinoma treatment. Int J Nanomedicine. https://doi.org/10.2147/IJN.S150610
Wang Z et al (2018b) Inhibition of TRPA1 attenuates doxorubicin-induced acute cardiotoxicity by suppressing oxidative stress, the inflammatory response, and endoplasmic reticulum stress. Oxid Med Cell Longev. https://doi.org/10.1155/2018/5179468
Wanke E, Restano-Cassulini R (2007) Toxins interacting with ether-a-go-go-related gene voltage-dependent potassium channels. Toxicon. https://doi.org/10.1016/j.toxicon.2006.09.025
Weaver AK, Liu X, Sontheimer H (2004) Role for calcium-activated potassium channels (BK) in growth control of human malignant glioma cells. J Neurosci Res. https://doi.org/10.1002/jnr.20240
Wondergem R et al (1998) Membrane potassium channels and human bladder tumor cells: II. Growth properties. J Membr Biol. https://doi.org/10.1007/s002329900332
Wong DT, Bymaster FP, Engleman EA et al (1995) Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. https://doi.org/10.1016/0024-3205(95)00209-o
Wood K et al (2018) Capecitabine and celecoxib as a promising therapy for thymic neoplasms. Am J Clin Oncol. https://doi.org/10.1097/COC.0000000000000400
Wu W et al (2012) Human ether-a-go-go gene potassium channels are regulated by EGFR tyrosine kinase. Biochim Biophys Acta. https://doi.org/10.1016/j.bbamcr.2011.10.010
Wu T et al (2019) Spider venom peptides as potential drug candidates due to their anticancer and antinociceptive activities. J Venom Anim Toxins Incl Trop Dis. https://doi.org/10.1590/1678-9199-JVATITD-14-63-18
Wulff H et al (2019) Antibodies and venom peptides: new modalities for ion channels. Nat Rev Drug Discov. https://doi.org/10.1038/s41573-019-0013-8
Xiang Y et al (2011) Chloride channel-mediated brain glioma targeting of chlorotoxin-modified doxorubicine-loaded liposomes. J Control Release. https://doi.org/10.1016/j.jconrel.2011.03.014
Xiao X et al (2010) Targeting CREB for cancer therapy: friend or foe. Curr Cancer Drug Targets
Xie J et al (2016) SOCE and cancer: recent progress and new perspectives. Int J Cancer. https://doi.org/10.1002/ijc.29840
Xie J et al (2019) Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials. https://doi.org/10.1016/j.biomaterials.2019.119491
Xu Y et al (2018a) Expression of CLIC1 as a potential biomarker for oral squamous cell carcinoma: a preliminary study. Onco Targets Ther. https://doi.org/10.2147/OTT.S181936
Xu J et al (2018b) Astemizole promotes the anti-tumor effect of vitamin D through inhibiting miR-125a-5p-meidated regulation of VDR in HCC. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2018.08.153
Xue X, Liang XJ (2012) Overcoming drug efflux-based multidrug resistance in cancer with nanotechnology. Chin J Cancer. https://doi.org/10.5732/cjc.011.10326
Xue H et al (2018) Inhibition of transient receptor potential vanilloid 6 channel, elevated in human ovarian cancers, reduces tumour growth in a xenograft model. J Cancer 9(17):3196–3207. https://doi.org/10.7150/jca.20639
Yagoda N et al (2007) RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. https://doi.org/10.1038/nature05859
Yamaci RF et al (2017) Neonatal Nav1.5 protein expression in normal adult human tissues and breast cancer. Pathol Res Pract. https://doi.org/10.1016/j.prp.2017.06.003
Yamamoto S et al (2008) TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. https://doi.org/10.1038/nm1758
Yang WH et al (2016) Imipramine blue halts head and neck cancer invasion through promoting F-box and leucine-rich repeat protein 14-mediated Twist1 degradation. Oncogene. https://doi.org/10.1038/onc.2015.291
Yang MY et al (2019) Carrier-free nanodrug: a novel strategy of cancer diagnosis and synergistic therapy. Int J Pharm. https://doi.org/10.1016/j.ijpharm.2019.118663
Ye Y et al (2015) CLIC1 a novel biomarker of intraperitoneal metastasis in serous epithelial ovarian cancer. Tumor Biol. https://doi.org/10.1007/s13277-015-3052-8
Yu C et al (2017) Mitochondrial calcium uniporter as a target of microRNA-340 and promoter of metastasis via enhancing the Warburg effect. Oncotarget. https://doi.org/10.18632/oncotarget.19747
Yusa K, Tsuruo T (1989) Reversal mechanism of multidrug resistance by verapamil: direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells. Cancer Res 49(18):5002–5006
Zeng X et al (2010) Novel role for the transient receptor potential channel TRPM2 in prostate cancer cell proliferation. Prostate Cancer Prostatic Dis. https://doi.org/10.1038/pcan.2009.55
Zeng B et al (2013) TRPC channels and their splice variants are essential for promoting human ovarian cancer cell proliferation and tumorigenesis. Curr Cancer Drug Targets 13(1):103–116
Zhang S et al (1999) Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. Circ Res. https://doi.org/10.1161/01.res.84.9.989
Zhang Y et al (2007) Effects of celecoxib on voltage-gated calcium channel currents in rat pheochromocytoma (PC12) cells. Pharmacol Res. https://doi.org/10.1016/j.phrs.2007.07.004
Zhang L et al (2018) Curcumin induces endoplasmic reticulum stress-associated apoptosis in human papillary thyroid carcinoma BCPAP cells via disruption of intracellular calcium homeostasis. Medicine (United States) 97(24):e11095. https://doi.org/10.1097/MD.0000000000011095
Zhao Y et al (2011) Analgesic-antitumor peptide inhibits proliferation and migration of SHG-44 human malignant glioma cells. J Cell Biochem. https://doi.org/10.1002/jcb.23166
Zhao W et al (2013) 1B50-1, a mAb raised against recurrent tumor cells, targets liver tumor-initiating cells by binding to the calcium channel α2δ1 subunit. Cancer Cell. https://doi.org/10.1016/j.ccr.2013.02.025
Zhao W, Lu M, Zhang Q (2015) Chloride intracellular channel 1 regulates migration and invasion in gastric cancer by triggering the ROS-mediated p38 MAPK signaling pathway. Mol Med Rep. https://doi.org/10.3892/mmr.2015.4459
Zhao LY et al (2016) The overexpressed functional transient receptor potential channel TRPM2 in oral squamous cell carcinoma. Sci Rep. https://doi.org/10.1038/srep38471
Zheng Z et al (2019) The Xc− inhibitor sulfasalazine improves the anti-cancer effect of pharmacological vitamin C in prostate cancer cells via a glutathione-dependent mechanism. Cell Oncol. https://doi.org/10.1007/s13402-019-00474-8
Zhou Z et al (1999) Block of HERG potassium channels by the antihistamine astemizole and its metabolites desmethylastemizole and norastemizole. J Cardiovasc Electrophysiol. https://doi.org/10.1111/j.1540-8167.1999.tb00264.x
Zhou C et al (2016) Icaritin activates JNK-dependent mPTP necrosis pathway in colorectal cancer cells. Tumor Biol. https://doi.org/10.1007/s13277-015-4134-3
Zhu X et al (2013) Loss and reduced expression of PTEN correlate with advanced-stage gastric carcinoma. Exp Ther Med. https://doi.org/10.3892/etm.2012.749
Zoratti M, Szabò I (1995) The mitochondrial permeability transition. Biochim Biophys Acta. https://doi.org/10.1016/0304-4157(95)00003-a
Zou L et al (2016) Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics. https://doi.org/10.7150/thno.14988
Acknowledgments
Conflict of Interest: The authors declare no conflict of interests. Contributions: AR, JG-Q, and LAA-E wrote the first draft of the manuscript. YS-P and JC revised the first draft and contributed to manuscript writing of the following versions. All authors read and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ramírez, A., García-Quiroz, J., Aguilar-Eslava, L., Sánchez-Pérez, Y., Camacho, J. (2020). Novel Therapeutic Approaches of Ion Channels and Transporters in Cancer. In: Stock, C., Pardo, L.A. (eds) Targets of Cancer Diagnosis and Treatment. Reviews of Physiology, Biochemistry and Pharmacology, vol 183. Springer, Cham. https://doi.org/10.1007/112_2020_28
Download citation
DOI: https://doi.org/10.1007/112_2020_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-03993-5
Online ISBN: 978-3-031-03994-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)