Abstract
Prostate cancer is the fourth most commonly diagnosed cancer, and although it is often a slow-growing malignancy, it is the second leading cause of cancer-associated deaths in men and the first in Europe and North America. In many forms of cancer, when the disease is a solid tumor confined to one organ, it is often readily treated. However, when the cancer becomes an invasive metastatic carcinoma, it is more often fatal. It is therefore of great interest to identify mechanisms that contribute to the invasion of cells to identify possible targets for therapy. During prostate cancer progression, the epithelial cells undergo epithelial-mesenchymal transition that is characterized by morphological changes, a loss of cell-cell adhesion, and invasiveness. Dysregulation of pH has emerged as a hallmark of cancer with a reversed pH gradient and with a constitutively increased intracellular pH that is elevated above the extracellular pH. This phenomenon has been referred to as “a perfect storm” for cancer progression. Acid-extruding ion transporters include the Na+/H+ exchanger NHE1 (SLC9A1), the Na+HCO3− cotransporter NBCn1 (SLC4A7), anion exchangers, vacuolar-type adenosine triphosphatases, and the lactate-H+ cotransporters of the monocarboxylate family (MCT1 and MCT4 (SLC16A1 and 3)). Additionally, carbonic anhydrases contribute to acid transport. Of these, several have been shown to be upregulated in different human cancers including the NBCn1, MCTs, and NHE1. Here the role and contribution of acid-extruding transporters in prostate cancer growth and metastasis were examined. These proteins make significant contributions to prostate cancer progression.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acid extrusion
- Carbonic anhydrase
- Cell invasion
- Metastasis
- Monocarboxylate transporter
- Na+/H+ exchanger
- Prostate cancer
- Proton transport
- Sodium bicarbonate cotransporter
- Vacuolar adenosine triphosphatases
1 Introduction
1.1 Prostate Cancer, General
Prostate cancer is the fourth most commonly diagnosed cancer, and although prostate cancer is often a slow-growing malignancy, it is the second leading cause of cancer-associated deaths in men and the first in Europe and North America (Siegel et al. 2014). Therapy by androgen ablation results in tumor regression, but patients are still at risk of androgen refractory tumors (Zhu et al. 2018). In many forms of cancer, including prostate cancer, when the disease is a solid tumor confined to one organ, it is often readily treated. However, when the cancer transforms to an invasive carcinoma that is metastatic, it is more often fatal. It is therefore of great interest to identify mechanisms that contribute to the invasion of cells to identify possibly targets for therapy (Dykes et al. 2017a). During prostate cancer progression, the epithelial cells undergo epithelial-mesenchymal transition (EMT) that is characterized by morphological changes from a cuboidal to a spindle-shaped cell (Grant and Kyprianou 2013). In prostate cancer and in several other types of cancers, there is a demonstrated downregulation of epithelial markers such as E-cadherin and occludins that leads to loss of cell-cell adhesion. Mesenchymal markers such as vimentin and N-cadherin are upregulated, and this allows the cells to migrate or metastasize to different organs (Lang et al. 2002; Sethi et al. 2010; Singh et al. 2003; Xu et al. 2009). EMT is affected by various growth factors and cytokines, and this induces various transcription factors such as Snail, Twist, and Zeb1/2 (Odero-Marah et al. 2018; Smith and Odero-Marah 2012). Most patients with prostate cancer are killed not by the cancer directly in the prostate but by the tumor metastasizing to critical organs such as the lungs or the liver. Prostate cancer in particular tends to metastasize to bone (Nauseef and Henry 2011). EMT and transcription factor expression play an important critical role in this progression and metastasis (Odero-Marah et al. 2018; Saha et al. 2008; Yuen et al. 2008).
1.2 Tumor Microenvironments, General
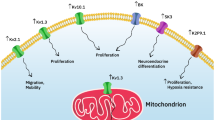
Cells within a solid tumor are in a different microenvironment than cells in normal tissues. This affects their gene expression and their physiological properties. The tumor microenvironment is different from other cell microenvironments in that most tumors are hypoxic or anoxic, with reduced glucose and ATP levels, elevated extracellular lactate levels, and an acidic extracellular pH (pHe) (Andersen et al. 2014; Parks et al. 2011; Vaupel 2004). In addition, tumor cells have a very interesting shift in their metabolism called the Warburg effect. Here, aside from the hypoxic environment, and even in the presence of enough oxygen, they are shifted away from oxidative phosphorylation and favor use of glycolytic metabolism (Koppenol et al. 2011). This elevated level of glycolysis and ATP hydrolysis causes the highly proliferative and anabolic cancer cells to produce more acid than normal cells (Andersen et al. 2014). A more alkaline intracellular pH (pHi) is suggested to be a prerequisite for growth, proliferation, and motility (Fig. 1) (Schwab et al. 2012; Webb et al. 2011). For example, studies in neutrophils (Hayashi et al. 2008) have shown that cellular alkalinization of 0.26 pH units occurs despite increased acid production, and this plays a permissive role in cell motility. Additionally, in fibroblasts, a pH threshold exists of about 7.2, below which growth factors cannot set in motion a G0 to S phase transition (Pouysségur et al. 1985), and intracellular pH controls protein synthesis and G0/G1 transition of fibroblasts (Chambard and Pouyssegur 1986). Conversely, inhibition of acid-extruding proteins, which raise intracellular pH, has been shown to be inhibitory to cell growth, migration, or invasion in several different cell types including in hepatocellular cancer, breast cancer, and gastric cancer (Amith et al. 2016a; Lu et al. 2005; Xie et al. 2017; Yang et al. 2010).
Dysregulation of intracellular and extracellular pH creates a “perfect storm” for cancer progression (Cardone et al. 2005; Webb et al. 2011). While tumor pH is not uniformly acidic (Helmlinger et al. 1997; Vaupel et al. 1981), with oncogenic transformation there is a general decrease in extracellular pH and an increase in intracellular pH (reviewed in Gillies et al. 2002). These events tend to promote cellular remodeling and invasive behavior that promote cell proliferation and metastatic behavior
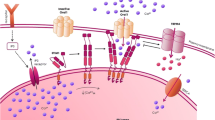
Additionally, not only is an elevated pHi an important part of tumor cell growth and metastasis, a more acidic external microenvironment has recently been thought to be an important part of metastatic behavior (Fig. 1) (Reshkin et al. 2014; Stock et al. 2008), which may occur through more than one mechanism. The acidic external microenvironment may result in protonation of chemotherapeutic drugs through a mechanism called “ion trapping,” which can result in decreased cellular uptake and decreased effectiveness (Lebelo et al. 2019; Mahoney et al. 2003; Peppicelli et al. 2017; Raghunand et al. 2003). Alternatively, acidification of the extracellular matrix may promote proteolysis of peri-invadopodial space (Fig. 2) (Busco et al. 2010; Greco et al. 2014).
Proposed role of extracellular acidification facilitating proteolysis and metastasis of tumor cells. In malignant cells acid is extruded by various mechanisms, and this results in decreased extracellular pH in a localized leading-edge region. This causes enhanced extracellular matrix digestions and increased cell motility and migration through this region (Cardone et al. 2005). Left panel, normal cell surrounded by extracellular matrix. Centre panel, malignant cell surrounded by extracellular matrix and enlargement of the leading edge of the cell, indicating reversed pH gradient. Right panel, enhanced mobility of malignant cell leads to migration
The abnormalities of intracellular alkalinization along with extracellular acidification of tumors appear to be a specific hallmark of malignancy. Dysregulation of pH has emerged as a hallmark of cancer with a reversed pH gradient and with a constitutively increased intracellular pH that is elevated above the extracellular pH. In fact, this phenomenon has been referred to as “a perfect storm” for cancer progression (Fig. 1). It facilitates various characteristics of cancers such as being permissive for cell proliferation, helps evade apoptosis, and facilitates metabolic adaptation that is obligatory for cancer cells. Additionally, cellular migration is enhanced (Webb et al. 2011). Degradation of the extracellular matrix is a critical step of tumor cell invasion. It requires protease-dependent proteolysis concentrated at invadopodia, where proteolysis of the extracellular matrix occurs (Busco et al. 2010; Greco et al. 2014). The pH optimum of these extracellular proteases is acidic, and proton extrusion out of cancer cells makes an acidic external microenvironment that facilitates their activity (Cardone et al. 2005; Greco et al. 2014) (Fig. 2).
Therefore, various methods of producing intracellular acidification or inhibiting extracellular acidification are becoming a new therapeutic concept for cancer treatment sometimes referred to as “the pH-centric anticancer paradigm”(Harguindey et al. 2017, 2018; Harguindey and Reshkin 2017). While tumor pH is not uniformly acidic (Helmlinger et al. 1997; Vaupel et al. 1981), the general abnormality in cancer of intracellular alkalinization, along with extracellular acidification, results in “proton gradient reversal” of solid tumors and leukemic cells and is becoming recognized as a specific and selective hallmark of cell malignancy. Attempts to induce cellular acidification using treatments such as proton transport inhibitors are becoming a new therapeutic concept for treatment of cancers (De Milito et al. 2010; De Milito and Fais 2005; Harguindey et al. 2018; Koltai 2017).
By modifying the pH characteristics of cancer cells, it is suggested that it may be possible to decrease proliferation and invasion. The origin of extracellular acidity is the excess of protons generated by cancer cells’ metabolism that are extruded from cancer cells by membrane proton transporters (or their equivalent). As noted above, the excess of protons is due to increased lactate and acid production because tumor cells metabolize more glucose than normal cells, through the glycolytic pathway, which is called the Warburg effect (Koltai 2017).
1.3 Acid-Extruding Ion Transporters
Mediators of increased acid extrusion in tumor cells include the Na+/H+ exchanger NHE1 (SLC9A1), Na+HCO3− cotransporters NBCn1 (SLC4A7) and NBCe1 (SLC4A4), anion exchangers, vacuolar-type adenosine triphosphatases (V-ATPases), carbonic anhydrases (CA), and the lactate-H+ cotransporters of the monocarboxylate family (MCT1 and MCT4 (SLC16A1 and 3)) (Amith and Fliegel 2016, 2017; Boedtkjer et al. 2012; Koltai 2017; Lauritzen et al. 2010; Le Floch et al. 2011; Mcintyre and Harris 2016; Parks et al. 2011) (Fig. 3). Of these, several have been shown to be upregulated in different forms of human cancers including the Na+HCO3− transporter, MCTs, and NHE1 (Amith and Fliegel 2016; Boedtkjer et al. 2013; Chiche et al. 2012; Dhup et al. 2012; Parks et al. 2011).
pH control systems in tumor cells for metabolically produced acid. Increased glycolysis leads to elevated lactate and proton production which are removed from the cell by various transporters. Carbonic anhydrases II, IX, and XII also make significant contributions to extracellular acidity (Harguindey et al. 2009; Webb et al. 2011). NHE1, Na+/H+ exchanger isoform 1; MCT1/4, monocarboxylate transporter 1 and 4; V-ATPase, vacuolar-type H+-adenosine triphosphatase; Cl/HCO3−, chloride/bicarbonate anion exchangers; NaHCO3−, sodium-dependent bicarbonate cotransporters; CA, carbonic anhydrase with isoforms indicated. The putative cycle of proton release through the actions of CAs is shown. V-ATPase is shown at the plasma membrane where it is mistargeted in certain tumor types and contributes to pH regulation of tumor cells (Cotter et al. 2015; Martinez-Zaguilan et al. 1993)
1.4 Acid-Extruding Transporters in Prostate Cancer
Given that a number of different pathways can contribute to acid extrusion in various types of cancer (Fig. 3), here, the roles of various acid-extruding transporters in prostate cancer are examined, with a brief presentation of their role in some other types of cancer. Table 1 (supplementary) summarizes studies which have suggested a role for various acid-extruding proteins in prostate cancer.
1.5 Na+/H+ Exchangers
Na+/H+ exchangers have been implicated as playing key roles in a number of types of cancers. This has been extensively documented in several cell types, in notable detail in breast cancer cells. There, it has been shown that cytoplasmic alkalinization and extracellular acidification occur as a result of increased NHE1 activity (Amith and Fliegel 2013; Reshkin et al. 2013). The extracellular acidification leads to proteolytic digestion of the extracellular matrix (Busco et al. 2010). Knockout of overactive NHE1 protein in the triple negative breast cancer cell MDA-MB-231 leads to impaired in vivo xenograft tumor growth and impaired invasive behavior. In addition, NHE1 inhibition or knockout leads to increased sensitivity to paclitaxel treatment (Amith et al. 2015b). Several regulatory loci on the NHE1 regulatory cytoplasmic domain have been identified as modulating metastatic and invasive potential, including phosphorylation at serine 703. Additionally, NHE1 regulation, through phosphorylation of its regulatory cytosolic domain, modifies EMT of triple negative breast cancer cells (Amith et al. 2016b).
Inhibition of NHE1 prevents extracellular acidification in breast cancer cells and is suggested to be a treatment that may reduce chemotherapy resistance (Amith and Fliegel 2017; Harguindey et al. 2013). Conversely, increased expression of NHE1 is associated with poor outcomes in several forms of cancer including breast cancer (Amith et al. 2017), ovarian cancer (Wang et al. 2018), and gliomas (Guan et al. 2018).
NHE1 has been studied to some degree in prostate cancer, but much is unknown. NHE1 mRNA expression has been shown to be elevated in prostate cancer relative to normal tissue (Amith et al. 2015a). Expression of NHE1 in DU145 prostate cancer cells correlates with Zeb1 expression, a transcription factor that promotes EMT (Dykes et al. 2017a). The NHE3 isoform message has also been shown to be present in the prostate gland (Brant et al. 1995) and in prostate cancer cells (Brant et al. 1995). Additionally, the protein was also found on the cell surface of prostate cancer cells (Gonzalez-Gronow et al. 2005).
Hormonal regulation of NHEs in prostate cancer cells is of interest. Hepatocyte growth factor, which is found in the prostate tumor microenvironment, triggers invasion, metastasis, and EMT and induces NHE activity in DU145 prostate cancer cells (Steffan et al. 2010). Hepatocyte growth factor induced trafficking of lysosomes to the cell surface and increased extracellular acidification, which was thought to be important in promoting invasiveness (Fig. 4). In this study, NHE activity was suggested to directly extrude protons and was also shown to promote lysosomal migration to the cell surface in the DU145 prostate cancer cell line (Steffan et al. 2010). It should also be noted that two isoforms of NHE, NHE1 and NHE3, were shown to contribute to lysosomal trafficking and extracellular pH acidification in both DU145 and PC3 prostate cancer cells and inhibition of both together had an additive effect in preventing lysosomal exocytosis (Steffan et al. 2009). Steffan et al. (2010) suggested that other isoforms, aside from NHE1 and NHE3, may also be involved in HGF-induced lysosome movement in DU145 cells. However, this has yet to be investigated further.
Lysosomal trafficking to the cell periphery is induced in prostate cancer cells by treatment with EGF (epidermal growth factor) or HGF (hepatocyte growth factor). The schematic diagram illustrates the effect of hormone treatment of prostate cancer cells (Dykes et al. 2017b; Steffan et al. 2010). Lysosome redistribution (and NHE concentration at the tip of pseudopods) is thought to result in local extrusion of acid and protease causing focalized proteolysis of the extracellular matrix. This is believed to compromise cell attachment resulting in increased cellular motility (Cardone et al. 2005). Lysosomes are represented by black filled circles. NHE1, NHE3, or other NHE isoforms may contribute (Steffan et al. 2010)
Epidermal growth factor is another hormone that stimulates lysosomal trafficking and invasion by DU145 cells that is dependent on NHE activity. Epidermal growth factor was shown to stimulate lysosomal trafficking, protease secretion, and invasion via a p38MAPK regulated pathway that was different from hepatocyte growth factor stimulation. Hepatocyte growth factor required PI3K and ERK (Dykes et al. 2017b).
Several other forms of regulation of NHEs also stimulate prostate cancer cell invasion. The plasminogen activation system comprises the serine protease urokinase plasminogen activator (uPA), its cell surface receptor, and two endogenous serpin inhibitors, plasminogen activator inhibitors 1 and 2. Plasminogen II can be converted to the active serine protease plasmin upon binding to cell surface receptors. Plasminogen type II binding to dipeptidyl peptidase IV on the surface of highly invasive 1-LN (prostate cancer) cells stimulated a NHE3-mediated rise in intracellular pH that promoted cell invasiveness (Gonzalez-Gronow et al. 2005).
The uPA receptor controls plasticity of prostate cancer cell movement (Margheri et al. 2014). uPA expression is higher in patients with prostate cancer (Akudugu et al. 2015; Bohm et al. 2013; Lippert et al. 2016), and its receptor is a biomarker for prostate cancer aggressiveness (Skovgaard et al. 2017a, b). It is important to note that knockdown of uPA and its receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo and uPA and its receptor levels correlate with invasive potential of prostate cancer cell lines. For example, knockdown of uPA and its receptor inhibits invasion and proliferation by PC3 cells (Pulukuri et al. 2005).
Membrane androgen receptors are expressed in several kinds of tumor cells including prostate cells. It is interesting to note that when stimulated with testosterone-albumin conjugates, membrane androgen receptors rapidly activate NHE1 in DU145 and LNCaP prostate cancer cells, an effect specifically blocked by NHE1 inhibitors and by inhibitors of glucocorticoid-inducible kinase (SGK1) and Rho-associated protein kinase (ROCK) (Chatterjee et al. 2014).
It should be noted that Chatterjee et al. (Chatterjee et al. 2014) also suggested a smaller amount of NHE2 is present in DU145 cells which agreed with Northern blot analysis of NHE2 distribution in whole tissues (Malakooti et al. 1999). However, NHE2’s role in promoting invasiveness and acidifying extracellular pH is not well studied.
Overall, there is a large amount of evidence implicating Na+/H+ exchangers in prostate cancer metastasis, primarily the NHE1 isoform and also the NHE3 isoform, and possibly there is a contribution of other isoforms.
1.6 Na+HCO3− Cotransporter NBCn1 (SLC4A7)
The Na+HCO3− transporter (NBCn1) is present in the prostate (Romero and Boron 1999; Sun and Bonanno 2003), but it has not been studied in the context of prostate cancer. It is worth briefly reviewing the role of NBCn1 in other forms of cancer, as a possible means of predicting possible roles in prostate cancer. In human MCF-7 breast cancer cells, NBCn1 mRNA and protein levels are increased by introduction of the constitutively active ErbB2 receptor, which is upregulated in cancers and associated with increased metastasis (Lauritzen et al. 2010), while in MCF-7 cells NHE1, not NBCn1, was important in enhancing cell motility in culture (Lauritzen et al. 2012). NBCn1 was critical in ErbB2-induced breast carcinogenesis in mice, and its knockout decelerated tumor growth by approximately 1/3 (Lee et al. 2018b). In other breast cancer types, NBCn1 may also be important. In primary breast carcinomas, it was notable that NBCn1 expression was upregulated and this protein was the major determinant of intracellular pH (Boedtkjer et al. 2013). In the triple negative breast cancer cell line MDA-MB-231 cells, shRNA-mediated knockdown of NBCn1 reduced pHi and the ability to form primary tumors in xenografts (as did knockdown of NHE1 and MCT4). Additionally, knockdown of NBCn1 (or MCT4) but not of NHE1 increased tumor-free survival and decreased cell proliferation and colony growth in soft agar (Andersen et al. 2018).
These studies indicate that NBCn1 can certainly have a critical role in some types of tumor growth and survival, though this seems to vary with the precise cell type, even in the same general cancer. Studies have also shown that other cancer types such as melanoma (Yang and Loh 2019), pancreatic (Kong et al. 2014), and renal cell carcinomas (Yamada et al. 2003) are dependent on bicarbonate transporters for intracellular pH regulation. The role of NBCn1 in various forms of prostate cancer would thus seem to be a priority for investigation.
The Na+HCO3− cotransporter NBCe1 (SLC4A4) is an electrogenic transporter with a stoichiometry of 1:2 or 1:3 Na+:HCO3−. It has been less studied than NBCn1, but one interesting study examined its role in growth and migration of breast cancer and colon cancer cells. Hypoxia induced NBCe1 mRNA expression in colon adenocarcinoma cells. NBCe1 contributed to pH regulation, and knockdown of the protein reduced cell proliferation. In MDA-MB-231 breast cancer cells, knockdown of NBCe1 also decreased proliferation, migration, and invasion. These results suggested that NBCe1 contributes to HCO3− transport in tumor cell phenotypes (Parks and Pouyssegur 2015). One preliminary publication has also suggested that hypoxia-induced acidification causes a small but significant upregulation of the protein (Lee et al. 2018a). Additionally, it is interesting to note that mutations in the SLC4A4 gene have recently been found in patients with prostate cancer (Liang et al. 2019). However, further studies in this area are needed.
1.7 Anion Exchangers
Anion exchangers comprise a family of transport proteins typified by AE1 (anion exchanger type 1, SLC4A1). The protein functions in mediating the exchange of Cl− for HCO3− and was originally studied as Band 3 in red blood cell membranes (Cordat and Casey 2009; Cordat and Reithmeier 2014). The bicarbonate transport proteins include over a dozen members, including a SLC26 family (Cordat and Casey 2009). AE1–3 are electroneutral transporters. There are few studies on the anion exchangers aside from those on NBCs as noted above. The SLC4A1 message is overexpressed in primary tumors and metastasis compared with normal prostate tissue as is the SLC26A8 ion transporter (Marin-Aguilera et al. 2015). However, there has been little to implicate most of these anion exchangers directly in promoting metastatic behavior in prostate cancers or other cancers, aside from studies on NBCs. In fact, the conclusive identification of the HCO3− transporter as responsible for uptake is mostly unclear. The large diversity of the family, as well as lack of clear roles in tumors, plus the lack of specific inhibitors for the transporters, has delayed progress in this area. There have been earlier suggestions that HCO3− uptake occurs via a Cl−/HCO3− exchanger, but more study is required in this area (Parks et al. 2013). In a tumor cell, where intracellular pH tends to be elevated, and extracellular pH tends to be decreased compared to normal cells, bicarbonate concentrations would tend to be more elevated, possibly because of reduced CA activity (Fig. 3). Thus, there would be less of a propensity for uptake of bicarbonate through anion exchangers (Parks et al. 2013).
1.8 Lactate-H+ Cotransporters (MCT1 and MCT4)
Lactate-H+ cotransporters (or symporters) are part of a fairly large SLC16 gene family. Monocarboxylate transporters, MCT1 and MCT4, are two of the four members of the family (MCT1 (SLC16A1), MCT2 (SLC16A7), MCT3 (SLC16A8), and MCT4 (SLC16A3)) that transport monocarboxylates such as L-lactate, pyruvate, and ketone bodies across the plasma membrane. The MCTs facilitate proton-linked transport of monocarboxylates across the plasma membrane, and L-lactate is the predominant substrate (Halestrap 2013). These proteins are driven mainly by substrate availability and were not thought of as normal pH regulatory proteins. Therefore, until recently they tended to be overlooked in relation to tumor pHi regulation. However, since tumors produce excess acid and protons, excretion of acid via this transport mechanism can provide important contributions to pHi regulation of tumors (Parks et al. 2013).
The discovery that MCT4 is induced by hypoxia provided part of the drive for studying these transporters in cancer (Parks and Pouyssegur 2017; Ullah et al. 2006). Combined genetic disruption of MCT1/2 and MCT4 caused significant decreases in pHi in colon adenocarcinoma and glioblastoma human cell lines (Marchiq et al. 2015). As noted above, MCT4 appears to also play an important role in breast cancer. MCT4 levels are elevated in breast cancer, and it is associated with poor patient survival correlating with lymph node status. Knockdown of MCT4 attenuated tumor growth in triple negative (MDA-MB-231) mouse xenografts and reduced proliferation rates, invasion, and anchorage-independent colony formation of MDA-MB-231 cells (Andersen et al. 2018). Similar results were found in invasive bladder cancer and urothelial carcinoma (Todenhofer et al. 2018).
In prostate cancer, MCTs are not well studied, but there are some interesting studies suggesting an important role for MCT4. Elevated MCT4 expression was associated with castration-resistant prostate cancer (Pertega-Gomes et al. 2011). Additionally, knockdown of MCT4 levels with antisense oligonucleotides in PC3, DU145, and C4-2 cells decreased proliferation of these prostate cancer cell types. Antisense oligonucleotides against MCT4 also inhibited tumor growth in PC3 tumor-bearing mice (Choi et al. 2016). Another group (Pertega-Gomes et al. 2015) showed the presence of MCT1 and MCT4 in human prostate samples. Of course, cancer cells exhibit high glycolytic rates with consequent lactate production. When a large cohort of human prostate tissue cancers were examined, increased glycolytic rates correlated with a poor prognosis, and MCT4 was elevated in metastatic tumors, supporting a role for MCT4 as a target in the disease (Pertega-Gomes et al. 2015). Further, a recent study examined a murine model of prostate cancer examining extracellular pH of low- and high-grade tumor regions. A low extracellular pH correlated with elevated MCT4 gene expression, which suggested that MCT4 is a contributor to extracellular acidosis. Low-grade tumors also possessed low lactate dehydrogenase activity (lactate dehydrogenase catalyzes the production of lactate from pyruvate) (Korenchan et al. 2019). In summary, several studies have implicated MCT4 as being an important contributor to tumor effectiveness and as a cause of extracellular acidosis.
1.9 Vacuolar-Type H+-ATPase
In addition to NHEs, MCTs, and bicarbonate transporters, vacuolar-type H+-ATPases (V-ATPases) are also proposed to contribute to pH regulation of tumors (Parks et al. 2011). V-ATPases are multi-subunit, ATP-driven proteins that play roles in processes such as endocytosis, trafficking, and lysosomal acidification. They are comprised of 13 distinct subunits that are part of the membrane integral or cytosolic domain. They are oriented to either proton pumps from the cytosol to organelles or, if on the plasma membrane, to the extracellular space (Whitton et al. 2018). Aside from acidification of organelles, they play a role in acid secretion at the plasma membrane (Fig. 3) in a variety of cell types including renal intercalated cells, osteoclasts, and epididymal cells (Sennoune et al. 2004b). Certain tumor types express V-ATPases at the plasma membrane, possibly because of missorting to the plasma membrane (Cotter et al. 2015; Martinez-Zaguilan et al. 1993; Smith et al. 2016).
Overexpression of V-ATPases in tumor cells has been documented in several cases, and these H+ pumps are thought to be important removers of acid in these tumor cells (Parks et al. 2013). In human breast cancer cells, highly metastatic cells have more V-ATPase than lowly metastatic cells (Sennoune et al. 2004a). Similarly, when comparing highly metastatic melanoma cells to lowly metastatic cells, the highly metastatic cells expressed V-ATPases at the plasma membrane, while the lowly metastatic cells did not (Nishisho et al. 2011). Overexpression of V-ATPase was demonstrated in breast cancer; cervical cancer; gastric, lung, esophageal, and oral squamous cell carcinoma; and ovarian and pancreatic cancer (Whitton et al. 2018). In many cases, elevated expression in patients was correlated with poorer survival. Inhibition of V-ATPase has been associated with inhibition of EMT (Merk et al. 2017). Activity of plasma membrane V-ATPases is important in invasion of MDA-MB231 breast cancer cells (Cotter et al. 2015).
As V-ATPases have many subunits, for physiological analysis by knockdowns, a multitarget approach may be required. For example, disruption of subunit a4, or a3 subunits, inhibited migration and invasion of MDA-MB-231 breast cancer cells (Hinton et al. 2009). Similarly, another study examined MCF10CA1a cells and found that knockdown of a3 (but not a1, a2, or a4 subunits) decreased invasion of these cells (Capecci and Forgac 2013). The a3 isoform has also been localized specifically to the plasma membrane of breast cancer cells (Cotter et al. 2016). Recently however, it should be noted that invasion and migration of 4T1-12B breast cancer cells was disrupted by ablation of the a4-encoding gene alone. This also inhibited targeting of the V-ATPase to the leading edge of the cell, suggesting it is important in the invasive cancer phenotype in these cells. Knockdown or knockout of other isoforms (a1, a2, a3) did not inhibit cell invasion (Mcguire et al. 2019). Clearly, there may be some cell-specific dependencies on different isoforms of the V-ATPase.
Studies on the role of V-ATPase in prostate cancer are fewer and further between than for other types of cancer but support a role for the V-ATPase in disease. It was recently demonstrated that expression of the C subunit of the V-ATPase was higher in prostate cancer cell lines with greater metastatic potential. Further, downregulation of the V-ATPase C subunit using siRNA, in the PC-3M-1E8 human prostate cancer cell line, decreased V-ATPase activity and invasion in vitro by these cells (Zou et al. 2018). Conversely, upregulation of V-ATPase activity was thought to be responsible for increased invasion and metastasis which occurred with downregulation of the tumor metastasis suppressor gene LASS2/TMSG1 of PC-3M-2B4 prostate cancer cells (Xu et al. 2014a, b). No database information appears to be available linking overexpression or amplification of the V-ATPase subunits to prostate cancer; however there is some such linkage for one subunit in breast cancer. No doubt this type of analysis is complicated by the presence of the 13 distinct subunits (Mcconnell et al. 2017; Whitton et al. 2018).
It is worth mentioning that proton pump (V-ATPase) inhibitors have been tried as a therapeutic target, for various forms of cancers, with mixed success. The chemical inhibitors such as bafilomycin A and concanamycin A bind to the subunit responsible for proton translocation. V-ATPase inhibition results in reduced cancer cell growth in a number of cell types (reviewed in (Whitton et al. 2018)). There has been a suggestion that the effects of bafilomycin on tumor cells are through induction of HIF-1α (hypoxia-inducible factor) expression and subsequent p21 induction and cell cycle arrest, rather than through effects on pH regulation (Lim et al. 2006; Parks et al. 2011). However, despite this possibility, proton pump inhibitors are being pursued to reduce tumor growth and have showed promise in many studies (Parks et al. 2011; Whitton et al. 2018). Proton pump inhibitors have an interesting advantage for cancer therapy as their activation requires acidity which is found in the tumor microenvironment (Parks et al. 2013), so they may be cancer specifically targeted to tumors. Proton pump inhibitors have been shown to inhibit tumor cell growth by disrupting acidosis in vivo in several models and may improve the efficacy of chemotherapy agents (Avnet et al. 2016; De Milito et al. 2010; Spugnini and Fais 2017). Of interest, Martins et al. recently found a plant-derived monoterpene that inhibits V-ATPases and had therapeutic potential in malignant melanoma (Martins et al. 2019).
Studies on the effect of proton pump inhibitors on prostate cancer are rarer. The proton pump inhibitors bafilomycin A and concanamycin A have been shown to decrease migration and invasion of the PC-3 prostate cancer cell line, though in this study it was suggested that the effects were mediated through intracellular pumps, and plasma membrane levels of V-ATPase were negligible (Licon-Munoz et al. 2017). A different study examined LNCaP and C4-2B cells and found that the same inhibitors reduced invasion by both cell types, regardless of the different levels of V-ATPase in these cell lines. The V-ATPase was predominant at the plasma membrane in the androgen-independent C4-2B cells, and only traces were found on the plasma membrane of androgen-dependent and low metastatic LNCaP cells (Michel et al. 2013). Clearly, more studies in this area would be of interest, along with a characterization of the subunits of the V-ATPase subunits that are present in prostate cancer cells.
A class of proton pump inhibitors (including omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole) inhibit gastric acid pump (H+, K+-ATPase) but also directly inhibit vacuolar V-ATPases (H+-ATPase). In 2005 it was suggested that these compounds may be of use to reduce tumor resistance (De Milito and Fais 2005). Of these lansoprazole was investigated in some detail by the Fais group. A small study examined the effect of adding high doses of lansoprazole to their chemotherapy regimen in cats and dogs with advanced neoplasms. The drug was generally well tolerated with an indication that tumor chemoresistance was reduced by the treatment (Spugnini et al. 2011, 2014). Additionally, lansoprazole pretreatment enhanced the sensitivity of human melanoma cells to suboptimal doses of paclitaxel in vitro, and also, in SCID mice lansoprazole increased the sensitivity of subcutaneously injected human tumor cells to paclitaxel (Azzarito et al. 2015). A similar effect was shown with combination treatment using the reverse transcriptase inhibitor efavirenz and lansoprazole (Lugini et al. 2017). Lansoprazole also had an antitumor effect, a cytotoxic action, against human multiple myeloma cells (Canitano et al. 2016), and the combination of lansoprazole and CA inhibitors inhibited cell proliferation and induced cell death in human melanoma cells (Federici et al. 2016). Lansoprazole appears to have greater cytotoxicity toward tumor cells than other proton pump inhibitors of this type (rabeprazole, pantoprazole, omeprazole, esomeprazole) both in vitro and in xenografts (Lugini et al. 2016). A review in 2017 summarizes clinical studies in this area. In one study, patients receiving proton pump inhibitors experience the highest response rates to chemotherapy and longest survivals (Spugnini and Fais 2017; Wang et al. 2015). While these reports strongly support the idea that proton pump inhibitors may be useful in clinical treatment of cancers, a recent large-scale study examined individuals with incidences of breast cancer, prostate cancer, and malignant melanoma in Iceland (Halfdanarson et al. 2019). Here there was no correlation between exposure to proton pump inhibitors and any chemoprotective effect. It should be noted however that this study examined a chemopreventive effect, and not the effect of combination therapies in disease. Studies in that area would still be of high interest.
1.10 Carbonic Anhydrases
Carbonic anhydrases are a family of 15 proteins that catalyze the hydration of CO2 to generate basic HCO3− and H+ (Fig. 3) (Pastorekova and Gillies 2019). Of the family members, CAIX is rapidly induced by hypoxia-inducible factor and is present on the extracellular surface of tumor cells. Normally, it is only expressed in limited parts of the gastrointestinal tracts. It, together with CAXII on the extracellular surface, can acidify extracellular pH of tumor cells and promote tumor cell growth (Parks et al. 2013; Parks and Pouyssegur 2017; Svastova et al. 2004), in addition to raising intracellular pH (Chiche et al. 2009). CAIX has been localized to tumor invadopodia where it promotes matrix degeneration (Debreova et al. 2019). Studies have looked at the effect of knockdown of CAs on tumor growth. In 4T1 breast cancer cells, depletion of CAIX resulted in regression of orthotopic mammary tumors and metastasis formation. Stable depletion of CAIX also resulted in attenuation of primary growth in MDA-MB-231 cells (Lou et al. 2011). Knockout of CAIX can reduce xenograft tumor growth of LS174Tr colon carcinoma cells, and knockout of both CAIX and CAXII gives a much better reduction in tumor growth (Chiche et al. 2009). Similarly, another group showed knockout of CAIX in LS174 cells reduced xenograft tumor growth which was more pronounced in hypoxic conditions (Parks et al. 2017), and a third group showed that knockout of CAIX in MDA-MB-231 breast cancer cells also attenuated xenograft tumor growth (Lou et al. 2011). It is of note that examination of the effects of CAIX on tumor extracellular pH in vivo has shown that CAIX acidifies the extracellular environment (Lee et al. 2018c). CAs are also known to bind to some of the other proton transport proteins such as MCT4 (Noor et al. 2015) and NHE1 (Li et al. 2002). This enables rapid H+ availability and enhances transport of protons. The presence of intracellular CAII was shown to enhance MCT transport (Klier et al. 2014) and NHE1 activity (Li et al. 2006). CAs are associated with poor prognosis in various cancer types, and this is through regulation of EMT (Hyuga et al. 2017; Lock et al. 2013).
Aside from these direct, experimental studies, there have also been links to elevated CAIX expression and various forms of cancer. In human glioblastoma, elevated CAIX expression level was associated with a significant decrease in survival time (Proescholdt et al. 2012), and a large-scale meta-analysis confirmed that CAIX is a marker of poor tumor prognosis in the majority of tumor types studied (Pastorekova and Gillies 2019).
There appears to be fewer studies on CAs and prostate cancer, compared with some other cancer types, but there is some key work that linked CAs with prostate cancer. Lin et al. have recently shown that there is a genetic polymorphism of CAIX that is associated with increased risk of lymph node invasion and metastasis in prostate cancer (Lin et al. 2019). While another study showed an association with CAIX expression levels and with expression levels in high-grade tumors, the localization of the protein was mostly nuclear in these tissue samples (Ambrosio et al. 2016). In contrast, a different earlier study could not find an association of CAIX expression and prostate cancer (Smyth et al. 2010). CAXII and CAIX mRNA are found in PC3 prostate cancer cells but not in DU145 cells (Ivanov et al. 2001). CAIX in PC3 cells was inducible by hypoxia (Li et al. 2009). Interestingly, the pivotal work of Fiaschi et al. (Fiaschi et al. 2013) showed that CAIX is upregulated in cancer-associated fibroblasts from prostate cancer cells, and this leads to extracellular acidification that is blocked by CAIX inhibition. Also, exposure of prostate cancer cells to conditioned medium from cancer-associated fibroblasts increased expression of CAIX. It is of note that CAIX expression was dependent on hypoxia-inducible factor-1 expression. Elevated CAIX activity enhanced matrix metalloproteinase (MMP) activity which is thought to be a trigger for metastatic behavior. Further, the cancer-associated fibroblasts enhanced EMT in prostate cancer cells, as indicated by decreased E-cadherin levels. Silencing of CAIX prevented the ability of conditioned medium from cancer-associated fibroblasts, to decrease E-cadherin levels (Fiaschi et al. 2013).
There are a large number of publications that have explored translation into the clinic of CA work in various cancer types (reviewed in (Pastorekova and Gillies 2019)). Different families of sulfonamides, sulfamates, or related compounds show anticancer potency in animal models (Lou et al. 2011). Briefly, in a breast cancer model of 4T1 mammary tumor cells, 4-{[(3′-nitrophenyl)carbamoyl]amino}benzenesulfonamide significantly inhibited the formation of metastases (Pacchiano et al. 2011). Similarly, a small molecule CAIX inhibitor treatment of mice with 4T1 tumors resulted in attenuation of tumor growth and metastasis formation (Lou et al. 2011). The sulfamide drug DH348 was also shown to reduce hypoxia-induced extracellular acidosis and to sensitize tumors to radiation therapy in CAIX-expressing tumors (Dubois et al. 2013). It should be noted however that adverse side effects and compensation by other proteins are a concern in these studies (Pastorekova and Gillies 2019).
In prostate cancer cells, little has been done in this regard. However, a CAIX isoform-specific selenazole compound recently showed antitumor activity against human prostate cancer (PC3) cells (Angeli et al. 2018).
2 Summary and Future Directions
The above review of the literature makes it clear that with regard to prostate cancer, there has been a significant amount of important work in the area of the tumor microenvironment and the pH-centric hypothesis, but still there are a lot of unanswered questions. Clearly some of the acid transport proteins have been shown to play notable roles in prostate cancer cells, especially Na+/H+ exchangers, MCTs, V-ATPases, and CAs. They have all been shown to be present in prostate cancers, and when expression is reduced or knocked out, or inhibited, there are positive effects on prostate tumor growth, or invasive properties can occur. For NHE1 and MCTs, databases have shown an association with expression and poor prognosis in prostate cancers. CA expression is also known to be associated with poor prognosis in several types of cancer.
Inhibitors of these acid transport proteins have been used in many studies and are being developed for clinical use. For example, CA inhibitors are being developed as are V-ATPase inhibitors. NHE1 inhibitors have potential in this area (Amith et al. 2016a) but still require further refinement. As noted above, one of the critical steps in cancer invasion is the acidification of the microenvironment at the leading edge of invasive cancer cells. This leads to activation of MMP in that microenvironment and subsequent matrix digestion (Fiaschi et al. 2013; Greco et al. 2014). If that is truly a critical key, then it seems obvious that whether the acidifying protons originate from NHEs, V-ATPases or other transport mechanisms might be irrelevant to the disease, presuming a similar acid load is present in the same extracellular location. Additionally, it supports the suggestion that combinations of proton transport inhibitors might create a very hostile environment for tumor growth, invasion, and metastasis (Harguindey et al. 2018). Thus, it seems that combinations of proton transport inhibitors might be a path to explore in the search for the prevention of metastatic behavior.
Several of the proton transport systems have more than one isoform of transport protein. For example, MCT1 and MCT4 are both thought to be important in cancer cells, NHE1 and NHE3 sometimes coexist in the same tumorigenic cell type, and V-ATPases have the involvement of multiple subunits and subtypes. It was also of interest that the presence and importance of different isoforms of the same transport proteins varies, even in the same general type of cancer. For example, CAIX and CAXII mRNA were detectable in one prostate cancer cell line and not another though induction by hypoxia may be required to demonstrate expression. And, as noted above, knockdown of CAIX can reduce xenograft tumor growth of LS174Tr colon carcinoma cells, while knockout of both CAIX and CAXII gives a much better reduction in tumor growth (Chiche et al. 2009). Thus, it is evident that different isoforms of the same proton transport proteins can contribute more, or less, and both can have significant contributions in tumorigenic cells.
Variations in the importance of different isoforms of proton transport proteins also exist. For example, disruption of the a4 subunit of V-ATPase inhibited invasion by MDA-MB-231 cells, but did not in another breast cancer cell type (Capecci and Forgac 2013; Hinton et al. 2009). Complicating this is the knowledge that some different isoforms of the same proton transport activity are insensitive to inhibitors that affect other isoforms. For example, in the case of Na+/H+ exchanger inhibitors, many are isoform specific, inhibiting NHE1 and not NHE3 (Pedersen et al. 2007). This makes the prospect of targeted therapies, based on knockout or inhibition of one type of proton transporter, difficult. It would seem that a general catalog of the various isoforms of proton transporters in many examples of prostate (and other) cancers could prove of use. It may be that in the era of “personalized medicine,” if clinical targeting of proton transporters comes to a fruition, it might require characterization of the proton transporters present in tumors, prior to treatment. An interesting related study of Meehan et al. (Meehan et al. 2017) examined three different types of breast cancer cell lines. They found that induction of expression of CAIX with hypoxia was cell line dependent. In the breast cancer cell lines MDA-MB-231 and HBL-100, CAIX was highly induced by hypoxia in 2D or 3D cultures, whereas in MCF-7 cells it was not. In contrast, NHE1 was induced in MCF7 cells by hypoxia but not in the other cell types. Differences in effects of CA inhibition on invasion were also noted between the cell lines. In another study comparing MCF7 and MDA-MB-231 cells, inhibition or knockdown of NHE1 had no effect on spheroid growth of MCF7 cells, while in contrast, these treatments decreased spheroid growth of MDA-MB-231 cells (Andersen et al. 2016). These results highlight the differences that can occur between cell lines from the same general type of cancer and emphasize the putative need for individual assessment.
If more than one proton extrusion pathway is important in prostate cancers, it may be that a cocktail of proton transport inhibitors is required to be effective in treatment (Iessi et al. 2017). In this regard one group examined the effect of dual admission of lansoprazole (V-ATPase inhibitor) and CA inhibitors against malignant melanomas. The combination of use of the two inhibitors was more effective than use of one alone (Federici et al. 2016). Presently, V-ATPase inhibitors and CA inhibitors are more developed as therapeutic agents, and the combination of these inhibitors seems a more likely starting point than some other proton transporters (Iessi et al. 2017). Other putative combination therapies could involve NHEs, MCTs, or even uPA inhibitors as anticancer drugs (Buckley et al. 2018). A recent study investigated whether use of proton pump inhibitors by humans is associated with a decreased risk of breast cancer, prostate cancer, and malignant melanoma. The results did not show any chemopreventive effect (Halfdanarson et al. 2019). The authors of this paper noted that the lack of a protective effect could be due to continued extracellular acidification by other means such as NHEs, MCT, CA, and HCO3−-based transporters, supporting the idea that a cocktail of inhibitors may be an improved alternative for treatment. It should be noted that one recent study found that knockout of NHE1 and CAIX led to induction of expression of CAXII in colon cancer cells (Parks et al. 2017). Thus, care needs to be taken to check for induction of expression of potentially compensating proteins during knockouts of other such proteins. Compensation by other isoforms does occur in prostate cancer cells. For example, knockout of both forms of lactate dehydrogenase, A and B, was necessary to ablate lactate production in DU145 tumors (Liu et al. 2018).
With the above in mind, it might be useful to suggest some more immediate future avenues of research in prostate cancer. It would be useful to examine the isoforms of the various proton transport proteins in different prostate cancer cell lines and compare that to the content of patient samples. Additionally, the effect of combinations of inhibitors of different proton transport proteins could be examined in vitro and in vivo. This could be coupled with an examination of the effect of knockout or downregulation of these proteins on invasion and metastatic behavior. Together these studies would provide useful information on potential clinical treatments for the disease.
While the challenges remain considerable, the background knowledge about the proteins involved in regulation of intracellular and extracellular pH has increased greatly in the area and points to many future areas of research that can be explored, and it has great potential to develop clinically useful strategies for treatment.
Abbreviations
- CA:
-
Carbonic anhydrase
- EMT:
-
Epithelial-mesenchymal transition
- MCT:
-
Monocarboxylate transporter
- MMP:
-
Matrix metalloproteinase
- NBC:
-
Sodium bicarbonate cotransporter
- NHE1:
-
Na+/H+ exchanger isoform 1
- pHe:
-
Extracellular pH
- pHi:
-
Intracellular pH
- uPA:
-
Urokinase plasminogen activator
- V-ATPases:
-
Vacuolar-type adenosine triphosphatases
References
Akudugu J, Serafin A, Bohm L (2015) Further evaluation of uPA and PAI-1 as biomarkers for prostatic diseases. J Cancer Res Clin Oncol 141:627–631
Ambrosio MR, Di Serio C, Danza G et al (2016) Carbonic anhydrase IX is a marker of hypoxia and correlates with higher Gleason scores and ISUP grading in prostate cancer. Diagn Pathol 11:45
Amith SR, Fliegel L (2013) Regulation of the Na+/H+ exchanger (NHE1) in breast cancer metastasis. Cancer Res 73:1259–1264
Amith SR, Fliegel L (2016) The Na+/H+ exchanger in metastasis. Aging 8:1291
Amith SR, Fliegel L (2017) Na(+)/H(+) exchanger-mediated hydrogen ion extrusion as a carcinogenic signal in triple-negative breast cancer etiopathogenesis and prospects for its inhibition in therapeutics. Semin Cancer Biol 43:35–41
Amith SR, Fong S, Baksh S et al (2015a) Na (+)/H (+)exchange in the tumour microenvironment: does NHE1 drive breast cancer carcinogenesis? Int J Dev Biol 59:367–377
Amith SR, Wilkinson JM, Baksh S et al (2015b) Na+/H+ exchanger (NHE1) as a novel co-adjuvant target in paclitaxel therapy of triple-negative breast cancer cells. Oncotarget 6:1262–1275
Amith SR, Wilkinson JM, Fliegel L (2016a) KR-33028, a potent inhibitor of the Na+/H+ exchanger NHE1, suppresses metastatic potential of triple-negative breast cancer cells. Biochem Pharmacol 118:31–39
Amith SR, Wilkinson JM, Fliegel L (2016b) Na+/H+ exchanger NHE1 regulation modulates metastatic potential and epithelial-mesenchymal transition of triple-negative breast cancer cells. Oncotarget 7:21091–21113
Amith SR, Vincent KM, Wilkinson JM et al (2017) Defining the Na(+)/H(+) exchanger NHE1 interactome in triple-negative breast cancer cells. Cell Signal 29:69–77
Andersen AP, Moreira JM, Pedersen SF (2014) Interactions of ion transporters and channels with cancer cell metabolism and the tumour microenvironment. Philos Trans R Soc Lond B Biol Sci 369:20130098
Andersen AP, Flinck M, Oernbo EK et al (2016) Roles of acid-extruding ion transporters in regulation of breast cancer cell growth in a 3-dimensional microenvironment. Mol Cancer 15:45
Andersen AP, Samsoe-Petersen J, Oernbo EK et al (2018) The net acid extruders NHE1, NBCn1 and MCT4 promote mammary tumor growth through distinct but overlapping mechanisms. Int J Cancer 142:2529–2542
Angeli A, Trallori E, Ferraroni M et al (2018) Discovery of new 2, 5-disubstituted 1,3-selenazoles as selective human carbonic anhydrase IX inhibitors with potent anti-tumor activity. Eur J Med Chem 157:1214–1222
Avnet S, Lemma S, Cortini M et al (2016) Altered pH gradient at the plasma membrane of osteosarcoma cells is a key mechanism of drug resistance. Oncotarget 7:63408–63423
Azzarito T, Venturi G, Cesolini A et al (2015) Lansoprazole induces sensitivity to suboptimal doses of paclitaxel in human melanoma. Cancer Lett 356:697–703
Boedtkjer E, Bunch L, Pedersen SF (2012) Physiology, pharmacology and pathophysiology of the pH regulatory transport proteins NHE1 and NBCn1: similarities, differences, and implications for cancer therapy. Curr Pharm Des 18:1345–1371
Boedtkjer E, Moreira JM, Mele M et al (2013) Contribution of Na+, HCO3(−)-cotransport to cellular pH control in human breast cancer: a role for the breast cancer susceptibility locus NBCn1 (SLC4A7). Int J Cancer 132:1288–1299
Bohm L, Serafin A, Akudugu J et al (2013) uPA/PAI-1 ratios distinguish benign prostatic hyperplasia and prostate cancer. J Cancer Res Clin Oncol 139:1221–1228
Brant SR, Yun CHC, Donowitz M et al (1995) Cloning, tissue distribution, and functional analysis of the human Na+/H+ exchanger isoform, NHE3. Am J Physiol 269:C198–C206
Buckley BJ, Aboelela A, Minaei E et al (2018) 6-substituted hexamethylene amiloride (HMA) derivatives as potent and selective inhibitors of the human urokinase plasminogen activator for use in cancer. J Med Chem 61:8299–8320
Busco G, Cardone RA, Greco MR et al (2010) NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. FASEB J 24:3903–3915
Canitano A, Iessi E, Spugnini EP et al (2016) Proton pump inhibitors induce a caspase-independent antitumor effect against human multiple myeloma. Cancer Lett 376:278–283
Capecci J, Forgac M (2013) The function of vacuolar ATPase (V-ATPase) a subunit isoforms in invasiveness of MCF10a and MCF10CA1a human breast cancer cells. J Biol Chem 288:32731–32741
Cardone RA, Casavola V, Reshkin SJ (2005) The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer 5:786–795
Chambard JC, Pouyssegur J (1986) Intracellular pH controls growth factor-induced ribosomal protein S6 phosphorylation and protein synthesis in the G0----G1 transition of fibroblasts. Exp Cell Res 164:282–294
Chatterjee S, Schmidt S, Pouli S et al (2014) Membrane androgen receptor sensitive Na+/H+ exchanger activity in prostate cancer cells. FEBS Lett 588:1571–1579
Chiche J, Ilc K, Laferriere J et al (2009) Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res 69:358–368
Chiche J, Le Fur Y, Vilmen C et al (2012) In vivo pH in metabolic-defective Ras-transformed fibroblast tumors: key role of the monocarboxylate transporter, MCT4, for inducing an alkaline intracellular pH. Int J Cancer 130:1511–1520
Choi SY, Xue H, Wu R et al (2016) The MCT4 gene: a novel, potential target for therapy of advanced prostate cancer. Clin Cancer Res 22:2721–2733
Cordat E, Casey JR (2009) Bicarbonate transport in cell physiology and disease. Biochem J 417:423–439
Cordat E, Reithmeier RA (2014) Structure, function, and trafficking of SLC4 and SLC26 anion transporters. Curr Top Membr 73:1–67
Cotter K, Capecci J, Sennoune S et al (2015) Activity of plasma membrane V-ATPases is critical for the invasion of MDA-MB231 breast cancer cells. J Biol Chem 290:3680–3692
Cotter K, Liberman R, Sun-Wada G et al (2016) The a3 isoform of subunit a of the vacuolar ATPase localizes to the plasma membrane of invasive breast tumor cells and is overexpressed in human breast cancer. Oncotarget 7:46142–46157
De Milito A, Fais S (2005) Proton pump inhibitors may reduce tumour resistance. Expert Opin Pharmacother 6:1049–1054
De Milito A, Canese R, Marino ML et al (2010) pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int J Cancer 127:207–219
Debreova M, Csaderova L, Burikova M et al (2019) CAIX regulates invadopodia formation through both a pH-dependent mechanism and interplay with actin regulatory proteins. Int J Mol Sci 20:2745
Dhup S, Dadhich RK, Porporato PE et al (2012) Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des 18:1319–1330
Dubois L, Peeters SG, Van Kuijk SJ et al (2013) Targeting carbonic anhydrase IX by nitroimidazole based sulfamides enhances the therapeutic effect of tumor irradiation: a new concept of dual targeting drugs. Radiother Oncol 108:523–528
Dykes SS, Gao C, Songock WK et al (2017a) Zinc finger E-box binding homeobox-1 (Zeb1) drives anterograde lysosome trafficking and tumor cell invasion via upregulation of Na+/H+ exchanger-1 (NHE1). Mol Carcinog 56:722–734
Dykes SS, Steffan JJ, Cardelli JA (2017b) Lysosome trafficking is necessary for EGF-driven invasion and is regulated by p38 MAPK and Na+/H+ exchangers. BMC Cancer 17:672
Federici C, Lugini L, Marino ML et al (2016) Lansoprazole and carbonic anhydrase IX inhibitors sinergize against human melanoma cells. J Enzyme Inhib Med Chem 31:119–125
Fiaschi T, Giannoni E, Taddei ML et al (2013) Carbonic anhydrase IX from cancer-associated fibroblasts drives epithelial-mesenchymal transition in prostate carcinoma cells. Cell Cycle 12:1791–1801
Gillies RJ, Raghunand N, Karczmar GS et al (2002) MRI of the tumor microenvironment. J Magn Reson Imaging 16:430–450
Gonzalez-Gronow M, Misra UK, Gawdi G et al (2005) Association of plasminogen with dipeptidyl peptidase IV and Na+/H+ exchanger isoform NHE3 regulates invasion of human 1-LN prostate tumor cells. J Biol Chem 280:27173–27178
Grant CM, Kyprianou N (2013) Epithelial mesenchymal transition (EMT) in prostate growth and tumor progression. Transl Androl Urol 2:202–211
Greco MR, Antelmi E, Busco G et al (2014) Protease activity at invadopodial focal digestive areas is dependent on NHE1-driven acidic pHe. Oncol Rep 31:940–946
Guan X, Luo L, Begum G et al (2018) Elevated Na/H exchanger 1 (SLC9A1) emerges as a marker for tumorigenesis and prognosis in gliomas. J Exp Clin Cancer Res 37:255
Halestrap AP (2013) The SLC16 gene family – structure, role and regulation in health and disease. Mol Aspects Med 34:337–349
Halfdanarson OO, Fall K, Ogmundsdottir MH et al (2019) Proton pump inhibitor use and risk of breast cancer, prostate cancer, and malignant melanoma: an Icelandic population-based case-control study. Pharmacoepidemiol Drug Saf 28:471–478
Harguindey S, Reshkin SJ (2017) “The new pH-centric anticancer paradigm in oncology and medicine”; SCB, 2017. Semin Cancer Biol 43:1–4
Harguindey S, Arranz JL, Wahl ML et al (2009) Proton transport inhibitors as potentially selective anticancer drugs. Anticancer Res 29:2127–2136
Harguindey S, Arranz JL, Polo Orozco JD et al (2013) Cariporide and other new and powerful NHE1 inhibitors as potentially selective anticancer drugs--an integral molecular/biochemical/metabolic/clinical approach after one hundred years of cancer research. J Transl Med 11:282
Harguindey S, Stanciu D, Devesa J et al (2017) Cellular acidification as a new approach to cancer treatment and to the understanding and therapeutics of neurodegenerative diseases. Semin Cancer Biol 43:157–179
Harguindey S, Koltai T, Reshkin SJ (2018) Curing cancer? Further along the new pH-centric road and paradigm. Oncoscience 5:132–133
Hayashi H, Aharonovitz O, Alexander RT et al (2008) Na+/H+ exchange and pH regulation in the control of neutrophil chemokinesis and chemotaxis. Am J Physiol Cell Physiol 294:C526–C534
Helmlinger G, Yuan F, Dellian M et al (1997) Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med 3:177–182
Hinton A, Sennoune SR, Bond S et al (2009) Function of a subunit isoforms of the V-ATPase in pH homeostasis and in vitro invasion of MDA-MB231 human breast cancer cells. J Biol Chem 284:16400–16408
Hyuga S, Wada H, Eguchi H et al (2017) Expression of carbonic anhydrase IX is associated with poor prognosis through regulation of the epithelialmesenchymal transition in hepatocellular carcinoma. Int J Oncol 51:1179–1190
Iessi E, Logozzi M, Mizzoni D et al (2017) Rethinking the combination of proton exchanger inhibitors in cancer therapy. Metabolites 8:2
Ivanov S, Liao SY, Ivanova A et al (2001) Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol 158:905–919
Klier M, Andes FT, Deitmer JW et al (2014) Intracellular and extracellular carbonic anhydrases cooperate non-enzymatically to enhance activity of monocarboxylate transporters. J Biol Chem 289:2765–2775
Koltai T (2017) Triple-edged therapy targeting intracellular alkalosis and extracellular acidosis in cancer. Semin Cancer Biol 43:139–146
Kong SC, Giannuzzo A, Novak I et al (2014) Acid-base transport in pancreatic cancer: molecular mechanisms and clinical potential. Biochem Cell Biol 92:449–459
Koppenol WH, Bounds PL, Dang CV (2011) Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer 11:325–337
Korenchan DE, Bok R, Sriram R et al (2019) Hyperpolarized in vivo pH imaging reveals grade-dependent acidification in prostate cancer. Oncotarget 10:6096–6110
Lang SH, Hyde C, Reid IN et al (2002) Enhanced expression of vimentin in motile prostate cell lines and in poorly differentiated and metastatic prostate carcinoma. Prostate 52:253–263
Lauritzen G, Jensen MB, Boedtkjer E et al (2010) NBCn1 and NHE1 expression and activity in DeltaNErbB2 receptor-expressing MCF-7 breast cancer cells: contributions to pHi regulation and chemotherapy resistance. Exp Cell Res 316:2538–2553
Lauritzen G, Stock CM, Lemaire J et al (2012) The Na+/H+ exchanger NHE1, but not the Na+, HCO3(−) cotransporter NBCn1, regulates motility of MCF7 breast cancer cells expressing constitutively active ErbB2. Cancer Lett 317:172–183
Le Floch R, Chiche J, Marchiq I et al (2011) CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc Natl Acad Sci U S A 108:16663–16668
Lebelo MT, Joubert AM, Visagie MH (2019) Warburg effect and its role in tumourigenesis. Arch Pharm Res 42:833–847
Lee S, Li JM, Choi I (2018a) Sodium bicarbonate cotransporter NBCe1 affects the growth and motility of prostate cancer cell lines LNCaP and PC3. FASEB J 32(1):IB411
Lee S, Axelsen TV, Jessen N et al (2018b) Na(+),HCO3(−)-cotransporter NBCn1 (Slc4a7) accelerates ErbB2-induced breast cancer development and tumor growth in mice. Oncogene 37:5569–5584
Lee SH, Mcintyre D, Honess D et al (2018c) Carbonic anhydrase IX is a pH-stat that sets an acidic tumour extracellular pH in vivo. Br J Cancer 119:622–630
Li X, Alvarez B, Casey JR et al (2002) Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. J Biol Chem 277:36085–36091
Li X, Liu Y, Alvarez BV et al (2006) A novel carbonic anhydrase II binding site regulates NHE1 activity. Biochemistry 45:2414–2424
Li Y, Wang H, Oosterwijk E et al (2009) Antibody-specific detection of CAIX in breast and prostate cancers. Biochem Biophys Res Commun 386:488–492
Liang C, Niu L, Xiao Z et al (2019) Whole-genome sequencing of prostate cancer reveals novel mutation-driven processes and molecular subgroups. Life Sci:117218. https://doi.org/10.1016/j.lfs.2019.117218
Licon-Munoz Y, Michel V, Fordyce CA et al (2017) F-actin reorganization by V-ATPase inhibition in prostate cancer. Biol Open 6:1734–1744
Lim JH, Park JW, Kim MS et al (2006) Bafilomycin induces the p21-mediated growth inhibition of cancer cells under hypoxic conditions by expressing hypoxia-inducible factor-1alpha. Mol Pharmacol 70:1856–1865
Lin CY, Wang SS, Yang CK et al (2019) Genetic polymorphism and carbonic anhydrase 9 expression can predict nodal metastatic prostate cancer risk in patients with prostate-specific antigen levels </=10 ng/ml at initial biopsy. Urol Oncol 37:814.e9–814.e16
Lippert S, Berg KD, Hoyer-Hansen G et al (2016) Copenhagen uPAR prostate cancer (CuPCa) database: protocol and early results. Biomark Med 10:209–216
Liu J, Chen G, Liu Z et al (2018) Aberrant FGFR tyrosine kinase signaling enhances the Warburg effect by reprogramming LDH isoform expression and activity in prostate cancer. Cancer Res 78:4459–4470
Lock FE, Mcdonald PC, Lou Y et al (2013) Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene 32:5210–5219
Lou Y, Mcdonald PC, Oloumi A et al (2011) Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res 71:3364–3376
Lu X, Qin W, Li J et al (2005) The growth and metastasis of human hepatocellular carcinoma xenografts are inhibited by small interfering RNA targeting to the subunit ATP6L of proton pump. Cancer Res 65:6843–6849
Lugini L, Federici C, Borghi M et al (2016) Proton pump inhibitors while belonging to the same family of generic drugs show different anti-tumor effect. J Enzyme Inhib Med Chem 31:538–545
Lugini L, Sciamanna I, Federici C et al (2017) Antitumor effect of combination of the inhibitors of two new oncotargets: proton pumps and reverse transcriptase. Oncotarget 8:4147–4155
Mahoney BP, Raghunand N, Baggett B et al (2003) Tumor acidity, ion trapping and chemotherapeutics. I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem Pharmacol 66:1207–1218
Malakooti J, Dahdal RY, Schmidt L et al (1999) Molecular cloning, tissue distribution, and functional expression of the human Na(+)/H(+) exchanger NHE2. Am J Physiol 277:G383–G390
Marchiq I, Le Floch R, Roux D et al (2015) Genetic disruption of lactate/H+ symporters (MCTs) and their subunit CD147/BASIGIN sensitizes glycolytic tumor cells to phenformin. Cancer Res 75:171–180
Margheri F, Luciani C, Taddei ML et al (2014) The receptor for urokinase-plasminogen activator (uPAR) controls plasticity of cancer cell movement in mesenchymal and amoeboid migration style. Oncotarget 5:1538–1553
Marin-Aguilera M, Reig O, Lozano JJ et al (2015) Molecular profiling of peripheral blood is associated with circulating tumor cells content and poor survival in metastatic castration-resistant prostate cancer. Oncotarget 6:10604–10616
Martinez-Zaguilan R, Lynch RM, Martinez GM et al (1993) Vacuolar-type H(+)-ATPases are functionally expressed in plasma membranes of human tumor cells. Am J Physiol 265:C1015–C1029
Martins BX, Arruda RF, Costa GA et al (2019) Myrtenal-induced V-ATPase inhibition – a toxicity mechanism behind tumor cell death and suppressed migration and invasion in melanoma. Biochim Biophys Acta Gen Subj 1863:1–12
Mcconnell M, Feng S, Chen W et al (2017) Osteoclast proton pump regulator Atp6v1c1 enhances breast cancer growth by activating the mTORC1 pathway and bone metastasis by increasing V-ATPase activity. Oncotarget 8:47675–47690
Mcguire CM, Collins MP, Sun-Wada G et al (2019) Isoform-specific gene disruptions reveal a role for the V-ATPase subunit a4 isoform in the invasiveness of 4T1-12B breast cancer cells. J Biol Chem 294:11248–11258
Mcintyre A, Harris AL (2016) The role of pH regulation in cancer progression. Recent Results Cancer Res 207:93–134
Meehan J, Ward C, Turnbull A et al (2017) Inhibition of pH regulation as a therapeutic strategy in hypoxic human breast cancer cells. Oncotarget 8:42857–42875
Merk H, Messer P, Ardelt MA et al (2017) Inhibition of the V-ATPase by Archazolid A: a new strategy to inhibit EMT. Mol Cancer Ther 16:2329–2339
Michel V, Licon-Munoz Y, Trujillo K et al (2013) Inhibitors of vacuolar ATPase proton pumps inhibit human prostate cancer cell invasion and prostate-specific antigen expression and secretion. Int J Cancer 132:E1–E10
Nauseef JT, Henry MD (2011) Epithelial-to-mesenchymal transition in prostate cancer: paradigm or puzzle? Nat Rev Urol 8:428–439
Nishisho T, Hata K, Nakanishi M et al (2011) The a3 isoform vacuolar type H(+)-ATPase promotes distant metastasis in the mouse B16 melanoma cells. Mol Cancer Res 9:845–855
Noor SI, Dietz S, Heidtmann H et al (2015) Analysis of the binding moiety mediating the interaction between monocarboxylate transporters and carbonic anhydrase II. J Biol Chem 290:4476–4486
Odero-Marah V, Hawsawi O, Henderson V et al (2018) Epithelial-mesenchymal transition (EMT) and prostate cancer. Adv Exp Med Biol 1095:101–110
Pacchiano F, Carta F, Mcdonald PC et al (2011) Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem 54:1896–1902
Parks SK, Pouyssegur J (2015) The Na(+)/HCO3(−) Co-transporter SLC4A4 plays a role in growth and migration of colon and breast cancer cells. J Cell Physiol 230:1954–1963
Parks SK, Pouyssegur J (2017) Targeting pH regulating proteins for cancer therapy-Progress and limitations. Semin Cancer Biol 43:66–73
Parks SK, Chiche J, Pouyssegur J (2011) pH control mechanisms of tumor survival and growth. J Cell Physiol 226:299–308
Parks SK, Chiche J, Pouyssegur J (2013) Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer 13:611–623
Parks SK, Cormerais Y, Durivault J et al (2017) Genetic disruption of the pHi-regulating proteins Na+/H+ exchanger 1 (SLC9A1) and carbonic anhydrase 9 severely reduces growth of colon cancer cells. Oncotarget 8:10225–10237
Pastorekova S, Gillies RJ (2019) The role of carbonic anhydrase IX in cancer development: links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev 38:65–77
Pedersen SF, King SA, Nygaard EB et al (2007) NHE1 inhibition by amiloride- and benzoylguanidine-type compounds. Inhibitor binding loci deduced from chimeras of NHE1 homologues with endogenous differences in inhibitor sensitivity. J Biol Chem 282:19716–19727
Peppicelli S, Andreucci E, Ruzzolini J et al (2017) The acidic microenvironment as a possible niche of dormant tumor cells. Cell Mol Life Sci 74:2761–2771
Pertega-Gomes N, Vizcaino JR, Miranda-Goncalves V et al (2011) Monocarboxylate transporter 4 (MCT4) and CD147 overexpression is associated with poor prognosis in prostate cancer. BMC Cancer 11:312
Pertega-Gomes N, Felisbino S, Massie CE et al (2015) A glycolytic phenotype is associated with prostate cancer progression and aggressiveness: a role for monocarboxylate transporters as metabolic targets for therapy. J Pathol 236:517–530
Pouysségur J, Franchi A, L’allemain G et al (1985) Cytoplasmic pH, a key determinant of growth factor-induced DNA synthesis in quiescent fibroblasts. FEBS Lett 190:115–119
Proescholdt MA, Merrill MJ, Stoerr EM et al (2012) Function of carbonic anhydrase IX in glioblastoma multiforme. Neuro Oncol 14:1357–1366
Pulukuri SM, Gondi CS, Lakka SS et al (2005) RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo. J Biol Chem 280:36529–36540
Raghunand N, Mahoney BP, Gillies RJ (2003) Tumor acidity, ion trapping and chemotherapeutics. II. pH-dependent partition coefficients predict importance of ion trapping on pharmacokinetics of weakly basic chemotherapeutic agents. Biochem Pharmacol 66:1219–1229
Reshkin SJ, Cardone RA, Harguindey S (2013) Na+-H+ exchanger, pH regulation and cancer. Recent Pat Anticancer Drug Discov 8:85–99
Reshkin SJ, Greco MR, Cardone RA (2014) Role of pHi, and proton transporters in oncogene-driven neoplastic transformation. Philos Trans R Soc Lond B Biol Sci 369:20130100
Romero MF, Boron WF (1999) Electrogenic Na+/HCO3− cotransporters: cloning and physiology. Annu Rev Physiol 61:699–723
Saha B, Kaur P, Tsao-Wei D et al (2008) Unmethylated E-cadherin gene expression is significantly associated with metastatic human prostate cancer cells in bone. Prostate 68:1681–1688
Schwab A, Fabian A, Hanley PJ et al (2012) Role of ion channels and transporters in cell migration. Physiol Rev 92:1865–1913
Sennoune SR, Bakunts K, Martinez GM et al (2004a) Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol 286:C1443–C1452
Sennoune SR, Luo D, Martinez-Zaguilan R (2004b) Plasmalemmal vacuolar-type H+-ATPase in cancer biology. Cell Biochem Biophys 40:185–206
Sethi S, Macoska J, Chen W et al (2010) Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res 3:90–99
Siegel R, Ma J, Zou Z et al (2014) Cancer statistics, 2014. CA Cancer J Clin 64:9–29
Singh S, Sadacharan S, Su S et al (2003) Overexpression of vimentin: role in the invasive phenotype in an androgen-independent model of prostate cancer. Cancer Res 63:2306–2311
Skovgaard D, Persson M, Kjaer A (2017a) Imaging of prostate cancer using urokinase-type plasminogen activator receptor PET. PET Clin 12:243–255
Skovgaard D, Persson M, Kjaer A (2017b) Urokinase plasminogen activator receptor-PET with (68)Ga-NOTA-AE105: first clinical experience with a novel PET ligand. PET Clin 12:311–319
Smith BN, Odero-Marah VA (2012) The role of Snail in prostate cancer. Cell Adh Migr 6:433–441
Smith GA, Howell GJ, Phillips C et al (2016) Extracellular and luminal pH regulation by vacuolar H+-ATPase isoform expression and targeting to the plasma membrane and endosomes. J Biol Chem 291:8500–8515
Smyth LG, O’hurley G, O’grady A et al (2010) Carbonic anhydrase IX expression in prostate cancer. Prostate Cancer Prostatic Dis 13:178–181
Spugnini E, Fais S (2017) Proton pump inhibition and cancer therapeutics: a specific tumor targeting or it is a phenomenon secondary to a systemic buffering? Semin Cancer Biol 43:111–118
Spugnini EP, Baldi A, Buglioni S et al (2011) Lansoprazole as a rescue agent in chemoresistant tumors: a phase I/II study in companion animals with spontaneously occurring tumors. J Transl Med 9:221
Spugnini EP, Buglioni S, Carocci F et al (2014) High dose lansoprazole combined with metronomic chemotherapy: a phase I/II study in companion animals with spontaneously occurring tumors. J Transl Med 12:225
Steffan JJ, Snider JL, Skalli O et al (2009) Na+/H+ exchangers and RhoA regulate acidic extracellular pH-induced lysosome trafficking in prostate cancer cells. Traffic 10:737–753
Steffan JJ, Williams BC, Welbourne T et al (2010) HGF-induced invasion by prostate tumor cells requires anterograde lysosome trafficking and activity of Na+-H+ exchangers. J Cell Sci 123:1151–1159
Stock C, Cardone RA, Busco G et al (2008) Protons extruded by NHE1: digestive or glue? Eur J Cell Biol 87:591–599
Sun XC, Bonanno JA (2003) Identification and cloning of the Na/HCO(3−) cotransporter (NBC) in human corneal endothelium. Exp Eye Res 77:287–295
Svastova E, Hulikova A, Rafajova M et al (2004) Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett 577:439–445
Todenhofer T, Seiler R, Stewart C et al (2018) Selective inhibition of the lactate transporter MCT4 reduces growth of invasive bladder cancer. Mol Cancer Ther 17:2746–2755
Ullah MS, Davies AJ, Halestrap AP (2006) The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem 281:9030–9037
Vaupel P (2004) Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol 14:198–206
Vaupel PW, Frinak S, Bicher HI (1981) Heterogeneous oxygen partial pressure and pH distribution in C3H mouse mammary adenocarcinoma. Cancer Res 41:2008–2013
Wang BY, Zhang J, Wang JL et al (2015) Intermittent high dose proton pump inhibitor enhances the antitumor effects of chemotherapy in metastatic breast cancer. J Exp Clin Cancer Res 34:85
Wang H, Long X, Wang D et al (2018) Increased expression of Na(+)/H(+) exchanger isoform 1 predicts tumor aggressiveness and unfavorable prognosis in epithelial ovarian cancer. Oncol Lett 16:6713–6720
Webb BA, Chimenti M, Jacobson MP et al (2011) Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 11:671–677
Whitton B, Okamoto H, Packham G et al (2018) Vacuolar ATPase as a potential therapeutic target and mediator of treatment resistance in cancer. Cancer Med 7:3800–3811
Xie R, Wang H, Jin H et al (2017) NHE1 is upregulated in gastric cancer and regulates gastric cancer cell proliferation, migration and invasion. Oncol Rep 37:1451–1460
Xu J, Lamouille S, Derynck R (2009) TGF-beta-induced epithelial to mesenchymal transition. Cell Res 19:156–172
Xu X, Liu B, Zou P et al (2014a) Silencing of LASS2/TMSG1 enhances invasion and metastasis capacity of prostate cancer cell. J Cell Biochem 115:731–743
Xu X, You J, Pei F (2014b) LASS2/TMSG1 gene silencing promotes the invasiveness and metastatic of human prostatic carcinoma cells through increase in vacuolar ATPase activity. Zhonghua Bing Li Xue Za Zhi 43:177–183
Yamada H, Yamazaki S, Moriyama N et al (2003) Localization of NBC-1 variants in human kidney and renal cell carcinoma. Biochem Biophys Res Commun 310:1213–1218
Yang OCY, Loh SH (2019) Acidic stress triggers sodium-coupled bicarbonate transport and promotes survival in A375 human melanoma cells. Sci Rep 9:6858
Yang X, Wang D, Dong W et al (2010) Inhibition of Na(+)/H(+) exchanger 1 by 5-(N-ethyl-N-isopropyl) amiloride reduces hypoxia-induced hepatocellular carcinoma invasion and motility. Cancer Lett 295:198–204
Yuen HF, Kwok WK, Chan KK et al (2008) TWIST modulates prostate cancer cell-mediated bone cell activity and is upregulated by osteogenic induction. Carcinogenesis 29:1509–1518
Zhu WB, Zhao ZF, Zhou X (2018) AMD3100 inhibits epithelial-mesenchymal transition, cell invasion, and metastasis in the liver and the lung through blocking the SDF-1alpha/CXCR4 signaling pathway in prostate cancer. J Cell Physiol 234:11746–11759
Zou P, Yang Y, Xu X et al (2018) Silencing of vacuolar ATPase c subunit ATP6V0C inhibits the invasion of prostate cancer cells through a LASS2/TMSG1-independent manner. Oncol Rep 39:298–306
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Supplementary Table 1
Summary of studies that directly point to a role for, or upregulation of, net acid-extruding transporters (and urokinase plasminogen activator) in prostate cancer. (PC, prostate cancer; uPA, urokinase plasminogen activator) (DOCX 53 kb))
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fliegel, L. (2020). Role of pH Regulatory Proteins and Dysregulation of pH in Prostate Cancer. In: Stock, C., Pardo, L.A. (eds) From Malignant Transformation to Metastasis. Reviews of Physiology, Biochemistry and Pharmacology, vol 182. Springer, Cham. https://doi.org/10.1007/112_2020_18
Download citation
DOI: https://doi.org/10.1007/112_2020_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99799-1
Online ISBN: 978-3-030-99800-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)