Abstract
The Transient receptor potential (TRP) family of cation channels is a large protein family, which is mainly structurally uniform. Proteins consist typically of six transmembrane domains and mostly four subunits are necessary to form a functional channel. Apart from this, TRP channels display a wide variety of activation mechanisms (ligand binding, G-protein coupled receptor dependent, physical stimuli such as temperature, pressure, etc.) and ion selectivity profiles (from highly Ca2+ selective to non-selective for cations). They have been described now in almost every tissue of the body, including peripheral and central neurons. Especially in the sensory nervous system the role of several TRP channels is already described on a detailed level. This review summarizes data that is currently available on their role in the central nervous system. TRP channels are involved in neurogenesis and brain development, synaptic transmission and they play a key role in the development of several neurological diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Obsessive Compulsive Disorder
- Neurite Outgrowth
- Growth Cone
- Transient Receptor Potential
- Transient Receptor Potential Channel
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 The Transient Receptor Potential Family of Cation Channels
The transient receptor potential (TRP) multigene superfamily encodes integral membrane proteins that function as ion channels. Members of this family are conserved in yeast, invertebrates and vertebrates. All members TRP channels are subdivided into seven subfamilies: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPP (poly-cystin), TRPML (mucolipin), TRPA (ankyrin) and TRPN (NOMPC-like), which is only found in invertebrates. Of the 6 mammalian subfamilies, 28 members are known, with only 27 in humans (TRPC2 is a pseudogene; see Fig. 1) (Nilius and Owsianik 2011).
It is clear from the current state of the literature that almost all 28 members of mammalian TRP channel play a key role in establishing the five classical senses described in De Anima (book II, 350 B.C.) by Aristotle, which allow humans to perceive the outside world: sight (visus), hearing (auditus), smell (olfactus), taste (gustus) and touch (contactus). For instance, recent studies have firmly established the role of temperature-sensitive TRPs (thermoTRPs) as the principal molecular thermometers in the peripheral sensory system, and provided the first molecular insight into the mechanisms underlying the exquisite thermo- and chemosensitivity of these channels. However, also for balance (or equilibrioreception), which is now also considered as a sixth exteroceptive sense, and the interoceptive senses, that provide information from within the body (e.g. proprioception informs the brain about the relative position of muscles and joints), TRP channels play an essential role (for an extensive review see Damann et al. 2008).
Now, it is widely recognized that TRP channels play a much wider role in the nervous system. They are involved in many homeostatic functions and, importantly, play an essential role in our brain much beyond their function as cell sensors (see Fig. 2).
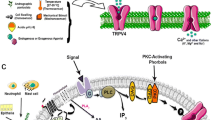
Schematic representation of the proposed roles of TRP channels in neurons. TRP channels are cation channels that constitute an influx pathway for Ca2+, Na+ and/or Mg2+. Most TRP channels are Ca2+ permeable, except TRPM4 and TRPM5, which permeate exclusively monovalent cations. TRPM6 and TRPM7 are Mg2+ permeable. TRP channels are activated by endogenous ligands (e.g. Endocannabinois, pregnenolonsulphate), physical and mechanical stimuli (heat, cold, stretch) and/or through receptor-activated Gq coupled intracellular signalling pathways. Basically, TRP channels influence Ca2+ signalling by allowing Ca2+ to enter the cell directly, or through membrane depolarisation which provides the trigger for voltage-gated Ca2+ channels to activate, or which limits the driving force for Ca2+ entry. A depolarisation mediated by TRP channels as such will influence the firing of action potentials in neurons. All these principal effects will lead to downstream signalling events mediated by other proteins (including exocytosis, gene expression, growth cone migration, etc.). For more details, see the text
TRPCs are highly expressed in various parts of the brain (for a complete overview, see Table 1). They function generally as receptor-activated ion channels and have been implicated in the formation of synapses in the developing brain, amongst others. Among all 28 mammalian TRPs, TRPV1 is probably the best-studied TRP channel in neurons. In the peripheral nervous system it is critically involved in nociception via sensory C and A∂ fibres, and is activated by the ‘hot’ and pungent capsaicin and heat. This channel is also expressed in central neurons and plays a very important ‘non-sensory role’ in brain. The expression of other Vanilloid TRP channels has also been reported in different brain structures. TRPV2 expression has been shown in hippocampal neurons cultures and co-localized with TRPV1 in rat cortex (Liapi and Wood 2005). TRPV4 is detected in rat and mouse hippocampus (Gao et al. 2004; Shibasaki et al. 2007) and in substancia nigra (Guatteo et al. 2005).
The TRPM subfamily has eight members and has been named after the first identified member “Melastatin”. Some of these channels are expressed in the central nervous system. TRPM2 is a Ca2+ permeable ion channel, expressed in hippocampal pyramidal neurons (Bai and Lipski 2010; Xie et al. 2012) and in dopaminergic neurons in substantia nigra (Freestone et al. 2009; Chung et al. 2011; Mrejeru et al. 2011). TRPM3 is highly expressed in the dentate gyrus, the hippocampus and likely plays a role during the development of the cerebellum (Lee et al. 2010) (Zamudio-Bulcock et al. 2011; Zamudio-Bulcock and Valenzuela 2011). TRPM4 and TRPM5 mRNA are also detected in the central nervous system. RT PCR experiments showed TRPM4 and TRPM5 expression in brain extracts from mouse and rat (Launay et al. 2002; Crowder et al. 2007; Yoo et al. 2010). TRPM5 is highly detectable by ISH and using reporter mice in the olfactory bulb and to a lesser extent in the thalamus (Lin et al. 2007). TRPM7 was detected on the mRNA and protein level in cell bodies from hippocampal neurons, cerebral neurons and cerebrospinal-fluid contacting neurons (Fonfria et al. 2006; Wei et al. 2007; Cook et al. 2009; Coombes et al. 2011; Zhang et al. 2011a). Finally, also TRPA1, TRPP1 and TRP-ML have been reported in the brain (see Table 1).
2 Clues for the Role of TRP Channels in the Development of the Brain and Neuronal Function
2.1 Axon Guidance, Growth Cone Tuning and TRPC’s
Axon guidance and neurite outgrowth are essential processes in the developing brain. Establishment of functional and morphological polarity of the neuronal cell is an important step in the formation of synapses and neuronal networks. Several essential signalling pathways have been identified already in this process, including Gq coupled receptors and tyrosine kinase linked receptors, but a key feature is obviously the regulation of the intracellular Ca2+ signaling in the growth cone. In neuronal growth cones, spatiotemporally distinct Ca2+waves can be detected upon receptor stimulation, and in their absence normal neuronal differentiation is prevented. Thus, these Ca2+ signals are in effect the link between external stimuli and processes such as growth-cone protrusion, axonal pathfinding and formation of synaptic contacts. These Ca2+ waves are largely dependent on the activity of Ca2+permeable ion channels, and it’s clear that TRPC channels are important candidates for a role in the developing brain (Tai et al. 2009). Indeed, Ca2+ influx via TRPC channels appears to be a critical component of the signalling cascade that mediates the guidance of growth cones and survival of neurons in response to chemical cues such as neurotrophins or Netrin-1 (Wang and Poo 2005) (Talavera et al. 2008). The role of TRPC in growth cone path finding has been reviewed already by several groups (Bezzerides et al. 2004; Moran et al. 2004; Wang and Poo 2005).
The first report on a TRPC channel as a regulator of neurite length and growth cone morphology (Greka et al. 2003) showed that TRPC5 expression is inversely related to hippocampal neurite length. Knockdown of channel activity by overexpressing a dominant-negative mutant channel allowed significantly longer neuritis and filopodia to form. TRPC5 knockout mice harbour long, highly branched granule neuron dendrites with impaired dendritic claw differentiation in the cerebellar cortex. Apparently, TRPC5 regulates dendrite morphogenesis in the cerebellar cortex in a cell-autonomous manner. Behavioral analyses reveal that TRPC5 knockout mice have deficits in gait and motor coordination and display diminished fear-levels in response to aversive stimuli. The protein kinase calcium/calmodulin-dependent kinase II beta (CaMKIIβ) is a critical effector of TRPC5 function in neurons. TRPC5 forms a complex specifically with CaMKIIβ, but not the closely related kinase CaMKIIα, and thereby induces the CaMKIIβ-dependent phosphorylation of the ubiquitin ligase Cdc20-APC at the centrosome. Accordingly, centrosomal CaMKIIβ signaling mediates the ability of TRPC5 to regulate dendrite morphogenesis in neurons (Puram et al. 2011). A role of TRPC5 in growth cone regulation also seems to involve Semaphorin 3A, a member of a class of growth-cone guidance – proteins. This protein mediates growth cone collapse, which is reduced in hippocampal neurons from Trpc5 −/− mice. This effect is due to an inhibition of the calcium-sensitive protease calpain in wild-type neurons but not in Trpc5 −/− neurons. Calpain-1 and calpain-2 cleave and functionally activate TRPC5. Semaphorin 3A initiates growth cone collapse via activation of calpain that in turn potentiates TRPC5 activity. Thus, TRPC5 acts downstream of semaphorin signaling and modulates neuronal growth cone morphology and neuron development (Kaczmarek et al. 2012).
Other TRPC channels implicated in modulating neurite outgrowth, include TRPC1 and TRPC6 (Li et al. 2005; Shim et al. 2009; Tai et al. 2009). Interestingly, though these ion channels, like TRPC5, each constitute Ca2+ permeable channels, their role in regulation of neurite outgrowth is often opposite; indicating that spatio-temporal regulation of these channels is critical for proper regulation of neuronal morphogenesis (Kumar et al. 2012).
TRPC1 seems to be specifically essential for early neurogenesis. In hippocampal development, proliferation of an adult neural progenitor cell (aNPC) is a critical first step. TRPC1 is the most significantly upregulated TRPC channel during neurogenesis and knockdown of TRPC1 markedly reduced the degree of aNPC proliferation. Specifically, suppression of aNPC proliferation was found to be associated with cell cycle arrest in G0/G1 phase (Li et al. 2012). Hence, TRPC1 plays probably an important role in hippocampal neurogenesis. Importantly, this mechanism is discussed as a tool for improving adult hippocampal neurogenesis and treating cognitive deficits (Li et al. 2012).
Furthermore, in a model system for neuritogenesis, i.e. nerve growth factor (NGF)-differentiated rat pheochromocytoma 12 (PC12) cells, it was shown that NGF markedly up-regulated TRPC1 and TRPC6 expression, but down-regulated TRPC5 expression, while promoting neurite outgrowth. Overexpression of TRPC1 augmented, whereas TRPC5 overexpression decelerated NGF-induced neurite outgrowth. Conversely, shRNA-mediated knockdown of TRPC1 decreased, whereas shRNA-mediated knockdown of TRPC5 increased NGF-induced neurite extension. TRPC6 overexpression slowed down neuritogenesis, whereas dominant negative TRPC6 (DN-TRPC6) facilitated neurite outgrowth in NGF-differentiated PC12 cells. Using pharmacological and molecular biological approaches, it was shown that NGF up-regulated TRPC1 and TRPC6 expression via a p75(NTR) -IKK(2) -dependent pathway that did not involve TrkA receptor signalling in PC12 cells. Similarly, NGF up-regulated TRPC1 and TRPC6 via an IKK(2) dependent pathway in primary cultured hippocampal neurons. Thus, it can be suggested that a balance of TRPC1, TRPC5, and TRPC6 expression determines neurite extension rate in neural cells, with TRPC6 emerging as an NGF-dependent “molecular damper” maintaining a submaximal velocity of neurite extension (Kumar et al. 2012).
In another study, the effects of TRPC channels and Stromal Interaction Molecule (STIM)1-induced store-operated Ca2+ entry on neurite outgrowth of PC12 cells were investigated. In general, it is now firmly established that upon depletion of intracellular Ca2+ stores, STIM1 activates store-operated channels in the plasma membrane (mainly members of the ORAI family). STIM1 and Orai assemble in puncta in the ER membrane upon Ca2+ store depletion and during growth cone turning. STIM1 knockdown perturbed growth cone turning responses to BDNF and semaphorin-3a (Sema-3a) (Mitchell et al. 2012). It was also shown that PC12 cell differentiation down-regulates TRPC5 expression, whereas TRPC1 expression is retained and transfection of TRPC1 and TRPC5 increased the receptor-activated Ca2+ influx that was in turn markedly augmented by the co-expression of STIM1. Accordingly, overexpression of TRPC1 in PC12 cells increased neurite outgrowth while that of TRPC5 suppressed it. Clearly, suppression of neurite outgrowth by TRPC5 requires the channel function of TRPC5. Strikingly however, multiple lines of evidence show that the TRPC1-induced neurite outgrowth was independent of TRPC1-mediated Ca2+ influx. Thus, TRPC1 and TRPC5 similarly increased Ca2+ influx but only TRPC1 induced neurite outgrowth, the constitutively STIM1(D76A) mutant that activates Ca2+ influx by TRPC and Orai channels did not increase neurite outgrowth, and a channel-dead pore mutant of TRPC1 increased neurite outgrowth to the same extent as WT TRPC1. Regulation of neurite outgrowth by TRPC1 thus seems independent of Ca2+ influx and TRPC1-promoted neurite outgrowth depends on the surface expression of TRPC1. Therefore, the possibility remains that TRPC1 merely acts as a scaffold at the cell surface to assemble a signaling complex to stimulate neurite outgrowth (Heo et al. 2012).
Golli proteins, products of the myelin basic protein gene (MBP), function as a new type of modulator of intracellular Ca2+ levels in oligodendrocyte progenitor cells (OPCs). They affect a number of Ca2+-dependent functions, such as OPC migration and process extension. Pharmacologically induced Ca2+ release from intracellular stores evokes a significant extracellular Ca2+ entry after store depletion in OPCs, and Golli promoted activation of Ca2+ influx by SOCCs in cultured OPCs as well as in tissue slices. Strikingly, using a small interfering RNA knockdown approach, it was shown that TRPC1 is involved in SOCC in OPCs and is modulated by golli. Golli is physically associated with TRPC1 at OPC processes and TRPC1 expression is essential for the effects of golli on OPC proliferation. Thus, Ca2+ uptake through TRPC1 is an essential component in the mechanism of OPC proliferation (Paez et al. 2011).
It is also know that bone morphogenic proteins (BMPs) are involved in axon pathfinding. Indeed, a BMP7 gradient causes bidirectional turning responses from nerve growth cones. This effect is due to activation of the kinase LIM (LIMK) and the phosphatase Slingshot (SSH). Both enzymes regulate actin dynamics by modulating the actin-depolymerizing factor (ADF)/cofilin-mediated actin dynamics. This interaction requires the expression of TRPC1. It was suggested that TRPC1 mediated Ca2+ signals thus support, through calcineurin phosphatase, SSH activation and growth cone repulsion (Wen et al. 2007).
Another important player in the developing brain is Wnt5a. It has been shown in vivo that Wnt5a gradients surround the corpus callosum and guide callosal axons by Wnt5a induced repulsion, which also involves Ryk receptors. Application of pharmacological inhibitors to acute brain slices revealed a signalling pathway involving Ca2+release through IP3 receptors and calcium entry, presumably through TRPCs. Expression of Ryk siRNA revealed that knock-down of the Ryk receptor reduced outgrowth rates of postcrossing but not precrossing axons by 50 % and caused axon misrouting. In the corpus callosum CaMKII inhibition reduced the outgrowth rate of postcrossing (but not precrossing) axons and caused severe guidance errors, which resulted from reduced CaMKII-dependent repulsion downstream of Wnt/calcium signalling (Hutchins et al. 2010). Wnt5a is thought to propel cortical axons down the corticospinal tract and through the corpus callosum by repulsive mechanisms. In cultured dissociated early postnatal cortical neurons from hamsters, exposure to a gradient of Wnt5a is a model for studying the mechanism of Wnt5a effects. Turning assays indicated that cortical axons were repelled away from a point source of Wnt5a. Surprisingly, during the 1-h turning assay, axons exposed to Wnt5a also increased their growth rates by almost 50 %. Ryk receptors but not Frizzled (Fz) receptors were required for Wnt5a-promoted axon outgrowth, whereas both Ryk and Fz receptors were required for repulsive growth-cone turning. Both Ryk and Fz receptors mediated calcium signalling, which is required for axon outgrowth and repulsive turning. Treatments with pharmacological inhibitors revealed that distinct Ca2+ signalling mechanisms were involved in Wnt5a-dependent axon outgrowth versus repulsive guidance. Ca2+ release from intracellular stores through inositol 1,4,5-trisphosphate receptors was required for Wnt5a-induced axon outgrowth but not for repulsive turning. In contrast, Ca2+ entry through TRPCs was required for both repulsive growth-cone turning and Wnt5a-increased axon outgrowth. Taken together, these results indicate that a guidance cue can induce increased rates of axon outgrowth simultaneously with repulsive guidance and may provide an understanding of how cortical axons may be repelled down the spinal cord in vivo (Hutchins et al. 2010; Li et al. 2010).
As mentioned above, the action of many extracellular guidance cues on axon pathfinding requires Ca2+ influx at the growth cone (Hong et al. 2000; Nishiyama et al. 2003; Henley and Poo 2004; Henley et al. 2004), but how activation of guidance cue receptors leads to opening of plasmalemmal ion channels remains largely unknown. Recent findings reveal that PI(3,4,5)P3 elevation polarizes to the growth cone’s leading edge and can serve as an early regulator during chemotactic guidance (Henle et al. 2011). A gradient of a chemoattractant triggered rapid asymmetric PI(3,4,5)P3 accumulation at the growth cone’s leading edge, as detected by the translocation of a GFP-tagged binding domain of Akt, in Xenopus laevis spinal neurons. Growth cone chemoattraction requires in this setting PI(3,4,5)P3 production and Akt activation, and genetic perturbation of polarized Akt activity disrupted axon pathfinding in vitro and in vivo. Furthermore, patch-clamp recording from growth cones revealed that exogenous PI(3,4,5)P3 rapidly activated cation currents, with properties reminiscent of TRPC channels, and asymmetrically applied PI(3,4,5)P3 was sufficient to induce chemoattractive growth cone turning in a manner that required downstream Ca2+ signalling. Which TRPC channels are specifically involved remains unclear from this work.
Immunophilins, including FK506-binding proteins (FKBPs), are protein chaperones with peptidyl-prolyl isomerase (PPIase) activity. FKBPs are most highly expressed in the nervous system, where their physiological function remains however unclear. Interestingly, FKBP12 and FKBP52 catalyze cis/trans isomerization of regions of the TRPC1 protein, which is implicated in controlling channel opening. FKBP52, on the other hand, mediates stimulus-dependent TRPC1 gating through isomerization, which is required for chemotropic turning of neuronal growth cones to netrin-1 and myelin-associated glycoprotein and for netrin-1/DCC-dependent midline axon guidance of commissural interneurons in the developing spinal cord. FKBP12 mediates opening of TRPC1 is not required for growth cone responses to netrin-1. This study demonstrates a novel physiological function of proline isomerases in chemotropic nerve guidance through TRPC1 gating and may have significant implication in clinical applications of immunophilin-related therapeutic drugs (Shim et al. 2009).
TRPV1 is expressed in the neurites and in the filopodia of central neurons. Several data indicate that it regulates growth cone morphology and growth cone movement. Activation of TRPV1 results in growth cone retraction and formation of varicosities along the neuritis (Goswami and Hucho 2007). In relation with this, it is interesting to consider that MYCBP2 is upregulated in the cerebellum and hippocampus, during the major synaptogenic period in these structures. MYCBP2 has been demonstrated to influence neuronal outgrowth and synaptogenesis by regulating the p38 MAPK-signaling pathways. Surprisingly, in the peripheral nervous system, the loss of MYCBP2 inhibits the internalization of TRPV1. Since both TRPV1 and MYCBP2 are involved in the neuronal growth in brain, this effect of MYBPC2 on TRPV1 might be a part of the mechanism regulating neuronal growth in hippocampus and cerebellum (Holland and Scholich 2011).
TRPV1 could be also involved in CNS regeneration after lesions, i.e. in the leech CNS: exposure to TRPV1 agonists after a nerve cut enhances neurite outgrowth, while capsazepine exposure produces this opposite effect (Meriaux et al. 2011).
Using siRNA interference to control TRPV4 expression in DRG neurons cultures, Jang et al. (2012) showed that TRPV4 can mediate neurite outgrowth via the regulation of neurtrophic factors. This regulation of neurite outgrowth could also occur in brain structures where TRPV4 is largely expressed. More than this, this study suggests than aberrant activity of TRPV4 could lead to some pathologies due to neuritogenesis defects.
Another vanilloid TRP channel, TRPV2, is also involved in growth cone guidance probably via sensing of membrane stretch during development (Shibasaki et al. 2010).
TRPM3 is activated by pregnolone sulfate (PS), a neurosteroid which is retrogradly released in cerebellum and in hippocampus. Interestingly, during development, PS release potentiates and refines the glutamatergic synapses in brain. Pharmacological experiments using a TRPM3 antagonist has demonstrated an inhibition of the PS induced glutamatergic synapse potentiation (Zamudio-Bulcock et al. 2011; Zamudio-Bulcock and Valenzuela 2011). Although there is no direct evidence, since the trmp3 KO mice have not been analysed in these studies, it might be suggested that TRPM3 acts a modulator of glutamatergic transmission in brain and therefore might play a role in synaptic contact establishment.
2.2 A Role for TRP Channels in Synaptic Plasticity and Behaviour
TRPC are widely expressed in the brain and play several roles in development and normal neuronal function. Members of the TRPC family are generally coupled to activation of Gq coupled receptors. Activation of phospholipase C leads to production of IP3 and diacylglycerol (DAG). The latter is described as a specific activator of TRPC3, TRPC6 and TRPC7. TRPC1 and TRPC4 are reported to be store-operated, i.e. activated by depletion of IP3 sensitive stores, or receptor operated and finally TRPC5 is activated by increases of intracellular [Ca2+]. Thus it can be anticipated that TRPC channels are players when Gq coupled neuronal receptors are stimulated. This class of receptors includes metabotropic muscarinic, glutamate and GABA receptors. With this in mind, it is not surprising that TRPC channels have been implicated in processes such as spine formation and modulation of synaptic transmission through membrane depolarization (Tai et al. 2009).
For instance, it is known that group I metabotropic glutamate receptors (mGluRs) play an essential role in cognitive function. Group 1 mGluR activation induced in CA1 pyramidal neurons intracellular Ca2+ waves and a biphasic electrical response composed of a transient Ca2+ -dependent SK channel-mediated hyperpolarization and a (possibly TRPC-mediated) sustained depolarization. The generation and magnitude of the SK channel-mediated hyperpolarization depended solely on the rise in intracellular Ca2+ concentration whereas the TRPC channel-mediated depolarization required both a small rise in [Ca2+]i and mGluR activation. Surprisingly in this study, TRPC-mediated current were suppressed by forskolin-induced rises in cAMP. Thus, SK- and TRPC-mediated currents robustly modulate pyramidal neuron excitability by decreasing and increasing their firing frequency. Apparently, cAMP levels provide an additional level of regulation by modulating TRPC-mediated sustained depolarization that might stabilize periods of sustained firing (El-Hassar et al. 2011). The mGluR1 receptor is particularly important for synaptic signalling and plasticity in the cerebellum. Unlike ionotropic glutamate receptors that mediate rapid synaptic transmission, mGluR1s produce in cerebellar Purkinje cells a complex postsynaptic response consisting of two distinct signal components, namely a local dendritic calcium signal and a slow excitatory postsynaptic potential. The basic mechanisms underlying these synaptic responses were clarified in recent years. Dendritic calcium signal results from IP3 receptor-mediated calcium release from internal stores. mGluR1-mediated slow excitatory postsynaptic potentials are mediated by the transient receptor potential channel TRPC3. This surprising finding established TRPC3 as a novel postsynaptic channel for glutamatergic synaptic transmission (Hartmann et al. 2011).
It is a common feature that neurons sum their input by spatial and temporal integration. Temporally, presynaptic firing rates are converted to dendritic membrane depolarizations by postsynaptic receptors and ion channels. In several regions of the brain, including higher association areas, the majority of firing rates are low. For rates below 20 Hz, the ionotropic receptors alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor and N-methyl-d-aspartate (NMDA) receptor will not produce effective temporal summation. Interestingly, TRP channels activated by metabotropic glutamate receptors would be more effective, owing to their slow kinetics. Using a computational model of the TRP channel and its intracellular activation pathway, it was suggested that synaptic input frequencies down to 3–4 Hz and inputs consisting of as few as three to five pulses can be effectively summed. Temporal summation characteristics of TRP channels may be important at distal dendritic arbors, where spatial summation is limited by the number of concurrently active synapses. It may be particularly important in regions characterized by low and irregular rates (Petersson et al. 2011).
Finally, activation of muscarinic receptors on pyramidal cells of the cerebral cortex induces the appearance of a slow afterdepolarization that can sustain autonomous spiking after a brief excitatory stimulus. This phenomenon has been hypothesized to allow for the transient storage of memory traces in neuronal networks. Muscarinic receptors have the ability to induce the inward aftercurrent underlying the slow afterdepolarization which is inhibited by expression of a Gq-11 dominant negative mutant and which is also markedly reduced in a phospholipase C ß1 (PLCβ1) knock-out mouse. These results indicate that the Gq-11/PLCß1 cascade plays a key role in the ability of muscarinic receptors to signal the inward current. Muscarinic afterdepolarizations might be mediated by a calcium-activated nonselective cation current. Surprisingly, it was found that expression of a TRPC dominant negative protein inhibits, and overexpression of wild-type TRPC5 or TRPC6 enhances, the amplitude of the muscarinic receptor-induced inward aftercurrent. Furthermore, coexpression of TRPC5 and T-type calcium channels is sufficient to reconstitute a muscarinic receptor-activated inward current in human embryonic kidney HEK-293 cells. These results indicate that TRPC channels might mediate the muscarinic receptor-induced slow afterdepolarization seen in pyramidal cells of the cerebral cortex and might suggest a possible role for TRPC channels in mnemonic processes (Yan et al. 2009).
TRPC6 is reportedly localized post-synaptically in excitatory synapses and promotes their formation via a Ca2+/calmodulin-dependent kinase IV (CaMKIV) – cAMP-response-element binding protein (CREB)-dependent pathway. Overexpression of TRPC6 increases the number of spines in hippocampal neurons and TRPC6 knockdown with RNAi decreases the number. Transgenic mice overexpressing trpc6 showed enhancement in spine formation, and a better spatial learning and memory in Morris water maze. These results reveal a previously unknown role of TRPC6 in synaptic and behavioral plasticity (Zhou et al. 2008). These results were confirmed in a second study (Tai et al. 2008). Interestingly, it was shown that the peak expression of TRPC6 in rat hippocampus was between postnatal day 7 and 14, a period known to be important for maximal dendritic growth. Mechanistically, these authors suggest that Ca2+ influx through the TRPC6 channel leads to CaMKIV and CREB. Overexpression of TRPC6 increased phosphorylation of both factors and promoted dendritic growth in hippocampal cultures. Downregulation of TRPC6 suppressed phosphorylation of both CaMKIV and CREB and impaired dendritic growth. Expressing a dominant-negative form of CaMKIV or CREB blocked the TRPC6-induced dendritic growth. Furthermore, inhibition of Ca2+ influx suppressed the TRPC6 effect on dendritic growth. In transgenic mice overexpressing Trpc6, the phosphorylation of CaMKIV and CREB was enhanced and the dendritic growth was also increased. Thus it seems that TRPC6 plays an important role during the development of the central nervous system (CNS) and has a profound impact on learning and memory through the regulation of spine formation (Tai et al. 2008).
In the cerebellum, Purkinje cell TRPC3 channels underlie the slow excitatory postsynaptic potential (EPSP) observed following parallel fibre stimulation. TRPC3 channel opening requires stimulation of metabotropic glutamate receptor 1 (mGluR1), activation of which can also lead to the induction of long term depression (LTD), which underlies cerebellar motor learning. LTD induction requires protein kinase C (PKC) and protein kinase G (PKG) activation, and whilst PKC phosphorylation targets are well established, virtually nothing is known about PKG targets in LTD. TRPC3 channels are inhibited following phosphorylation by PKC and PKG in expression systems, we examined whether native TRPC3 channels in Purkinje cells are a target for PKG or PKC, thereby contributing to cerebellar LTD. In Purkinje cells, activation of TRPC3-dependent currents is not inhibited by conventional PKC or PKG to any significant extent and that inhibition of these kinases does not significantly impact on TRPC3-mediated currents, TRPC3-dependent currents may differ significantly in their regulation from those overexpressed in expression systems (Nelson and Glitsch 2012).
TRPV1 is largely expressed in brain and plays a surprisingly important ‘non-sensory role’ in brain. The expression of other Vanilloid TRP proteins has been reported in different brain structures (Kauer and Gibson 2009) and role is strikingly versatile and is involved in the general “excitability” of the cortex (Mori et al. 2012). Indeed, TRPV1 activation induces long-term depression at CA1 interneurons synapses (Gibson et al. 2008). Activation of TRPV1 by capsaicin and capsazepin led to the depression of the communication at interneuron synapses. This capsaicin induced LTD was absent in the trpv1 KO mouse. This synaptic depression apparently is mediated via a presynaptic activation of calcineurin, a phosphatase known to decrease neurotransmission probably linked in DRG to TRPV1 (Wu et al. 2005). This inhibition of the excitatory transmission via TRPV1 activation has also been reported in the dentate granule cells. Chávez et al. (2010) showed that this depression of the synaptic communication was due to an internalization of the AMPA receptor in a calcineurin dependent manner.
Surprisingly, application of capsaicin also enhances the long-term potentiation of pyramidal neurons in the CA1 of hippocampus. Bennion et al. (2011) proposed that this modulation of synaptic plasticity by TRPV1 is mediated by its effects on the inhibitory GABAergic system (Bennion et al. 2011). The enhancement of LTP in CA1 neurons would be then the consequence of the depression of the synaptic communication of the inhibitory interneurons in the CA1 region previously reported by Gibson et al. (2008). The influence on synaptic plasticity is also important in the Nucleus Accumbens (NAc) which plays a key role in goal-directed behaviours and reward dependent learning and in amygdala. In NAc, as in dentate gyrus, TRPV1 can trigger LTD via the endocytosis of the AMPA receptors. Nevertheless, on the opposite of the modulation of the synaptic plasticity in hippocampus, in the NAc, the endocannabinoids act post synaptically through TRPV1 (Grueter et al. 2010). Remarkably and although the mechanism remains unclear, capsaicin application in amygdala increases the amplitude of the LTP, suggesting a role for TRPV1 in the modulation of synaptic plasticity in this structure.
This TRPV1 mediated synaptic plasticity in brain might explain some properties of Docosahexaenoic acid (DHA). DHA is known to enhance cognitive functions (Morley and Banks 1998). DHA supplementation in primary hippocampal neuron cultures regulates TRPV1 and TRPV2 expression in a dose dependent manner without altering TRPV3 or TRPV4 expression. This suggests that DHA positive effects on memory could be mediated by modulation of the endovanilloid receptors expression.
In accordance with this modulation of hippocampus synaptic plasticity, TRPV1 also presents a role in the memory consolidation. In vivo injection of capsazepine disrupted memory consolidation following a strong training protocol. This might highlight a possible synergic role of the endocannabinoid and endovanilloid system in memory consolidation (Genro et al. 2012).
TRPV1 could also control the anxiety-like behavior through its expression in the medial prefrontal cortex. Injection of capsaicin increases anxiogenic response in mice whereas capsazepine injection significantly exhibits an anxiolytic effect (Manna and Umathe 2011). This was confirmed by injections of capsazepine in prefrontal cortex of rats (Aguiar et al. 2009). Moreover, anandamide release has opposite effect on the anxiety behaviour: cannabinoid receptor type 1 (CB1) activation inhibits whereas TRPV1 activation enhances anxiety-like behaviour. The blockade of TRPV1 might be a functional tool to treat anxiety while preventing the risks associated with the long-term use of benzodiazepines (Moreira et al. 2011). Interestingly, another study reported that the trpv1 KO mice exhibit less stress or anxiety than WT mice (Marsch et al. 2007).
Although there is no direct evidence for an involvement of TRPV1 in the obsessive compulsive disorder (OCD), TRPV1 might be considered as a potential therapeutic target in such a depression syndrome. Indeed, Umathe et al. (2012) have reported that a TRPV1 antagonist produced a persistent inhibition of the OCD while capsaicin or anandamide produced the opposite effect. Inhibition of TRPV1 might be an effective tool in the treatment of OCD.
Finally however, some caution should be taken concerning the role of TRPV1 in brain. In 2011, Cavanaugh et al. created a trpv1 reporter mouse and actually showed that the expression of TRPV1 in brain is much more restricted than first reported. No expression, neither functional activity of TRPV1 could be detected in hippocampus, amygdala or cerebellum. This study puts previous studies involving TRPV1 in physiology of brain in a different perspective. Indeed, it should be noted also that previous studies showing an implication of TRPV1 in hippocampus synaptic plasticity via capsaicin application, but never recorded TRPV1 direct activation by calcium imaging or whole cell recording. These discrepancies could have several explanations. First, Chávez et al. (2010) and Grueter et al. (2010) suggested the role of TRPV1 in the synaptic plasticity in response to endocannabinoids. This endocannabinoid-induced plasticity could also be triggered by other TRP channels. Indeed Watanabe and colleagues showed that anandamine could activate TRPV4 via epoxyeicosatrienoic acid (Watanabe et al. 2003) and TRPV4 is known to be expressed in hippocampus (Shibasaki et al. 2007). Another possibility could be that TRPV1 triggers LTD via a non-conducting function (Kaczmarek 2006). In such conditions, some non-functional alternative splicing forms of TRPV1 could be expressed in hippocampus and respond to capsaicin without any calcium influx. Indeed, the authors reported a TRPV1 expression (Cavanaugh et al. 2011) in hippocampus interneurons, but did not report any capsaicin response in calcium imaging, suggesting a eventual non functional form of TRPV1.
TRPM channels have also been implicated in neuronal plasticity. TRPM2 is a Ca2+ permeable cation channel activated by oxidative stress and is involved in cell death. However, it may be also a modulator of hippocampal synaptic plasticity. Indeed, Olah et al. (2009) have described TRPM2 as a regulator of voltage-dependent Ca2+ channels and the NMDA receptors via a rise in the intracellular calcium concentration, and its depolarizing effect. The study of hippocampus slices in a trpm2 KO context showed that the LTD is selectively impaired because of inhibition of the kinase GSK3beta, confirming TRPM2 as a key player in hippocampal synaptic plasticity (Xie et al. 2012). Additionally, TRPM2 is linked to neuronal cell death after oxidative stress induced by glutathione (GSH). GSH inhibits TRPM2 channels through a thiol-independent mechanism, which plays an important role in aging and neurological diseases associated with depletion of GSH (Belrose et al. 2012).
Finally, it has been shown that TRPA1 is involved in the glycinergic neurotransmission generating IPSPs in the rat medullary dorsal horn (Substantia gelatinosa) and also as a presynaptic channel in the nucleus supraopticus regulating glutamate release (Yokoyama et al. 2011; Cho et al. 2012).
2.3 TRP Channels as Players in Neuronal Activity
Apart from the role of TRP channels in specific processes such as memory formation and neuronal development, an ever increasing number of studies links TRPC channels with specific neuronal receptor activity. Basically TRPC channels can provide a Ca2+ influx pathway, which couples to intracellular functions, or TRPC channels can support a depolarization, which would influence action potential triggering and bursting behavior. As such, TRPC channels have been linked with metabotropic glutamate, GABA and acetylcholine receptors (see above, and e.g. Berg et al. 2007), serotonin 2C and leptin receptors in pro-opiomelanocortin neurons and kisspeptin receptors in hypothalamic neurons (Qiu et al. 2011; Sohn et al. 2011; Williams et al. 2011).
In the mammalian central nervous system, slow synaptic excitation involves the activation of metabotropic glutamate receptors (mGluRs). TRPC3, but not TRPC1, is needed for mGluR-dependent synaptic signaling in mouse cerebellar Purkinje cells. TRPC3 is the most abundantly expressed TRPC subunit in Purkinje cells. In mutant mice lacking TRPC3, both slow synaptic potentials and mGluR-mediated inward currents are completely absent, while the synaptically mediated Ca2+ release signals from intracellular stores are unchanged. Importantly, trpc3 knockout mice exhibit an impaired walking behavior. Taken together, these results establish TRPC3 as a new type of postsynaptic channel that mediates mGluR-dependent synaptic transmission in cerebellar Purkinje cells and is crucial for motor coordination (Hartmann et al. 2008; Hartmann and Konnerth 2008).
Cholecystokinin (CCK) is one of the most abundant neuropeptides in the brain where it interacts with two G protein-coupled receptors (CCK-1 and CCK-2). Activation of both CCK receptors increases the activity of phospholipase C (PLC) resulting in increases in intracellular Ca2+ release and activation of protein kinase C (PKC). High density of CCK receptors has been detected in the superficial layers of the entorhinal cortex (EC). Effects of CCK on neuronal excitability of layer III pyramidal neurons in the EC include a remarkable increase of the firing frequency of action potentials, which are mediated via activation of CCK-2 receptors and required the functions of G proteins and PLC. In a recent study, CCK-mediated facilitation of neuronal excitability appeared independent of IP3 receptors and PKC, but relying on the activation of a cationic channel to generate membrane depolarization. This cationic channel shows a pharmacological profile which has been described for TRPC channels (but albeit relatively unselective): inhibition by 2-aminoethyldiphenyl borate (2-APB) and flufenamic acid (FFA) and potentiation by Gd3+ and 100 μM La3+. Furthermore, CCK-induced enhancement of neuronal excitability was significantly inhibited by intracellular application of the antibody to TRPC5 suggesting the involvement of TRPC5 channels (Wang et al. 2011).
Another interesting growth hormone whose receptor has been linked with TRPC channels is brain-derived neurotrophic factor (BDNF) (Jia et al. 2007; Sossin and Barker 2007). BDNF is believed to be an important regulator of striatal neuron survival, differentiation, and plasticity. Reduction of BDNF delivery to the striatum has been implicated in Huntington’s disease. With respect to TRP channels, an interesting study suggested that they might contribute to intracellular signaling pathways, which lead to short-term induction of striatal gene expression by BDNF. Indeed, gene expression responses to BDNF can be abolished by inhibitors of TrkB (K252a) and calcium (chelator BAPTA-AM) and the (non-selective) transient receptor potential cation channel [TRPC] antagonist SKF-96365 (Gokce et al. 2009). BDNF also induces synaptic potentiation at both neuromuscular junctions (NMJs) and synapses of the CNS through a Ca2+ dependent pathway. Pharmacological inhibition or morpholino-mediated knockdown of Xenopus TRPC1 (XTRPC1) can significantly attenuate the BDNF-induced potentiation of the frequency of spontaneous synaptic responses at the NMJ. XTRPC1 was required specifically in postsynaptic myocytes for BDNF-induced Ca2+ elevation and full synaptic potentiation at the NMJ, suggesting a previously underappreciated postsynaptic function of Ca2+ signalling in neurotrophin-induced synaptic plasticity (McGurk et al. 2011).
Persistent neuronal activity lasting seconds to minutes has been proposed to allow for the transient storage of memory traces in entorhinal cortex and thus could play a major role in working memory. Nonsynaptic plateau potentials, induced by acetylcholine, account for persistent firing in many cortical and subcortical structures. The expression of these intrinsic properties in cortical neurons involves the recruitment of a non-selective cation conductance of unknown origin. In layer V of rat medial entorhinal cortex, muscarinic receptor-evoked plateau potentials and persistent firing induced by carbachol require PLC, decrease of PI(4,5)P2, and a permissive [Ca2+]i. Plateau potentials and persistent activity were suppressed by the generic nonselective cation channel blockers FFA (100 μM) and 2-APB (100 μM), as well as by the TRPC channel blocker SKF-96365 (50 μM) and are not affected by the TRPV channel blocker ruthenium red (40 μM). The TRPC3/6/7 activator OAG did not induce or enhance persistent firing evoked by carbachol. Voltage clamp recordings revealed a carbachol-activated, nonselective cationic current with a heteromeric TRPC-like phenotype, including outward rectification and a reversal potential around 0 mV. Moreover, plateau potentials and persistent firing were inhibited by intracellular application of the peptide EQVTTRL that disrupts interactions between the C-terminal domain of TRPC4/5 subunits and associated PDZ proteins of the NHERF family and which has been reported to be important for TRPC4/C5 channel function (Harteneck et al. 2003), suggesting that TRPC4-5 mediated currents significantly contribute persistent depolarisation of neurons and thus controls the firing and mnemonic properties of projection neurons in the entorhinal cortex (Zhang et al. 2011b).
As mentioned above, TRPC6 is expressed in several types of neurons, including cerebrospinal-fluid contacting neurons (Wu et al. 2011), cortical neurons (Tu et al. 2009b), and in the substantia nigra of normal rat brain (Giampa et al. 2007).
Interestingly, Hyperforin, one of the main bioactive compounds of the medicinal plant Hypericum perforatum (St. John’s wort), activates TRPC6 without affecting the other TRPC channels (Tu et al. 2009a). A recent studies describes its impact on the BDNF receptor TrkB and on adult hippocampal neurogenesis, since they appear central to the mechanisms of action of antidepressants. Chronic hyperforin treatment on cortical neurons in culture and on the brain of adult mice led to increased expression of TRPC6 channels and TrkB via SKF-96365-sensitive channels controlling a downstream signaling cascade involving Ca2+, protein kinase A, CREB and p-CREB. Hyperforin augmented the expression of TrkB in the cortex but not in the hippocampus where neurogenesis remained unchanged (Gibon et al. 2012).
St. John’s Wort (SJW) has been used medicinally for over 5,000 years and first gained attention as the constituent of SJW responsible for its antidepressant effects. Since then, several of its neurobiological effects have been described, including neurotransmitter re-uptake inhibition, the ability to increase intracellular sodium and calcium levels, TRPC6 activation, NMDA receptor antagonism as well as antioxidant and anti-inflammatory properties. Until recently, its pharmacological actions outside of depression had not been investigated. Hyperforin has been shown to have cognitive enhancing and memory facilitating properties. Importantly, it has been shown to have neuroprotective effects against Alzheimer’s disease (AD) neuropathology, including the ability to disassemble amyloid-beta (Aβ) aggregates in vitro, decrease astrogliosis and microglia activation, as well as improve spatial memory in vivo (Griffith et al. 2010).
The analysis of Trpc6 −/− mice clearly shows that TRPC6 activity affects behaviour. Trpc6 −/− mice showed no significant differences in anxiety in a marble burying test, but demonstrated reduced exploration in the square open field and the elevated star maze (Beis et al. 2011).
Using electromyography and transcranial magnetic stimulation, Mori et al. (2012) described for the first time that some single nucleotide polymorphisms of trpv1 in human can regulate cortical excitability probably by modulation of glutamate release at synapses. In the striatum, TRPV1 regulates the release of the excitatory messenger glutamate. Capsaicin application enhances the frequency of glutamate-mediated spontaneous (sEPSCs) and miniature (mEPSC) excitatory postsynaptic currents (Musella et al. 2008, 2010). It also modulates GABA transmission, an inhibitory pathway, via endocannabinoids (eCBs). The effect of capsaicin application both on glutamate and GABA transmission is lacking in the trpv1 KO mice.
Therefore, TRPV1 modulation offers alternative therapeutic routes in disorders of striatal neurotransmission (Musella et al. 2008, 2010). Moreover other studies suggest that this regulation of synapse activity might occur in several other structures such as the pineal gland (Reuss et al. 2010).
Some brain neurons present a specific firing behaviour, called burst firing. This spiking behaviour is characterized by a sustained firing activity. Such a burst firing activity is involved in different brain processes like reward circuit, short-term memory in an emotional and experience dependent learning context, respiratory rhythms regulation. Ca2+ activated non-selective (CAN) currents are proposed to be key players of sustained firing activity mechanisms (Rubin et al. 2009). TRPM4 and TRPM5 are considered as the channel underlying CAN and could contribute in this reasoning to many brain processes (Launay et al. 2002; Hofmann et al. 2003). So far, the most investigated process of burst firing behaviour in which TRPM4 plays a role is described in the pre-Bötzinger Complex (preBötC) neurons (Pace et al. 2007). The preBötC is involved in the respiratory rythmogenesis (Feldman and Del Negro 2006). These neurons are characterized by an oscillating activity and by the synchronization of their burst firing. Only 20 % of these neurons present a pacemaker activity, meaning that most of the neurons generate inspiratory drive potentials by evoking post-synaptic currents that depend on intrinsic membrane properties (Del Negro et al. 2005). CAN currents have been proposed to be responsible for amplifying glutamatergic synaptic drive by transforming the glutamatergic synaptic inputs to membrane depolarization (Pace et al. 2007; Mironov 2008; Mironov and Skorova 2011). Pace et al. showed that calcium influx was able to induce some plateau potentials, and external sodium substitution and flufenamic acid exposure attenuated those plateau potentials. They also proposed CAN activation by glutamatergic inputs could direct (via NMDA-R calcium influx) or indirect (via mGluR induced IP3 dependent calcium release, or AMPA-R activation of voltage gated calcium channels). Crowder et al. (2007) detected by RT-PCR TRPM4 and TRPM5 expression in preBötC neurons and showed that excess of PIP2 augmented the inspiratory drive potential and the effect was modulated by flufenamic acid (FFA) application (Crowder et al. 2007). Thus, TRPM4 current could be activated by calcium waves in the soma and generate inspiratory bursts by boosting glutamatergic synaptic inputs. More recently, a novel pathway of activation of TRPM4 has been suggested in this system: the Epac/cAMP pathway. Epac agonist application on preBötC neurons sensitized calcium mobilization from IP3 internal calcium stores that stimulated TRPM4 and potentiated bursts of action potentials (Mironov and Skorova 2011). It remains unclear however, whether TRPM4 activity itself is regulated by this mechanism.
This mechanism of activation via glutamatergic synaptic inputs and the role of TRPM4/5 in burst firing activity might be conserved also in other brain structures. Mrejeru et al., have described a similar mechanism in dopaminergic (DA) neurons of substantia nigra (Mrejeru et al. 2011). Those neurons present two different behaviours, tonic firing and bursts of action potentials. They showed by electrophysiology that NMDA currents recruit a CAN current capable of generating a plateau potential. This CAN current can be blocked by flufenamic acid and 9-phenanthrol application. Since mRNA expression of TRPM2 and TRPM4 has been detected by RT-PCR (TRPM5 could not be detected), they hypothesized TRPM4 to be the channel involved in the burst firing behavior. Although TRPM4 current has not been directly recorded in dopaminergic neurons, and the specificity of flufenamic acid and 9-phenanthrol on brain slices has not been determined, Mrejeru et al. provide the first evidences that TRPM channels (TRPM2 and TRPM4) are expressed in substancia nigra neurons and could be a part of the reward circuit by boosting NMDA currents during burst firing.
The neurons of the lateral nucleus of amygdala also display such a sustained firing activity. The graded increase in firing is linked to a CAN current and is blocked by flufenamic acid application (Egorov et al. 2002). The Allen Brain Atlas shows TRPM4/5 mRNA expression in amygdala, leading to the conclusion that either one or the two channels are involved in a burst firing activity in the lateral nucleus and then are part of the mechanism for sustaining information about novel items in a short term memory in a context of emotional and experience dependent learning.
Although no direct evidence of endogenous TRPM4 or TRPM5 currents in neurons are now available, a similar process of sustained firing activity dependent on CAN channels exists in diverse structures such as the motoneurons of the nucleus ambiguous, the layer II neurons of the entorhinal cortex (Egorov et al. 2002), the sensory neurons of the olfactory bulbs (Pressler and Strowbridge 2006), indicating possibly a new role for TRPM4 and TRPM5 in firing behavior in brain physiology. However, see also data mentioned above that imply a more prominent role of TRPC4/C5 channels in this process (Wang et al. 2011; Zhang et al. 2011b). In the absence of a specific pharmacology, TRP specific knockout mice or knockdown strategies are clearly needed to clarify this issue.
Finally, an intriguing role for TRPA1 in astrocytes has been shown. Astrocytes contribute to the formation and function of synapses and are found throughout the brain, where they show intracellular store-mediated Ca2+ signals. Recently, using a membrane-tethered, genetically encoded calcium indicator (Lck-GCaMP3), it was reported that Ca2+ fluxes mediated by spontaneously open TRPA1 channels gave rise to frequent and highly localized ‘spotty’ Ca2+ microdomains near the membrane that contributed appreciably to resting Ca2+ levels in astrocytes. Work in cultured astrocytes and in brain slices showed that inhibiting these Ca2+ signals with a TRPA1 specific blocker, leads to decreased astrocyte resting Ca2+ concentrations, and decreased interneuron inhibitory synapse efficacy. It was shown that influx through TRPA1, reduces the activity of a GABA transporter in astrocytes, GAT-3, which leads to elevated extracellular GABA levels, and reduced miniature inhibitory post-synaptic currents (mIPSC’s) specifically in interneurons, but not in pyramidal neurons. This work highlights the housekeeping role of astrocytes in neuronal networks, and specifically the role of intracellular Ca2+ levels and TRPA1 therein (Clarke and Attwell 2011; Shigetomi et al. 2012).
3 TRP Channels Cause Neurological Diseases
3.1 TRP Channels Could Play a Role in Disease Mechanisms
Considering their function as Ca2+ influx channels, and considering the critical role of intracellular [Ca2+] dynamics in neuronal differentiation, functional signalling and survival it is clear that dysfunctional TRP channels can be expected to have a profound effect on the neuron’s health status.
In neurons, excessive Ca2+ entry occurs via over-activation of glutamate receptors (NMDA, AMPA, KA) or of a range of channels and transporters (TRPM2, TRPM7, NCX, ASICs, CaV1.2, and hemichannels). Potentially toxic cytoplasmic calcium concentrations can also occur due to release from internal stores, either through physical damage to mitochondria and the endoplasmic reticulum, or a malfunction of receptors and channels present in their membranes. Such increases of cytoplasmic calcium concentrations can trigger a range of downstream neurotoxic cascades, including the uncoupling mitochondrial electron transfer from ATP synthesis, and the activation and overstimulation of enzymes such as calpains and other proteases, protein kinases, nitric oxide synthase (NOS), calcineurin and endonucleases. Alterations in Ca2+ homeostasis have been suggested in the onset/progression of neurological diseases, such as Parkinson’s, Alzheimer’s, bipolar disorder, hereditary ataxia and Huntington’s or with neurological aspects of aging (Amaral et al. 2007; Amaral and Pozzo-Miller 2007a, b; Adachi et al. 2008; Poduslo et al. 2008, 2009; Roedding et al. 2009; Cucchiaroni et al. 2010; Becker et al. 2011).
TRP channels are also important regulators of membrane potential. They will support slow depolarization of the cell and shape burst firing patterns of neurons or support persistent activity of neurons. In this sense it can be anticipated that gain-of-function mutations of TRP channels will contribute to prolonged burst firing patterns and vice versa. Disease states which are associated with this in relation to TRP channels include ataxia and epilepsy (Adachi et al. 2008; Becker et al. 2009, 2011; Tai et al. 2009). Epilepsy is caused mainly by perturbances of the balance of excitation and inhibition within the central system. Because TRPV1 activation modulates activity dependent synaptic efficacy, TRPV1 blockade is now considered as a potential antiepilepsy treatment (Fu et al. 2009). Basically, all the TRPM could be key players in epilepsy. Indeed, the balance in ion homeostasis is important for the neuronal network activity. TRP channels could fine-tune this neuronal activity, so any perturbance of TRP physiology might be considered as an epileptogenic event (Stawicki et al. 2011). An epileptic seizure is composed of recurrent bursts of intense firing. For instance, Schiller Y (2004) recorded a Ca2+ activated cation (CAN) current in neocortex slices treated with bicuculline to induce seizure (Schiller 2004). This current was unaffected by changing chloride concentrations but was sensitive to intracellular calcium changes and was blocked by flufenamic acid application. This CAN current is activated by calcium influx through NDMA receptors and voltage gated calcium channels. This is the first direct evidence that CAN current is involved in a pathological process. Indeed this current could support sustained seizure like events (Schiller 2004). Interestingly the mechanism seems to be similar to what has been described in the preBötC and substancia nigra neurons. Since TRPM4 and TRPM5 have been shown to function as CAN channels, further investigation in trpm4 and trpm5 KO mice could improve the understanding of the pathophysiological process leading to epileptic seizure.
TRPV channels, in cooperation with the endocannabinoid system, influence GABAergic and glutamatergic synapses and play a modulatory function on dopamine transmission. Through these mechanisms TRPV and endocannabinoids have an important influence on various neurobiological processes (e.g., control of movement, motivation/reward) and, particularly, on different pathologies affecting these processes such as basal ganglia disorders, schizophrenia (Fernandez-Ruiz et al. 2010), and drug addiction.
TRPM4 is thought to be underlying the boosting of NMDA current in DA neurons (Mrejeru et al. 2011). Since those neurons are vulnerable to neurodegeneration, this CAN current boost mechanism may also explain the high sensitivity of DA neurons for excitotoxicity. In this case, TRPM4 could be considered as a potential drug target in Parkinson disease (PD). But TRPM4 is not the only TRPM that may be involved in PD. TRPM2 current has been recorded in DA neurons and the injection of Rotenone, used as a model of PD, induces a current that can be specifically inhibited by TRPM2 blockers. The ROS production induced by the rotenone injection is probably the key player in the activation of TRPM2 in this model (Freestone et al. 2009). Moreover, there is wide agreement that oxidative stress induced TRPM2 activation could lead to cell death. This highlights a possible relation between TRPM2 and the neurodegenerative part of PD (Belrose et al. 2012) described. Human genetic studies in western countries also revealed that some single nucleotides polymorphisms in trpm2 and trpm7 could be associated with risk factors for certain form of Parkinsonian Dementia Complex (Hermosura et al. 2005, 2008; Hermosura and Garruto 2007). Nevertheless, a Japanese study could not find any correlation between trpm7 SNP and PD (Hara et al. 2010). This tends to suggest that, mainly TRPM2 should be considered as a risk factor for neurodegenerative diseases as well as a potential therapeutic target.
3.2 TRPs Channels in Brain Injury and Stroke
TRPM7 is a potential target for neuroprotection after brain injury. Suppressing the expression of TRPM7 in hippocampal CA1 neurons causes resistance to ischemic cell death, preserved cell function and prevented ischemia-induced deficits in memory (Sun et al. 2009). Depletion of intracellular Mg2+, a symptom of traumatic brain injury and a reduction of extracellular Ca2+ are both associated with poor neurological outcome and are both conditions which activated TRPM7 thereby possibly increasing the Ca2+ load of neuronal cells. This leads to secondary injury processes and to cell death following brain injury, including stroke (Cook et al. 2009) TRPM7 has been implicated in ischemic brain damage. TRPM7 gene variation might play a role in the risk of ischemic stroke (Romero et al. 2009).
3.3 TRPs, Schizophrenia and Bipolar Disorders
TRP channels play a role in the pathogenesis of schizophrenia. TRPV1, in cooperation with the endocannabinoid system, influences GABAergic and glutamatergic synapses and play a modulatory function on dopamine transmission. Through these mechanisms, TRPV1 and endocannabinoids have an important influence on various neurobiological processes (e.g., control of movement, motivation/reward) and, particularly, on different pathologies affecting these processes like basal ganglia disorders, schizophrenia, and drug addiction (Fernandez-Ruiz et al. 2010).
Natural compounds, used in traditional medicine as anti-depressants, target TRP channels, e.g. Incensole acetate which is released by the burning of resin from the Boswellia plant has been used for religious and cultural ceremonies for millennia. It activates TRPV3, which is expressed in the brain and causes anxiolytic-like, antidepressive-like behavioral effects and protects against brain ischemia (Moussaieff et al. 2008, 2012). As mentioned above already, St. John’s Wort has been used medicinally for over 5,000 years. Recently, Hyperforin, an antidepressive compound obtained from St. John’s Wort, has been identified as effective activator of TRPC6 (Leuner et al. 2007). It causes cognitive enhancing, memory facilitating properties and has probably neuroprotective effects (Griffith et al. 2010). TRPM2, which is highly expressed in the striatum (caudate nucleus and putamen) is supposed to play a key role in bipolar disorders (Aita et al. 1999; Uemura et al. 2005; Xu et al. 2006, 2009; Roedding et al. 2012). Recent case-control studies implicate TRPM2 conferring risk for bipolar disorder (BD) and genetic variants of TRPM2 have been identified to be coupled with BD supporting a role for this channel in the pathogenesis of this disorder (Xu et al. 2009) (see for a review Chahl 2007).
3.4 Lessons from KO Mice
In the absence of a clear and selective pharmacology of TRP channels, TRP deficient mice remain the gold standard for delineating their functional role in neurons, and their possible contribution to disease states. Another possibility is the use of inbred mice with acquired mutations, which display a neurological phenotype which can be delineated to a mutation in a specific gene. An interesting example for this approach is TRPC3.
In Trpc3 −/− mice it has been shown that slow synaptic potentials, which are associated with metabotropic glutamate receptor mediated activation of an inward cation current are absent in cerebellar purkinje cells. This is associated with impaired walking behavior and suggests that defects in TRPC3 could contribute to impaired motor control and coordination also in human patients. Interestingly, shortly thereafter a mouse line was identified from a large-scale phenotype-driven mutagenesis, the Moonwalker mouse, which displays severe motor and coordination defects, including impaired gait and balance. Genome sequencing revealed that these mice have mutation in the trpc3 gene, which allegedly makes the channel more active. Thus, a gain of function and a loss of function of the same channel leads to similar defects in mice. Intriguingly, the gain-of-function mutant in the Moonwalker mice is associated with increased Purkinje cell loss and altered dendritic development, as displayed by decreased dendritic length and arborisation. Thus, one could unify these data by appreciating the loss of a depolarizing current in the KO mice, which leads to defect in mGluR signaling, and realizing that the gain of function mutant will disturb the normal Ca2+ and Na+ homeostasis at the developing dendrites which will lead to developmental abnormalities (Trebak 2010).
Interestingly, in another mouse model of cerebellar ataxia, the staggerer mouse, there was also a link with defective mGlu-TRPC3 signalling. Staggerer mutant mice have a functional loss of a transcription factor, Retinoid-related Orphan Receptor alpha (RORalpha), which is abundantly expressed in Purkinje cells (PCs) of the cerebellum. Homozygous staggerer (sg/sg) mice show cerebellar hypoplasia and congenital ataxia. Sg/sg mice serve as an important extreme mouse model of the hereditary spinocerebellar ataxia type 1 (SCA1), since it has been shown that RORalpha dysfunction is strongly correlated with SCA1 pathogenesis. The prominent synaptic dysfunction in these mice is that sg/sg mice lack metabotropic glutamate receptor (mGluR)-mediated slow EPSCs completely. Western blot analysis in the sg/sg cerebellum revealed expression of mGluR1 and TRPC3, both of which underlie mGluR-mediated slow currents in WT PCs. Immunohistochemical data demonstrated marked mislocalization of mGluR1 on sg/sg PCs. These results suggest that disruption of mGluR signalling at PF-PC synapses is one of the major synaptic defects in sg/sg mice and may manifest itself in SCA1 pathology and cerebellar motor control in general (Mitsumura et al. 2011).
3.5 Lessons from Human Disease
Until now, only one TRP channel has been linked causally with a human neuronal disease. Indeed, mutations in the TRPML1 gene are responsible for the development of the devastating lysosomal storage disease disorder Mucolipidosis type IV. Lysosomal storage diseases (LSDs) are caused by inability of cells to process the material captured during endocytosis (Kiselyov et al. 2010, 2011).
TRPML1, TRPML2 and TRPML3 belong to the mucolipin family of the TRP superfamily of ion channels. The founding member of this family, TRPML1 was cloned during the search for the genetic determinants of the lysosomal storage disease mucolipidosis type IV (MLIV). Mucolipins are predominantly expressed within the endocytic pathway where they appear to regulate membrane traffic and/or degradation of lysosomal storage vesicles. The physiology of TRPML proteins raises some of the most interesting questions of the modern cell biology. Their traffic and localization is a multi-step process involving a system of adaptor proteins, while their ion channel activity possibly exemplifies the rare cases of regulation of endocytic traffic and hydrolysis by ion channels (Puertollano and Kiselyov 2009).
Mucolipidosis type IV arises from mutations in TRPML1 (Bargal et al. 2000, 2001; Bassi et al. 2000; Slaugenhaupt 2002). The two other members, TRPML2 and TRPML3 multimerize with TRPML1, are involved in TRPML1 distribution and trafficking. TRPML1 functions as a Ca2+ and iron release channel in lysosomes (Dong et al. 2010; Shen et al. 2012). The pathogenic mechanism by which loss of TRPML1 leads to abnormal cellular storage and neuronal cell death is however still poorly understood. Yeast two-hybrid and co-immunoprecipitation experiments identified interactions between TRPML1 and Hsc70 as well as TRPML1 and Hsp40. Hsc70 and Hsp40 are members of a molecular chaperone complex required for protein transport into the lysosome during chaperone-mediated autophagy (CMA). Fibroblasts from MLIV patients show a defect in CMA in response to serum withdrawal. This defect in CMA was subsequently confirmed in purified lysosomes isolated from control and MLIV fibroblasts. The amount of lysosomal-associated membrane protein type 2A (LAMP-2A) is reduced in lysosomal membranes of MLIV fibroblasts. As a result of decreased CMA, MLIV fibroblasts have increased levels of oxidized proteins compared to control fibroblasts. Mechanistically, TRPML1 may act as a docking site for intralysosomal Hsc70 allowing it to more efficiently pull in substrates for CMA. It is also possible that TRPML1 channel activity may be required for CMA (Venugopal et al. 2009). More specifically, it was suggested that TRP-ML1 modulates postendocytic delivery to lysosomes by regulating interactions between late endosomes and lysosomes (Miedel et al. 2008).
Lysosomal lipid accumulation, defects in membrane trafficking and altered Ca2+ homoeostasis are common features in many lysosomal storage diseases. Interestingly, in fibroblasts from patients with another lysosomal storage disorder, Nieman Pick syndrome (NP), it was shown that sphingomyelins accumulate in lysosomes. Sphingomyelins (SMs) are plasma membrane lipids that undergo sphingomyelinase (SMase)-mediated hydrolysis in the lysosomes of normal cells. Patch-clamp analyses revealed that TRPML1 channel activity is inhibited by SMs, but potentiated by SMases. In NP-type C cells, increasing TRPML1’s expression or activity was sufficient to correct the trafficking defects and reduce lysosome storage and cholesterol accumulation. Thus, it was proposed that abnormal accumulation of luminal lipids causes secondary lysosome storage by blocking TRPML1- and Ca2+-dependent lysosomal trafficking, which might be a common feature in lysosomal storage disorders (Shen et al. 2012).
Finally, a Drosophila model with a defective Trpml gene recapitulates the key disease features, including abnormal intracellular accumulation of macromolecules, motor defects and neurodegeneration. The basis for the buildup of macromolecules was defective autophagy, which resulted in oxidative stress and impaired synaptic transmission. Late-apoptotic cells accumulated in trpml mutant brains suggesting diminished cell clearance. The accumulation of late apoptotic cells and motor deficits could be rescued by expression of trpml + in neurons, glia or hematopoietic cells. Thus, from this model it was concluded that the neurodegeneration and motor defects result primarily from decreased clearance of apoptotic cells, and it was suggested that bone marrow transplantation may limit the progression of MLIV, hematopoietic cells in humans are involved in clearance of apoptotic cells (Venkatachalam et al. 2008).
4 Conclusion
TRP channels are relatively new membrane proteins that are involved in a plethora of cell functions and are mainly appreciated as sensory ion channels. This review maps TRP channels as important players in the function of our brain including the forming of hard-wired connections in our developing brain by growth cone guidance, regulation of synaptogenesis, spine forming and modulation of synaptic plasticity. This new view on the function of TRP channels in our central nervous system has already identified some of these channels as potential pharmaceutical targets and has led to a new understanding of several brain diseases. However, we have just entered a new era of neurophysiology and we anxiously await exciting discoveries in a rapidly expanding field of brain research.
References
Adachi N, Kobayashi T, Takahashi H, Kawasaki T, Shirai Y, Ueyama T, Matsuda T, Seki T, Sakai N, Saito N (2008) Enzymological analysis of mutant protein kinase Cgamma causing spinocerebellar ataxia type 14 and dysfunction in Ca2+ homeostasis. J Biol Chem 283:19854–19863
Aguiar DC, Terzian AL, Guimaraes FS, Moreira FA (2009) Anxiolytic-like effects induced by blockade of transient receptor potential vanilloid type 1 (TRPV1) channels in the medial prefrontal cortex of rats. Psychopharmacology 205:217–225
Aita VM, Liu J, Knowles JA, Terwilliger JD, Baltazar R, Grunn A, Loth JE, Kanyas K, Lerer B, Endicott J, Wang Z, Penchaszadeh G, Gilliam TC, Baron M (1999) A comprehensive linkage analysis of chromosome 21q22 supports prior evidence for a putative bipolar affective disorder locus. Am J Hum Genet 64:210–217
Amaral MD, Pozzo-Miller L (2007a) BDNF induces calcium elevations associated with IBDNF, a non-selective cationic current mediated by TRPC channels. J Neurophysiol 98:2476–2482
Amaral MD, Pozzo-Miller L (2007b) TRPC3 channels are necessary for brain-derived neurotrophic factor to activate a nonselective cationic current and to induce dendritic spine formation. J Neurosci 27:5179–5189
Amaral MD, Chapleau CA, Pozzo-Miller L (2007) Transient receptor potential channels as novel effectors of brain-derived neurotrophic factor signaling: potential implications for Rett syndrome. Pharmacol Ther 113:394–409
Bai JZ, Lipski J (2010) Differential expression of TRPM2 and TRPV4 channels and their potential role in oxidative stress-induced cell death in organotypic hippocampal culture. Neurotoxicology 31:204–214
Bargal R, Avidan N, Ben-Asher E, Olender Z, Zeigler M, Frumkin A, Raas-Rothschild A, Glusman G, Lancet D, Bach G (2000) Identification of the gene causing mucolipidosis type IV. Nat Genet 26:118–123
Bargal R, Avidan N, Olender T, Ben Asher E, Zeigler M, Raas-Rothschild A, Frumkin A, Ben-Yoseph O, Friedlender Y, Lancet D, Bach G (2001) Mucolipidosis type IV: novel MCOLN1 mutations in Jewish and non-Jewish patients and the frequency of the disease in the Ashkenazi Jewish population. Hum Mutat 17:397–402
Bassi MT, Manzoni M, Monti E, Pizzo MT, Ballabio A, Borsani G (2000) Cloning of the gene encoding a novel integral membrane protein, mucolipidin-and identification of the two major founder mutations causing mucolipidosis type IV. Am J Hum Genet 67:1110–1120
Becker EBE, Oliver PL, Glitsch MD, Banks GT, Achillic F, Hardy A, Noland PM, Fisher EMC, Davies KE (2009) A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice. Proc Natl Acad Sci (USA) 106:6706–6711
Becker EB, Fogel BL, Rajakulendran S, Dulneva A, Hanna MG, Perlman SL, Geschwind DH, Davies KE (2011) Candidate screening of the TRPC3 gene in cerebellar ataxia. Cerebellum 10:296–299
Beis D, Schwarting RK, Dietrich A (2011) Evidence for a supportive role of classical transient receptor potential 6 (TRPC6) in the exploration behavior of mice. Physiol Behav 102:245–250
Belrose JC, Xie YF, Gierszewski LJ, Macdonald JF, Jackson MF (2012) Loss of glutathione homeostasis associated with neuronal senescence facilitates TRPM2 channel activation in cultured hippocampal pyramidal neurons. Mol Brain 5:11
Bennion D, Jensen T, Walther C, Hamblin J, Wallmann A, Couch J, Blickenstaff J, Castle M, Dean L, Beckstead S, Merrill C, Muir C, St Pierre T, Williams B, Daniel S, Edwards JG (2011) Transient receptor potential vanilloid 1 agonists modulate hippocampal CA1 LTP via the GABAergic system. Neuropharmacology 61:730–738
Berg AP, Sen N, Bayliss DA (2007) TRPC3/C7 and Slo2.1 are molecular targets for metabotropic glutamate receptor signaling in rat striatal cholinegrgic interneurons. J Neurosci 27:8845–8856
Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE (2004) Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol 6:709–720
Carreno O, Corominas R, Fernandez-Morales J, Camina M, Sobrido MJ, Fernandez-Fernandez JM, Pozo-Rosich P, Cormand B, Macaya A (2012) SNP variants within the vanilloid TRPV1 and TRPV3 receptor genes are associated with migraine in the Spanish population. Am J Med Genet B Neuropsychiatr Genet 159B:94–103
Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI (2011) Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 31:5067–5077
Chahl LA (2007) TRP’s: links to schizophrenia? Biochim Biophys Acta 1772:968–977
Chávez AE, Chiu CQ, Castillo PE (2010) TRPV1 activation by endogenous anandamide triggers postsynaptic LTD in dentate gyrus. Nat Neurosci 13:1511–1518
Cho JH, Jeong MY, Choi IS, Lee HJ, Jang IS (2012) TRPA1-like channels enhance glycinergic transmission in medullary dorsal horn neurons. J Neurochem 122(4):691–701
Chung YH, Kim D, Moon NJ, Oh CS, Lee E, Shin DH, Kim SS, Lee WB, Lee JY, Cha CI (2007) Immunohistochemical study on the distribution of canonical transient receptor potential channels in rat basal ganglia. Neurosci Lett 422:18–23
Chung KK, Freestone PS, Lipski JL (2011) The expression and functional properties of TRPM2 channels in dopaminergic neurons of the substantia nigra of the rat. J Neurophysiol 106:2865–2875
Clarke LE, Attwell D (2011) An astrocyte TRP switch for inhibition. Nat Neurosci 15:3–4
Cook NL, Van Den Heuvel C, Vink R (2009) Are the transient receptor potential melastatin (TRPM) channels important in magnesium homeostasis following traumatic brain injury? Magnes Res 22:225–234
Coombes E, Jiang J, Chu XP, Inoue K, Seeds J, Branigan D, Simon RP, Xiong ZG (2011) Pathophysiological relevant levels of hydrogen peroxide induces glutamate-independent neurodegeneration that involves activation of TRPM7 channels. Antioxid Redox Signal 14:1815–1827
Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V (2006) Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience 139:1405–1415
Crowder EA, Saha MS, Pace RW, Zhang H, Prestwich GD, Del Negro CA (2007) Phosphatidylinositol 4,5-biphosphate regulates inspiratory burst activity in the neonatal mouse preBotzinger complex. J Physiol 582:1047–1058
Cucchiaroni ML, Viscomi MT, Bernardi G, Molinari M, Guatteo E, Mercuri NB (2010) Metabotropic glutamate receptor 1 mediates the electrophysiological and toxic actions of the cycad derivative beta-N-Methylamino-L-alanine on substantia nigra pars compacta DAergic neurons. J Neurosci 30:5176–5188
Czondor K, Ellwanger K, Fuchs YF, Lutz S, Gulyas M, Mansuy IM, Hausser A, Pfizenmaier K, Schlett K (2009) Protein kinase D controls the integrity of Golgi apparatus and the maintenance of dendritic arborization in hippocampal neurons. Mol Biol Cell 20:2108–2120
Damann N, Voets T, Nilius B (2008) TRPs in our senses. Curr Biol 18:R880–R889
Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL (2005) Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci 25:446–453, United States
Dong XP, Wang X, Xu H (2010) TRP channels of intracellular membranes. J Neurochem 113:313–328
Du J, Yang X, Zhang L, Zeng YM (2009) Expression of TRPM8 in the distal cerebrospinal fluid-contacting neurons in the brain mesencephalon of rats. Cerebrospinal Fluid Res 6:3
Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA (2002) Graded persistent activity in entorhinal cortex neurons. Nature 420:173–178
El-Hassar L, Hagenston AM, Bertetto D'Angelo L, Yeckel M (2011) MGluRs regulate hippocampal CA1 pyramidal neuron excitability via Ca2+ wave-dependent activation of SK and TRPC channels. J Physiol 589:3211–3229
Feldman JL, Del Negro CA (2006) Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 7:232–242, England
Fernandez-Ruiz J, Hernandez M, Ramos JA (2010) Cannabinoid-dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther 16:e72–e91
Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S (2006) Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res 26:159–178
Fowler MA, Sidiropoulou K, Ozkan ED, Phillips CW, Cooper DC (2007) Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain. PLoS One 2:e573
Freestone PS, Chung KK, Guatteo E, Mercuri NB, Nicholson LF, Lipski J (2009) Acute action of rotenone on nigral dopaminergic neurons – involvement of reactive oxygen species and disruption of Ca homeostasis. Eur J Neurosci 30:849–859
Fu M, Xie Z, Zuo H (2009) TRPV1: a potential target for antiepileptogenesis. Med Hypotheses 73:100–102
Gasperini RJ, Choi-Lundberg D, Thompson MJ, Mitchell CB, Foa L (2009) Homer regulates calcium signalling in growth cone turning. Neural Dev 4:29
Gao YQ, Gao H, Zhou ZY, Lu SD, Sun FY (2004) Expression of transient receptor potential channel 4 in striatum and hippocampus of rats is increased after focal cerebral ischemia. Sheng li xue bao: Acta physiologica Sinica 56:153–157
Genro BP, de Oliveira Alvares L, Quillfeldt JA (2012) Role of TRPV1 in consolidation of fear memories depends on the averseness of the conditioning procedure. Neurobiol Learn Mem 97:355–360
Giampa C, Demarch Z, Patassini S, Bernardi G, Fusco FR (2007) Immunohistochemical localization of TRPC6 in the rat substantia nigra. Neurosci Lett 424:170–174
Gibon J, Deloulme JC, Chevallier T, Ladeveze E, Abrous DN, Bouron A (2012) The antidepressant hyperforin increases the phosphorylation of CREB and the expression of TrkB in a tissue-specific manner. Int J Neuropsychopharmacol 1–10
Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA (2008) TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 57:746–759
Gokce O, Runne H, Kuhn A, Luthi-Carter R (2009) Short-term striatal gene expression responses to brain-derived neurotrophic factor are dependent on MEK and ERK activation. PLoS One 4:e5292
Goswami C, Hucho T (2007) TRPV1 expression-dependent initiation and regulation of filopodia. J Neurochem 103:1319–1333
Greka A, Navarro B, Oancea E, Duggan A, Clapham DE (2003) TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci 6:837–845
Griffith TN, Varela-Nallar L, Dinamarca MC, Inestrosa NC (2010) Neurobiological effects of hyperforin and its potential in Alzheimer’s disease therapy. Curr Med Chem 17:391–406
Grueter BA, Brasnjo G, Malenka RC (2010) Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci 13:1519–1525
Guatteo E, Chung KK, Bowala TK, Bernardi G, Mercuri NB, Lipski J (2005) Temperature sensitivity of dopaminergic neurons of the substantia nigra pars compacta: involvement of transient receptor potential channels. J Neurophysiol 94:3069–3080
Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M (2002) Heat-evoked activation of the ion channel, TRPV4. J Neurosci 22:6408–6414
Hara K, Kokubo Y, Ishiura H, Fukuda Y, Miyashita A, Kuwano R, Sasaki R, Goto J, Nishizawa M, Kuzuhara S, Tsuji S (2010) TRPM7 is not associated with amyotrophic lateral sclerosis-parkinsonism dementia complex in the Kii peninsula of Japan. Am J Med Genet B Neuropsychiatr Genet 153B:310–313
Hartmann, J. & Konnerth, A. (2008). Mechanisms of metabotropic glutamate receptor-mediated synaptic signaling in cerebellar Purkinje cells. Acta Physiol (Oxf)
Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, Birnbaumer L, Konnerth A (2008) TRPC3 channels are required for synaptic transmission and motor coordination. Neuron 59:392–398
Hartmann J, Henning HA, Konnerth A (2011) mGluR1/TRPC3-mediated synaptic transmission and calcium signaling in mammalian central neurons. Cold Spring Harbor Perspect Biol 3:a00676
Harteneck C (2003) Proteins modulating TRP channel function. Cell Calcium 33:303–310
Henle SJ, Wang G, Liang E, Wu M, Poo MM, Henley JR (2011) Asymmetric PI(3,4,5)P3 and Akt signaling mediates chemotaxis of axonal growth cones. J Neurosci 31:7016–7027
Henley J, Poo MM (2004) Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol 14:320–330, England
Henley JR, Huang KH, Wang D, Poo MM (2004) Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron 44:909–916, United States
Heo DK, Chung WY, Park HW, Yuan JP, Lee MG, Kim JY (2012) Opposite regulatory effects of TRPC1 and TRPC5 on neurite outgrowth in PC12 cells. Cell Signal 24:899–906
Hermosura MC, Garruto RM (2007) TRPM7 and TRPM2-candidate susceptibility genes for western pacific ALS and PD? Biochim Biophys Acta 1772:822–835
Hermosura MC, Nayakanti H, Dorovkov MV, Calderon FR, Ryazanov AG, Haymer DS, Garruto RM (2005) A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc Natl Acad Sci USA 102:11510–11515
Hermosura M, Cui AM, Go G, Davenport B, Shetler C, Heizer J, Schmitz C, Mocz G, Garruto R, Perraud A-L (2008) Altered functional properties of a TRPM2 variant in Guamanian ALS and PD. Proc Natl Acad Sci (USA) 105:18029–18034
Hofmann T, Chubanov V, Gudermann T, Montell C (2003) TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr Biol 13:1153–1158
Holland S, Scholich K (2011) Regulation of neuronal functions by the E3-ubiquitinligase protein associated with MYC (MYCBP2). Commun Integr Biol 4:513–515
Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M (2000) Calcium signalling in the guidance of nerve growth by netrin-1. Nature 403:93–98
Hu J, Choo HJ, Ma SX (2012) Infrared heat treatment reduces food intake and modifies expressions of TRPV3-POMC in the dorsal medulla of obesity prone rats. Int J Hyperthermia 27:708–716
Hutchins BI, Li L, Kalil K (2010) Wnt/calcium signaling mediates axon growth and guidance in the developing corpus callosum. Dev Neurobiol 71:269–283
Iida T, Shimizu I, Nealen ML, Campbell A, Caterina M (2005) Attenuated fever response in mice lacking TRPV1. Neurosci Lett 378:28–33
Jang Y, Jung J, Kim H, Oh J, Jeon JH, Jung S, Kim KT, Cho H, Yang DJ, Kim SM, Kim IB, Song MR, Oh U (2012) Axonal neuropathy-associated TRPV4 regulates neurotrophic factor-derived axonal growth. J Biol Chem 287:6014–6024
Jia Y, Zhou J, Tai Y, Wang Y (2007) TRPC channels promote cerebellar granule neuron survival. Nat Neurosci 10:559–567
Jiang X, Newell EW, Schlichter LC (2003) Regulation of a TRPM7-like current in rat brain microglia. J Biol Chem 278:42867–42876
Kaczmarek LK (2006) Non-conducting functions of voltage-gated ion channels. Nat Rev Neurosci 7:761–771
Kaczmarek JS, Riccio A, Clapham DE (2012) Calpain cleaves and activates the TRPC5 channel to participate in semaphorin 3A-induced neuronal growth cone collapse. Proc Natl Acad Sci USA 109:7888–7892
Kauer JA, Gibson HE (2009) Hot flash: TRPV channels in the brain. Trends Neurosci 32:215–224
Kiselyov K, Yamaguchi S, Lyons CW, Muallem S (2010) Aberrant Ca(2+) handling in lysosomal storage disorders. Cell Calcium 47:103–111
Kiselyov K, Colletti GA, Terwilliger A, Ketchum K, Lyons CW, Quinn J, Muallem S (2011) TRPML: transporters of metals in lysosomes essential for cell survival? Cell Calcium 50:288–294
Koch M, Kreutz S, Bottger C, Grabiec U, Ghadban C, Korf HW, Dehghani F (2011) The cannabinoid WIN 55,212-2-mediated protection of dentate gyrus granule cells is driven by CB1 receptors and modulated by TRPA1 and Cav 2.2 channels. Hippocampus 21:554–564
Kumar S, Chakraborty S, Barbosa C, Brustovetsky T, Brustovetsky N, Obukhov AG (2012) Mechanisms controlling neurite outgrowth in a pheochromocytoma cell line: the role of TRPC channels. J Cell Physiol 227:1408–1419
Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H (2006) Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics 7:159
Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP (2002) TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109:397–407
Lee N, Chen J, Sun L, Wu S, Gray KR, Rich A, Huang M, Lin JH, Feder JN, Janovitz EB, Levesque PC, Blanar MA (2003) Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3). J Biol Chem 278:20890–20897
Lee KH, Cho JH, Choi IS, Park HM, Lee MG, Choi BJ, Jang IS (2010) Pregnenolone sulfate enhances spontaneous glutamate release by inducing presynaptic Ca2+−induced Ca2+ release. Neuroscience 171:106–116
Leuner K, Kazanski V, Muller M, Essin K, Henke B, Gollasch M, Harteneck C, Muller WE (2007) Hyperforin – a key constituent of St. John’s wort specifically activates TRPC6 channels. FASEB J 21:4101–4111
Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, Yuan XB (2005) Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 434:894–898
Li L, Hutchins BI, Kalil K (2010) Wnt5a induces simultaneous cortical axon outgrowth and repulsive turning through distinct signaling mechanisms. Sci Signal 3:pt2
Li M, Chen C, Zhou Z, Xu S, Yu Z (2012) A TRPC1-mediated increase in store-operated Ca(2+) entry is required for the proliferation of adult hippocampal neural progenitor cells. Cell Calcium 51:486–496
Liapi A, Wood JN (2005) Extensive co-localization and heteromultimer formation of the vanilloid receptor-like protein TRPV2 and the capsaicin receptor TRPV1 in the adult rat cerebral cortex. Eur J Neurosci 22:825–834
Lin W, Margolskee R, Donnert G, Hell SW, Restrepo D (2007) Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc Natl Acad Sci U S A 104:2471–2476
Lipski J, Park TI, Li D, Lee SC, Trevarton AJ, Chung KK, Freestone PS, Bai JZ (2006) Involvement of TRP-like channels in the acute ischemic response of hippocampal CA1 neurons in brain slices. Brain Res 1077:187–199
Lowry CA, Lightman SL, Nutt DJ (2009) That warm fuzzy feeling: brain serotonergic neurons and the regulation of emotion. J Psychopharmacol 23:392–400
Manna SS, Umathe SN (2011) Transient receptor potential vanilloid 1 channels modulate the anxiolytic effect of diazepam. Brain Res 1425:75–82
Marsch R, Foeller E, Rammes G, Bunck M, Kossl M, Holsboer F, Zieglgansberger W, Landgraf R, Lutz B, Wotjak CT (2007) Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J Neurosci 27:832–839
Martorana A, Giampa C, DeMarch Z, Viscomi MT, Patassini S, Sancesario G, Bernardi G, Fusco FR (2006) Distribution of TRPC1 receptors in dendrites of rat substantia nigra: a confocal and electron microscopy study. Eur J Neurosci 24:732–738
McGurk JS, Shim S, Kim JY, Wen Z, Song H, Ming GL (2011) Postsynaptic TRPC1 function contributes to BDNF-induced synaptic potentiation at the developing neuromuscular junction. J Neurosci 31:14754–14762
Meriaux C, Arafah K, Tasiemski A, Wisztorski M, Bruand J, Boidin-Wichlacz C, Desmons A, Debois D, Laprevote O, Brunelle A, Gaasterland T, Macagno E, Fournier I, Salzet M (2011) Multiple changes in peptide and lipid expression associated with regeneration in the nervous system of the medicinal leech. PLoS One 6:e18359
Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A (2000) Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci USA 97:3655–3660
Miedel MT, Rbaibi Y, Guerriero CJ, Colletti G, Weixel KM, Weisz OA, Kiselyov K (2008) Membrane traffic and turnover in TRP-ML1-deficient cells: a revised model for mucolipidosis type IV pathogenesis. J Exp Med 205:1477–1490
Mironov SL (2008) Metabotropic glutamate receptors activate dendritic calcium waves and TRPM channels which drive rhythmic respiratory patterns in mice. J Physiol 586:2277–2291
Mironov SL, Skorova EY (2011) Stimulation of bursting in pre-Botzinger neurons by Epac through calcium release and modulation of TRPM4 and K-ATP channels. J Neurochem 117:295–308
Mitchell CB, Gasperini RJ, Small DH, Foa L (2012) STIM1 is necessary for store operated calcium entry in turning growth cones. J Neurochem 122:1155–1166
Mitsumura K, Hosoi N, Furuya N, Hirai H (2011) Disruption of metabotropic glutamate receptor signalling is a major defect at cerebellar parallel fibre-Purkinje cell synapses in staggerer mutant mice. J Physiol 589:3191–3209
Moran MM, Xu H, Clapham DE (2004) TRP ion channels in the nervous system. Curr Opin Neurobiol 14:362–369
Moreira FA, Aguiar DC, Terzian AL, Guimaraes FS, Wotjak CT (2011) Cannabinoid type 1 receptors and transient receptor potential vanilloid type 1 channels in fear and anxiety-two sides of one coin? Neuroscience 204:186–192
Mori F, Ribolsi M, Kusayanagi H, Monteleone F, Mantovani V, Buttari F, Marasco E, Bernardi G, Maccarrone M, Centonze D (2012) TRPV1 channels regulate cortical excitability in humans. J Neurosci 32:873–879
Morley JE, Banks WA (1998) Lipids and cognition. J Alzheimers Dis 20:737–747
Moussaieff A, Rimmerman N, Bregman T, Straiker A, Felder CC, Shoham S, Kashman Y, Huang SM, Lee H, Shohami E, Mackie K, Caterina MJ, Walker JM, Fride E, Mechoulam R (2008) Incensole acetate, an incense component, elicits psychoactivity by activating TRPV3 channels in the brain. FASEB J 22:3024–3034
Moussaieff A, Yu J, Zhu H, Gattoni-Celli S, Shohami E, Kindy MS (2012) Protective effects of incensole acetate on cerebral ischemic injury. Brain Res 1443:89–97
Mrejeru A, Wei A, Ramirez JM (2011) CAN currents are involved in generation of tonic and bursting activity in dopamine neurons of the substantia nigra pars compacta. J Physiol 589:2497–2514
Musella A, De Chiara V, Rossi S, Prosperetti C, Bernardi G, Maccarrone M, Centonze D (2008) TRPV1 channels facilitate glutamate transmission in the striatum. Mol Cell Neurosci 40:89–97
Musella A, De Chiara V, Rossi S, Cavasinni F, Castelli M, Cantarella C, Mataluni G, Bernardi G, Centonze D (2010) Transient receptor potential vanilloid 1 channels control acetylcholine/2-arachidonoylglicerol coupling in the striatum. Neuroscience 167:864–871
Narayanan KL, Irmady K, Subramaniam S, Unsicker K, von Bohlen und Halbach O (2008) Evidence that TRPC1 is involved in hippocampal glutamate-induced cell death. Neurosci Lett 446:117–122
Nelson C, Glitsch MD (2012) Lack of kinase regulation of canonical transient receptor Potential (TRPC) 3 dependent currents in rat cerebellar Purkinje cells. J Biol Chem 287:6326–6335
Nilius B, Owsianik G (2011) The transient receptor potential family of ion channels. Genome Biol 12:218–229
Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, Tessier-Lavigne M, Poo MM, Hong K (2003) Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature 423:990–995, England
Olah ME, Jackson MF, Li H, Perez Y, Sun HS, Kiyonaka S, Mori Y, Tymianski M, Macdonald JF (2009) Ca2+−dependent induction of TRPM2 currents in hippocampal neurons. J Physiol 587:965–979
Pace RW, Mackay DD, Feldman JL, Del Negro CA (2007) Role of persistent sodium current in mouse preBotzinger complex neurons and respiratory rhythm generation. J Physiol 580:485–496
Paez PM, Fulton D, Spreuer V, Handley V, Campagnoni AT (2011) Modulation of canonical transient receptor potential channel 1 in the proliferation of oligodendrocyte precursor cells by the golli products of the myelin basic protein gene. J Neurosci 31:3625–3637
Petersson ME, Yoshida M, Fransen EA (2011) Low-frequency summation of synaptically activated transient receptor potential channel-mediated depolarizations. Eur J Neurosci 34:578–593
Philipp S, Hambrecht J, Braslavski L, Schroth G, Freichel M, Murakami M, Cavalie A, Flockerzi V (1998) A novel capacitative calcium entry channel expressed in excitable cells. EMBO J 17:4274–4282
Poduslo SE, Huang R, Huang J (2008) The frequency of the TRPC4AP haplotype in Alzheimer’s patients. Neurosci Lett 450:344–346
Poduslo SE, Huang R, Huang J, Smith S (2009) Genome screen of late-onset Alzheimer’s extended pedigrees identifies TRPC4AP by haplotype analysis. Am J Med Genet B Neuropsychiatr Genet 150B:50–55
Pressler RT, Strowbridge BW (2006) Blanes cells mediate persistent feedforward inhibition onto granule cells in the olfactory bulb. Neuron 49:889–904, United States
Puertollano R, Kiselyov K (2009) TRPMLs: in sickness and in health. Am J Physiol Renal Physiol 296:F1245–F1254
Puram SV, Riccio A, Koirala S, Ikeuchi Y, Kim AH, Corfas G, Bonni A (2011) A TRPC5-regulated calcium signaling pathway controls dendrite patterning in the mammalian brain. Genes Dev 25:2659–2673
Qiu J, Fang Y, Bosch MA, Ronnekleiv OK, Kelly MJ (2011) Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology 152:1503–1514
Reuss S, Disque-Kaiser U, Binzen U, Greffrath W, Peschke E (2010) ‘TRPing’ synaptic ribbon function in the rat pineal gland: neuroendocrine regulation involves the capsaicin receptor TRPV1. Neuroendocrinology 92:133–142
Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, Benham CD, Pangalos MN (2002) mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res 109:95–104
Roedding AS, Gao AF, Wu AM, Li PP, Kish SJ, Warsh JJ (2009) TRPC3 protein is expressed across the lifespan in human prefrontal cortex and cerebellum. Brain Res 1260:1–6
Roedding AS, Gao AF, Au-Yeung W, Scarcelli T, Li PP, Warsh JJ (2012) Effect of oxidative stress on TRPM2 and TRPC3 channels in B lymphoblast cells in bipolar disorder. Bipolar Disord 14:151–161
Romero JR, Ridker PM, Zee RY (2009) Gene variation of the transient receptor potential cation channel, subfamily M, member 7 (TRPM7), and risk of incident ischemic stroke. Prospective, nested, case–control study. Stroke 64:791–797
Rubin JE, Hayes JA, Mendenhall JL, Del Negro CA (2009) Calcium-activated nonspecific cation current and synaptic depression promote network-dependent burst oscillations. Proc Natl Acad Sci USA 106:2939–2944
Schiller Y (2004) Activation of a calcium-activated cation current during epileptiform discharges and its possible role in sustaining seizure-like events in neocortical slices. J Neurophysiol 92:862–872
Shen D, Wang X, Li X, Zhang X, Yao Z, Dibble S, Dong XP, Yu T, Lieberman AP, Showalter HD, Xu H (2012) Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun 3:731
Shibasaki K, Suzuki M, Mizuno A, Tominaga M (2007) Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci 27:1566–1575
Shibasaki K, Murayama N, Ono K, Ishizaki Y, Tominaga M (2010) TRPV2 Enhances Axon Outgrowth through Its Activation by Membrane Stretch in Developing Sensory and Motor Neurons. J Neurosci 30:4601–4612
Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS (2011) TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci 15:70–80
Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS (2012) TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci 15:70–80
Shim S, Yuan JP, Kim JY, Zeng W, Huang G, Milshteyn A, Kern D, Muallem S, Ming G-L, Worley PF (2009) Peptidyl-Prolyl Isomerase FKBP52 Controls Chemotropic Guidance of Neuronal Growth Cones via Regulation of TRPC1 Channel Opening. Neuron 64:471–483
Slaugenhaupt SA (2002) The molecular basis of mucolipidosis type IV. Curr Mol Med 2:445–450
Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK (2011) Serotonin 2C Receptor Activates a Distinct Population of Arcuate Pro-opiomelanocortin Neurons via TRPC Channels. Neuron 71:488–497
Sossin WS, Barker PA (2007) Something old, something new: BDNF-induced neuron survival requires TRPC channel function. Nat Neurosci 10:537–538
Stawicki TM, Zhou K, Yochem J, Chen L, Jin Y (2011) TRPM Channels Modulate Epileptic-like Convulsions via Systemic Ion Homeostasis. Curr Biol. 21:883–888
Stokes A, Wakano C, Koblan-Huberson M, Adra CN, Fleig A, Turner H (2006) TRPA1 is a substrate for de-ubiquitination by the tumor suppressor CYLD. Cell Signal 18:1584–1594
Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE (2001) TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29:645–655
Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, Kiyonaka S, Mori Y, Jones M, Forder JP, Golde TE, Orser BA, Macdonald JF, Tymianski M (2009) Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci 12:1300–1307
Sylvester JB, Mwanjewe J, Grover AK (2001) Transient receptor potential protein mRNA expression in rat substantia nigra. Neurosci Lett 300:83–86
Tai Y, Feng S, Ge R, Du W, Zhang X, He Z, Wang Y (2008) TRPC6 channels promote dendritic growth via the CaMKIV-CREB pathway. J Cell Sci 121:2301–2307
Tai Y, Feng S, Du W, Wang Y (2009) Functional roles of TRPC channels in the developing brain. Pflugers Archiv: European journal of physiology 458:283–289
Talavera K, Nilius B, Voets T (2008) Neuronal TRP channels: thermometers, pathfinders and life-savers. Trends Neurosci 31:287–295
Tian SL, Jiang H, Zeng Y, Li LL, Shi J (2007) NGF-induced reduction of an outward-rectifying TRPM7-like current in rat CA1 hippocampal neurons. Neurosci Lett 419:93–98
Trebak M (2010) The puzzling role of TRPC3 channels in motor coordination. Pflugers Archiv: European journal of physiology 459:369–375
Toth A, Boczan J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Edes I, Csiba L, Blumberg PM (2005) Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res 135:162–168
Tu P, Gibon J, Bouron A (2009a) The TRPC6 channel activator hyperforin induces the release of zinc and calcium from mitochondria. J Neurochem 112:204–213
Tu P, Kunert-Keil C, Lucke S, Brinkmeier H, Bouron A (2009b) Diacylglycerol analogues activate second messenger-operated calcium channels exhibiting TRPC-like properties in cortical neurons. J Neurochem 108:126–138
Uemura T, Kudoh J, Noda S, Kanba S, Shimizu N (2005) Characterization of human and mouse TRPM2 genes: identification of a novel N-terminal truncated protein specifically expressed in human striatum. Biochem Biophys Res Commun 328:1232–1243
Umathe SN, Manna SS, Jain NS (2012) Endocannabinoid analogues exacerbate marble-burying behavior in mice via TRPV1 receptor. Neuropharmacology 62:2024–2033
Venkatachalam K, Long AA, Elsaesser R, Nikolaeva D, Broadie K, Montell C (2008) Motor deficit in a Drosophila model of mucolipidosis type IV due to defective clearance of apoptotic cells. Cell 135:838–851
Venugopal B, Mesires NT, Kennedy JC, Curcio-Morelli C, Laplante JM, Dice JF, Slaugenhaupt SA (2009) Chaperone-mediated autophagy is defective in mucolipidosis type IV. J Cell Physiol 219:344–353
Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B (2004) Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA 101:396–401
Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, Oberwinkler J, Vennekens R, Gudermann T, Nilius B, Voets T (2011) TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70:482–494
Wainwright A, Rutter AR, Seabrook GR, Reilly K, Oliver KR (2004) Discrete expression of TRPV2 within the hypothalamo-neurohypophysial system: implications for regulatory activity within the hypothalamic-pituitary-adrenal axis. J Comp Neurol 474:24–42
Wang GX, Poo MM (2005). Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 434:898–904
Wang S, Zhang AP, Kurada L, Matsui T, Lei S (2011) Cholecystokinin Facilitates Neuronal Excitability in the Entorhinal Cortex via Activation of TRPC-like Channels. J Neurophysiol 106:1515–1524
Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B (2003) Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424:434–438
Wei WL, Sun HS, Olah ME, Sun X, Czerwinska E, Czerwinski W, Mori Y, Orser BA, Xiong ZG, Jackson MF, Tymianski M, Macdonald JF (2007) TRPM7 channels in hippocampal neurons detect levels of extracellular divalent cations. Proc Natl Acad Sci USA 104:16232–16328
Wen Z, Han L, Bamburg JR, Shim S, Ming GL, Zheng JQ (2007) BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF/cofilin. J Cell Biol 178:107–119
Williams KW, Sohn JW, Donato J Jr, Lee CE, Zhao JJ, Elmquist JK, Elias CF (2011) The acute effects of leptin require PI3K signaling in the hypothalamic ventral premammillary nucleus. J Neurosci 31:13147–13156
Wodarczyk C, Rowe I, Chiaravalli M, Pema M, Qian F, Boletta A (2009) A novel mouse model reveals that polycystin-1 deficiency in ependyma and choroid plexus results in dysfunctional cilia and hydrocephalus. PLoS One 4:e7137
Wu ZZ, Chen SR, Pan HL (2005) Transient receptor potential vanilloid type 1 activation down-regulates voltage-gated calcium channels through calcium-dependent calcineurin in sensory neurons. J Biol Chem 280:18142–18151
Wu TT, Zhao ZJ, Xu C, Zhang LC (2011) Distribution of trpc6 in the cerebrospinal fluid-contacting nucleus of rat brain parenchyma and its expression in morphine dependence and withdrawal. Neurochem Res 36:2316–2321
Xie YF, Belrose JC, Lei G, Tymianski M, Mori Y, Macdonald JF, Jackson MF (2012) Dependence of NMDA/GSK3beta mediated metaplasticity on TRPM2 channels at hippocampal CA3-CA1 synapses. Mol Brain 4:44
Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE (2002) TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418:181–186
Xu C, Macciardi F, Li PP, Yoon IS, Cooke RG, Hughes B, Parikh SV, McIntyre RS, Kennedy JL, Warsh JJ (2006) Association of the putative susceptibility gene, transient receptor potential protein melastatin type 2, with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet 141:36–43
Xu C, Li PP, Cooke RG, Parikh SV, Wang K, Kennedy JL, Warsh JJ (2009) TRPM2 variants and bipolar disorder risk: confirmation in a family-based association study. Bipolar Disord 11:1–10
Yan HD, Villalobos C, Andrade R (2009) TRPC channels mediate a muscarinic receptor-induced afterdepolarization in cerebral cortex. J Neurosci 29:10038–10046
Yin DM, Huang YH, Zhu YB, Wang Y (2008) Both the establishment and maintenance of neuronal polarity require the activity of protein kinase D in the Golgi apparatus. J Neurosci 28:8832–8843
Yokoyama T, Ohbuchi T, Saito T, Sudo Y, Fujihara H, Minami K, Nagatomo T, Uezono Y, Ueta Y (2011) Allyl isothiocyanates and cinnamaldehyde potentiate miniature excitatory postsynaptic inputs in the supraoptic nucleus in rats. Eur J Pharmacol 655:31–37
Yoo JC, Yarishkin OV, Hwang EM, Kim E, Kim DG, Park N, Hong SG, Park JY (2010) Cloning and characterization of rat transient receptor potential-melastatin 4 (TRPM4). Biochem Biophys Res Commun 391:806–811
Zamudio-Bulcock PA, Valenzuela CF (2011) Pregnenolone sulfate increases glutamate release at neonatal climbing fiber-to-Purkinje cell synapses. Neuroscience 175:24–36
Zamudio-Bulcock PA, Everett J, Harteneck C, Valenzuela CF (2011) Activation of steroid-sensitive TRPM3 channels potentiates glutamatergic transmission at cerebellar Purkinje neurons from developing rats. J Neurochem 119:474–485
Zechel S, Werner S, von Bohlen Und Halbach O (2007) Distribution of TRPC4 in developing and adult murine brain. Cell Tissue Res 328:651–656
Zhang J, Zhao F, Zhao Y, Wang J, Pei L, Sun N, Shi J (2011a) Hypoxia induces an increase in intracellular magnesium via TRPM7 channels in rat hippocampal neurons in vitro. J Biol Chem 286:20194–20207
Zhang Z, Reboreda A, Alonso A, Barker PA, Seguela P (2011b) TRPC channels underlie cholinergic plateau potentials and persistent activity in entorhinal cortex. Hippocampus 21:386–397
Zhou J, Du W, Zhou K, Tai Y, Yao H, Jia Y, Ding Y, Wang Y (2008) Critical role of TRPC6 channels in the formation of excitatory synapses. Nat Neurosci 11:741–743
Acknowledgments
The authors are supported by the FWO Vlaanderen and the Bijzonder Onderzoeksfonds of the KU Leuven. We would like to thank all members of the Laboratory of Ion Channel Research, KU Leuven, for stimulating discussions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Vennekens, R., Menigoz, A., Nilius, B. (2012). TRPs in the Brain. In: Nilius, B., et al. Reviews of Physiology, Biochemistry and Pharmacology, Vol. 163. Reviews of Physiology, Biochemistry and Pharmacology, vol 163. Springer, Berlin, Heidelberg. https://doi.org/10.1007/112_2012_8
Download citation
DOI: https://doi.org/10.1007/112_2012_8
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-33520-4
Online ISBN: 978-3-642-33521-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)