Abstract
Methane is produced usually from organic waste in a straightforward anaerobic digestion process. However, hydrogen production is technically more challenging as more stages are needed to convert all biomass to hydrogen because of thermodynamic constraints. Nevertheless, the benefit of hydrogen is that it can be produced, both biologically and thermochemically, in more than one way from either organic compounds or water. Research in biological hydrogen production is booming, as reflected by the myriad of recently published reviews on the topic. This overview is written from the perspective of how to transfer as much energy as possible from the feedstock into the gaseous products hydrogen, and to a lesser extent, methane. The status and remaining challenges of all the biological processes are concisely discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Dark fermentation
- Electrohydrogenesis
- Hydrogen productivity
- Hydrogen yield
- Mesophiles
- Photofermentation
- Thermodynamics
- Thermophiles
1 Introduction
In the light of creating a sustainable society, the interest in both hydrogen and biomethane is increasing. The global biogas market is expected to double between 2011 and 2022 from $ 17.3 to 33.1 billion [1]. There is increasing decentralized production for local demand (farmers and municipalities) and production for “greening” the natural gas grid. The global hydrogen market, on the other hand, is steadily increasing from about $ 87.5 billion (2011) to $ 118 billion in 2016 [2]. However, hydrogen is mainly produced thermochemically from petroleum and to a small extent through electrolysis of water, as industrial biological hydrogen processes (BHPs) are as yet non-existent. Today hydrogen is mainly used as an industrial reducing agent (oil, food, electronics, ammonia), for which a cost of about 1–2 € kg H2 −1 is set based on the estimated oil prices for 2020 [3]. The increasing demand for hydrogen is especially driven by ever stricter regulatory norms of removing sulfur from petroleum products. Hydrogen as an energy carrier is, as yet, only a niche market, mainly because of a lack of a comprehensible hydrogen fuel infrastructure and an effective hydrogen storage technology. Introducing CO2 taxes is seen as a driver on the long road to a hydrogen economy [3].
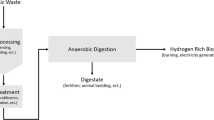
Apart from water used as the source for hydrogen in biophotolysis, feedstocks for hydrogen and methane can be derived as wastes from forestry, agriculture, industry (e.g., food industry), and domestic waste. In addition, special energy crops can be cultivated which do not compete with edible crops. Regarding the biological production of gaseous fuels, anaerobic digestion is the most common and widely applied process. The product biogas, mainly a mixture of methane and carbon dioxide, may need to be purified depending on its use (vehicle fuel or the natural gas grid). Anaerobic digestion (AD) occurs naturally in places rich in organic waste, and is a straightforward process which can be applied, depending on the investment, with low-tech installations. Interestingly, hydrogen is a temporary intermediate in the fermentation process as hydrogen producers are essential members of the microbial consortium. Thus, in principle the fermentation can be halted at hydrogen production by removing or inhibiting the methanogens. The drawback of this alternative process is that maximally only one-third of the energy content – on a hexose basis – is captured in the gaseous product. The remaining energy is left in the organic byproducts, but can be extracted in a second stage process consisting of photofermentation (PF), hydroelectrogenesis (HE), or methanogenesis (Fig. 1). Hence, a complete biomass conversion is accomplished by integration of two processes, i.e., a dark fermentation (DF) converting the organic feedstock to hydrogen and organic acids followed by a process that converts the organic acids to either hydrogen or methane. DF, a fermentation without light, comes in two variations depending on the type of bacteria used: (1) mesophilic, operating between 25 and 35 °C and (2) thermophilic, operating between 55 and 80 °C. The two-stage or hybrid hydrogen production process has been discussed earlier [4–6]. This process setup is required to maximize the energy yield contained in the biomass source to make the process sustainable (minimal waste!) and economically feasible.
Various configurations of hydrogen and/or biogas production from “cheap” feedstock (organic wastes) or dedicated energy crops. (a) Cheap feedstock may lead to simplification of gaseous energy carrier production, including emphasizing on hydrogen productivity, hence mesophilic dark fermentation. (b) Expensive feedstocks lead to emphasizing maximizing hydrogen yields, hence thermophilic dark fermentation combined with conversion steps of the organic acids that require either sunlight input (photofermentation) or electricity input (electrohydrogenesis). An alternative could be a hydrogen-methane two-step fermentation process
The choice of mesophilic or thermophilic DF depends on the choice of feedstock:
-
1.
If the feedstock is cheap then the hydrogen yield is less important; instead opt for high productivities for which mesophilic bacteria are the best choice
-
2.
If specific energy crops or biomass pretreatment is necessary, then efficacy lies in high product yields rather than productivities; hence the choice falls on thermophilic bacteria
Where necessary, pretreatment of biomass increases accessibility of the microorganisms to the substrates [7]. The majority of raw biomass, especially lignocellulosics, consists of rigid materials which have to undergo a thermochemical treatment to destroy the delicate intertwined, fiber structure of the various polymers, i.e., lignin, cellulose, and hemicellulose. In this step, chopped up biomass is treated with steam using acid (sulfuric acid or phosphoric acid) or alkaline (lime or ammonia) water. Often this is followed by a hydrolysis step with a cocktail of commercial enzymes, including cellulases and xylanases. Updated cost analyses related to these different biological hydrogen processes (BHPs) have been published in the last 4–8 years [8, 9].
This chapter looks into the current status of each BHP process and highlights challenges that are still to be faced before an economical feasible process is possible. These challenges are of microbial, physical, and technical nature and solutions have to be found with minimal environmental impact. That is the reason why not one BHP process has moved far beyond the lab scale, and experience has been gained only with some pilot-scale installations. Biophotolysis is a standalone BHP process and can be carried out either aerobically or anaerobically. Therefore, it is not part of an integrated process (Fig. 1), but can deliver surplus algae or cyanobacteria biomass as a feedstock for one of the fermentation processes.
2 Background Information
Essential background information is provided here in order to follow the discussion of each of the processes below.
In principle, there are two different types of electron sources to make hydrogen, i.e., H2O and organic compounds. The former is the sole original source in the biophotolysis process, whereas in the fermentation processes both electron sources are involved. This is demonstrated by the overall conversion reactions given below.
In biophotolysis, water is split, which demands a very high input of energy from solar radiation:
In the other BHP processes, sugar-based biomass is mainly used, consisting of both hexoses and pentoses. For the sake of convenience the reactions and hydrogen yields (Y H2) are all based on the hexose glucose. Therefore, the stoichiometrically maximum yield of 12 H2 per glucose according to [10]:
is endergonic and thus not thermodynamically feasible. However, ideally it is possible to extract one-third of this total in a fermentation reaction yielding acetate as a byproduct:
In mesophilic DF hydrogen can also be formed in the conversion of sugars to butyrate:
but at a lower stoichiometry, and is therefore not favored.
Conversion of the remaining two-thirds of the electrons stored in acetate to hydrogen is strongly endergonic:
and thus needs an external energy source to push this reaction to the right. Sustainable external energy sources can be either solar radiation (photofermentation) or electricity from, e.g., windpower, solar cells, or microbial fuel cells (electrohydrogenesis).
Acetate can also be favorably converted to methane by acetoclastic methanogens:
All these metabolic conversions proceed under mild conditions, i.e., 30–80 °C and neutral to slightly acidic pH [11].
In the large body of BHP literature many different units are used for productivity. For the sake of comparison in this chapter the unit for volumetric hydrogen productivity (Q H2) [mmol H2 L reactor−1 h−1] is used and [mol H2 mol substrate−1] for the hydrogen yield (Y H2). Only the best results obtained so far have been gathered here to judge the order of magnitude of each BHP technology (Table 1). For detailed lists see the references to reviews mentioned below.
3 Hydrogen Production Processes
3.1 Biophotolysis
With five decades of biophotolytic hydrogen production, investigations are still strong and ongoing, whereby two different research lines have been explored, i.e., direct biophotolysis and indirect biophotolysis with both algae and cyanobacteria. However, the majority of studies have remained at lab scale, of which only a few have progressed to pilot plant scale. These studies on photosynthetic metabolism, strategies for improvements and photobioreactor (PBR) development have been discussed recently in dedicated reviews and book chapters [19–23].
3.1.1 Oxygenic Photosynthetic Microbes
Biophotolysis is the only process where eukaryotes (algae) and prokaryotes (cyanobacteria) are exploited in BHP. Yet the hydrogen-producing algae and cyanobacteria share quite similar photosynthetic constitutions and pathways to channel electrons to hydrogen production (Fig. 2).
Principle of direct and indirect biophotolysis in the oxygenic photosynthetic microbes. The major purpose of the photosystem II (PS II) in algae and cyanobacteria is to generate electrons through water splitting. These electrons are transferred via electron carriers in the electron transport chain to photosystem I (PS I) in the thylakoid membranes. PS I reduces the electron carrier ferredoxin (Fd), which is a cofactor for various enzymes. For direct hydrogen production, reduced Fd passes its electrons in algae to [FeFe]-hydrogenase (H2ase) and in cyanobacteria to nitrogenase (N2ase). Second, Fd can recycle the electrons in the electron transport chain around PS I (cyclic electron flow, CEF), which competes with hydrogen production during anaerobiosis. Finally, reduced Fd can donate its electrons to ferredoxin:NAD(P)+ oxidoreductase (FNR) to generate NAD(P)H. The latter can be involved in cyanobacteria in direct hydrogen production by passing its electrons to an [NiFe]-hydrogenase. NAD(P)H is also important for biomass formation and starch (algae) or glycogen (cyanobacteria) production by donating its electrons to the electron transfer chain to produce ATP via the proton motive force (pmf) or to the Calvin-Benson cycle (CBC), using ribulose 1,5-bisphosphate carboxylase/oxygenase (RubisCO) as the key-enzyme to fix CO2. Indirect biophotolysis can take place in the dark through fermentation of starch (glycogen), whereby the electrons are carried via NAD(P)H to a hydrogenase. Blue arrows and text: algae only; green arrows and text: cyanobacteria only
There are differences (Fig. 2) and one of the most obvious is that cyanobacteria can use nitrogenase to produce H2 under non-nitrogen fixing conditions according to the following reaction:
which is an energy-demanding reaction. Hydrogen is a byproduct under nitrogen fixing conditions:
which is even more energy-expensive (fourfold) to gain H2 and thus should be avoided.
Overall, hydrogen production rates observed with photosynthesis are relatively low (Table 1), especially for direct biophotolysis. Because oxygen irreversibly inhibits the hydrogenase, light-to-hydrogen conversion efficiencies are <0.1%, which is considered impractical for commercial use [24]. Indirect biophotolysis indeed increased the Q H2 by an order of magnitude (Table 1). Yet the conversion efficiencies remained below 1%. A major breakthrough to increase hydrogen evolution has been obtained through creating conditions of sulfur limitation [25] as a means to deactivate PS II and thereby preventing oxygen generation. As a consequence, the environment becomes anaerobic, which induces the synthesis of an [FeFe]-hydrogenase that combines electrons and protons from the low active PS II and storage products [26]. Nevertheless, less than 10% of photosynthesis capacity is channeled to hydrogen production because of light saturation [11]. To improve Q H2 further, researchers have looked into engineering better strains based on the understanding of the metabolism of photosynthesis and H2 production.
Electrons liberated in PS II are distributed over at least three competing pathways, i.e., hydrogen production, cyclic electron flow (CEF), and the Calvin–Benson cycle (CBC) (Fig. 2). For direct biophotolysis, strategies to diminish CEF were indeed enhancing hydrogen production [27]. However, under direct biophotolysis conditions the CBC is essential for autotrophic growth [28], and thus deletion of this pathway might be lethal. Instead, it may be possible to lower the electron flux through the CBC by modifying the expression or engineering of RubisCO [26, 29]. Indirect biophotolysis relies on electrons being directed to storage product formation to enhance H2 production. This was successfully accomplished in an engineered C. reinhardtii strain accumulating large quantities of starch [30]. Another approach for directing electrons to hydrogen production would be to engineer hydrogenases for higher affinities for Fdred to better compete with the other pathways [31]. Likewise, introducing heterologous ferredoxins that show better affinities or protein engineering the interacting surfaces of the electron donor and acceptor are potential strategies for both algae and cyanobacteria [32]. Finally, making chimeric complexes between electron carriers and electron acceptors is a recent approach that resulted in successes only in in vitro systems, but similar fusions have shown in vivo successes only in Escherichia coli so far [32].
3.1.2 Photobioreactor
For the design of a PBR the light regime and light conversion efficiency are very important factors [33]. The reactor requires a large surface area to volume ratio for optimal light availability per cell in the reactor. Therefore, the choices for closed systems are usually tubular and flat-panel reactors, and for open systems the pond or pool configuration. In the case of H2 production, it is obvious that one should use closed, gas-tight systems to capture this gaseous product. For industrial-scale indirect biophotolysis a two-reactor in tandem system is likely to be used. As hydrogen production is disconnected from growth and oxygen production, the latter can take place in open systems, of which the raceway is a long-time favorite [22]. This system allows the best conditions for growth and carbon storage production and fewer variables need to be controlled (e.g., temperature and mixing conditions). To speed up growth, active supply of CO2 into the liquid is required for meeting the carbon demand and maintaining a correct pH. Grown cells are subsequently centrifuged and pumped into the second, closed reactor and kept under sulfur deprivation, allowing the storage products to be fermented to H2. For proper operation of the closed process it is essential to monitor many variables, including flow rates, pH, dissolved oxygen tension (DOT), H2, and sulfur content. Still, outdoor tests with C. reinhardtii reached Q H2 of only about 0.024 mmol H2 L−1 h−1 [34], whereas A. variabilis reached productivities as high as 1.68 mmol H2 L−1 h−1 (Table 1). In theory, indirect biophotolysis leads to about 40% of the energy efficiency from light to H2 of direct biophotolysis [35]. This is because of (1) more steps being involved to extract the captured energy, and (2) significant amounts of ATP are required for the nitrogenase (cyanobacteria only). Still, it more than compensates for the losses of direct biophotolysis with its inherent inhibitory nature of oxygen.
Because light conversion efficiencies tend to decrease at higher light intensities as a result of light saturation of the photosynthetic apparatus, light should be diluted by distribution over the entire reactor volume. Adequately mixing the culture therefore becomes essential to expose the cells only briefly to the light, plus it avoids sedimentation and nutrient gradients.
Because biophotolysis requires large surface areas, a detailed cost analysis is of the utmost importance to minimize material and operation costs. A strategic location of PBRs is part of this, as factors such as light environment, climate, land space, and availability of water should be considered. For upscaling, modular design is the most effective way to increase surface area, bringing flexibility of handling to the system, and minimizing efforts for mixing.
3.1.3 Conclusions and Challenges
The most crucial parameter of all photosynthetic processes is the photon conversion efficiency. Further, direct biophotolysis with oxygenic phototrophs is not a viable commercial option as the produced oxygen inhibits the hydrogenases. Therefore – next to sulfur deprivation – indirect biophotolysis is the best strategy to produce H2, but requires a more complex reactor configuration and process operation. Interestingly, hydrogen production via direct biophotolysis can be further improved using designed co-cultures of the oxygenic photosynthetic microorganism with another microorganism that removes oxygen through respiration. For instance, co-cultivation of C. reinhardtii with Bradyrhizobium japonicum, a symbiotic rhizobium of the soybean Glycine max, resulted in improved Y H2 and 14-fold higher Q H2 [36]. This is an interesting field which needs to be further explored.
At present there is great uncertainty as to what scaling effects lie in store when progressing to pilot scale, as current calculations are based on data gathered from lab-scale experiments. As large surface areas are required for PBRs, because of low photon conversion efficiencies, it involves high costs for investment (material and land area) and operation. Yet, for further development of this BHP, abundant pilot-scale experience is required.
3.2 Photofermentation
The advantages of purple non-sulfur bacteria (PNSB) are (1) they do not produce oxygen, (2) they convert a broad variety of organic substrates, and (3) they harvest photons at a wide light spectrum (300–1,000 nm). Photofermentation has been extensively investigated with synthetic media, various organic waste streams, hydrolysates, and effluents from dark fermentations (DFE), in both indoor and outdoor situations, and was recently reviewed [21, 37]. Many different waste streams of the food industry, such as dairy food, molasses, olive mill waste (especially in the Mediterranean), and tofu (especially in Asia), can be directly converted by PNBS using light as an external energy source. The choice of feedstock is generally strain dependent, meaning that a screening for an adequate species needs to precede the optimization of the fermentation process. Still, many biological and technological parameters need to be optimized to arrive at a sustained process, and are briefly discussed below.
3.2.1 Feedstock
Most studies have been performed using artificial media, partly to optimize the system and partly to determine possible maximum productivities and yields without complications associated with complex feedstocks. One of the important parameters of the feedstock is the carbon to nitrogen ratio (C/N ratio). It is essential that the concentration of the N-source is low enough to avoid repression of expression levels of nitrogenase. Ammonium is a strong inhibitor, but glutamate appeared to be an adequate alternative. A C/N-ratio of 25 for a feedstock containing mainly acetate and glutamate resulted in improved productivities and yields [38]. However, to reduce costs, it is essential to find cheap replacements for glutamate, most probably by using smart combinations of waste streams which are complementary in nutrients. Many feedstocks are short in particular nutrients, such as iron and molybdenum, which need to be added for optimal functioning of nitrogenase and proteins of the electron transport chain. In addition, the buffer capacity needs to be high enough to keep the pH between 6.5 and 8.0. This is of particular importance because it is very difficult to control the pH in large surface area bioreactors. Most probably the buffer capacity can best be increased with bicarbonate as phosphate is not a sustainable solution. However, this needs to be investigated as it might lead to higher CO2 concentrations which can become inhibitory [39].
Even though PNSB can theoretically convert all 24 electrons in glucose to H2, they prefer organic acids [40]. Moreover, in practice PNSB reach only a fraction of this maximum yield because of excretion of intermediates [41].
Raw feedstocks and hydrolysates are not transparent and contain particles that absorb precious light in the photobioreactor. For instance, the light penetration into the reactor to a depth of 1 cm is 51% for molasses dark fermentation effluent (DFE) compared to 89% for a clear artificial medium [42]. In addition, the absorption spectrum of the feedstock should not overlap too much with that of the PNSB. Therefore, a pretreatment step maybe required, such as filtration or decolorization. Clay treatment is a promising method as it removes the majority of light absorbing compounds though hardly affecting the preferred compounds [43]. Finally, the feedstock should be kept as anaerobic as possible. Oxygen does not kill the PNSB, but shifts its metabolism and thus decreases the hydrogen production rate and yield.
3.2.2 PNSB Strains
As in cyanobacteria, hydrogen production is catalyzed by a molybdenum nitrogenase (Mo-N2ase), which is abundantly present in the cytoplasm, as a compensation for its slow reactivity (electron turnover ~5 s−1). The latter explains its rate of H2 production (approx. 1.3 mmol H2 mg protein−1 min−1) being one order of magnitude lower than for hydrogenases, and matching the Q H2 of the hydrogen-utilizing oxygenic phototrophs [40]. Nitrogenase is expressed when the soluble N-source (NH4 +) is below a certain critical concentration. In the presence of N2 the Mo-N2ase catalyzes the fixation of this gas molecule into ammonia, thereby producing hydrogen as a byproduct (8), but under non-fixing conditions of N2 the energy demand, as with cyanobacteria, for hydrogen production by Mo-N2ase is fourfold lower (7). Genetic modification has been another approach [23], in addition to determining optimal environmental conditions.
Several genetic engineering strategies have been carried out for improving hydrogen yields and productivities (Fig. 3), i.e., (1) removing the uptake hydrogenase, (2) removing the CBC pathway, and (3) increasing the expression of the proteins involved in directing electrons to nitrogenase and overexpressing the latter. Knocking out of the gene (Hup) coding for the uptake hydrogenase improved hydrogen production by Rb. sphaeroides as tested indoors in an artificial medium [45]. In addition, one of the highest Q H2 (approx. 2 mmol H2 L−1 h−1) with a Y H2 of 3.1 mol H2 mol acetate−1 was measured for the Rb. capsulatus hup− mutant on DFE of molasses in an outdoors flat panel reactor [46]. Deleting the RuBisCO genes in a Rhodopseudomonas palustris, possessing no uptake hydrogenase and containing constitutively expressed nitrogenase, indeed improved Y H2 [44]. However, it was more effective for succinate and butyrate (both twofold increase) as substrates than for acetate (only 1.3-fold increase), because of the metabolism of the former two substrates being connected to a higher CBC flux than for acetate. Conveniently, the constitutively expressed nitrogenase made growth and hydrogen production possible in the presence of NH4 + [47]. Overexpressing the Rnf complex in Rb. capsulatus [48] and NifA, encoding the specific transcriptional regulator of all nif genes, in Rb. sphaeroides [49] did improve H2 production. The former study succeeded in increasing the electron flow to nitrogenase as it was rate-limiting in the wild type. In the latter study the expression of nitrogenase was made constitutive and perhaps increased its activity, whereby the H2 production increased by 20%.
Principle of photofermentation metabolism. Organic substrates donate electrons (e−) in a pool of reductants, which are used for several purposes. (1) Electrons transferred to nitrogenase are converted together with protons to H2, a reaction that requires ATP, making this reaction, in contrast to hydrogenases, quite irreversible (7). However, H2 can partly donate electrons to NADH by an uptake hydrogenase (H2aseup). (2) Electrons together with light – in a cyclic electron flow (CEF) in the electron transport chain in the cell membrane – can create a pmf to enable ATP production. (3) Electrons are used for producing biomass via CO2 fixation in the CBC and to some extent to produce polyhydroxybutyrate (PHB) for storage of carbon and electrons. Part of the organic substrates is C-source for biomass, PHB production, and excreted organic compounds (EOC). If the fermentation process is allowed enough time the EOC are taken up again and consumed [44]
3.2.3 Light Radiation
Even though the majority of studies have been carried out indoors with artificial light, for cost-effective operation photobioreactors should be outside using sunlight. The major results of a wide selection of these studies are listed in recent reviews [21, 37]. So far, only a few studies have been performed outdoors, where additional issues affect sustained operation: (1) day and night rhythm, (2) temperature, and (3) light intensity. As sunlight is the light source, the culture needs to adjust to the day-night regime. Indeed, delay in growth and H2 production for more than a week has been observed for outdoor conditions [50]. Second, sunlight contains infrared light and the biochemical reactions produce heat, and hence cooling is required to keep the temperature between 20 °C and 45 °C. This cooling is either accomplished internally [22] or by sprinkling water on the outer surface [36], although the latter may introduce cracks in the panels depending on the material.
3.2.4 Bioreactor and Operation Conditions
To allow as much light penetration per surface area, the best reactors are either of the tubular or flat panel type. Both types of reactors' configuration and operation have been discussed in detail [22]. The limitations of each reactor type for photofermentation are similar to those of biophotolysis. Light penetration is one of the most important parameters to gain high hydrogen yields, and therefore, the diameter of the tubular reactor should not be too big. To receive similar portions of light, high recirculation can be applied, which is also an appropriate way of mixing the culture [37].
High organic acid concentrations have a detrimental effect on the start up of the photofermentation process. Therefore, the substrate concentration requires dilution, which increases the water demand even though part of the water can come from recirculation of treated wastewater from the entire process. After a lag phase growth starts without hydrogen evolution, and once the culture reaches a critical mass, hydrogen production is observed. Optimal production is seen with a cell density of 0.5–0.7 g DW L−1 [51] and concentrations of 30–40 mM acetate [52], beyond which hydrogen production activity may decline again. Therefore, it is also important to regulate the optimal cell density for sustained operation.
3.2.5 Conclusions and Challenges
Comparison between different feedstocks revealed that PNSB prefer short-chain organic acids, particularly acetate, with which the highest yields and productivities are achieved [37]. This is an appropriate property for considering this process as a process step in tandem with the DF process. The best procedure would be to use a combination of mutations in one strain to maximize the electron flow in the cell to nitrogenase and preventing H2 being consumed.
The photofermentation process requires rigorous control (including light penetration, pH, temperature, substrate concentration, adequate mixing in reactors with high ratio of surface area to volume, and cell density). The optimum temperature of the process for PNSB is 30–35 °C [53], and consequently cooling is often required for much of the day. Interestingly, there are moderate thermophilic PNSB growing at 40–45 °C [54], but up till now they have not been tested for H2 production. It would be of interest whether these thermophiles may give higher productivities and yields.
3.3 Electrohydrogenesis
Biocatalyzed electrolysis, as performed in microbial electrolysis cells (MECs) or bio-electrochemical systems (BESs), is the most recent technique applied to renewable hydrogen production [55, 56]. It is a variant of the microbial fuel cell (MFC), but, instead of producing electricity, biochemical conversion takes place through addition of a low voltage from an external power source. In general, similar to conventional batteries and water electrolysis cells, MECs consist of two chambers separated from each other by a semi-permeable membrane to prevent diffusion of hydrogen to the anode chamber. An MEC needs to be completely anaerobic as oxygen would interfere with either chamber. In principle, microorganisms oxidize organic compounds in the anode chamber, thereby transferring electrons to the anode. Gaseous carbon dioxide leaves the anode chamber and the protons diffuse through a cation exchange membrane (CEM) to the cathode where, together with the electrons supplied by the cathode, they form hydrogen (Fig. 4).
Principle of the microbial electrolysis cell (MEC). Through oxidation of organic compounds (e.g., acetate Ac−) microbes transfer electrons to the anode via (1) direct contact or (2) indirectly by an electron shuttle (reduced shuttle, S RED). The shuttle is reoxidized at the anode and returns back into the culture (S OX). The protons (H+) diffuse through a cation exchange membrane (CEM) to the cathode where, together with the electrons from the cathode, it is converted to hydrogen. As the anodic reduction potential is higher than the cathodic one, a small voltage must be applied to drive the reaction. The power supply possesses a certain resistance (R EXT) which contributes to energy loss
3.3.1 Electrochemistry
A small input of electrical energy is required to accomplish the endothermic conversion of acetate under anaerobic conditions (3). The upper limit of the electromotive force (E EMF) of the MEC is set by the half reactions at the electrodes:
In theory, 0.14 V is adequate for H2 production through biocatalyzed electrolysis of acetate. This is according to the equilibrium potentials for the two half reactions, i.e., oxidation of acetate (1 mol L−1) and proton reduction. Thus, the supplied electricity enables the conversion of, for instance, acetate at the anode:
Together with the electrons of the external power source, the protons are converted to hydrogen at the cathode:
In practice, a higher voltage is required because of (1) the electrons being partly consumed by the bacteria for their growth and maintenance requirements, (2) the ohmic resistance of the electrochemical systems, and (3) the overpotentials of the electrodes. Hydrogen production is usually observed at the cathode at a voltage of >−0.2 V, corresponding with an applied voltage of at least 0.22 V instead of 0.14 V.
Ohmic voltage losses in MECs are determined by (1) the resistance to electron flow through electrical conductors (electrodes and external circuitry), (2) resistance to ion flow through ionic conductors (electrolyte and membrane), and (3) the reactor size and spatial configuration [57, 58]. Reducing electrode spacing, increasing electrolyte conductivity, and choice of the electrode material with low resistivity are therefore pivotal. Even more important, removing the membrane and turning the MEC into a single chamber has a most profound effect on the ohmic loss [18].
Electrode overpotentials in MECs are related to (1) activation losses, (2) coulombic losses, and (3) concentration losses [57]. To overcome activation energy of a redox reaction, additional energy is required. These activation losses are inherent to the mode of transfer of electrons to or from a substance reacting at the electrode surface (see below). To minimize activation losses the catalyst reaction kinetics should be improved together with increasing the operating temperature and increasing surface areas [57–59]. Concentration losses are related to ionic transport between anode and cathode, and when considering protons, the pH is of utmost importance. Most processes use bacteria that ferment optimally at neutral pH, but at the anode where proton formation is high the pH might drop several units. Likewise, at the cathode, conversion of the protons to H2 increases the pH to 11 [60]. At both electrodes, therefore, steep pH gradients may exist, which contribute to overpotentials. This can be prevented by enforcing the buffering capacity of the feedstock [61].
3.3.2 Bacteria Involved
The bacteria that transfer electrons to the anode are called electrigens and their mode of transfer is either direct or indirect. Direct electron transfer is accomplished by cells attached to the anode via (1) outer membrane c-type cytochromes and (2) nanowires. The dominating bacterial species attached to the anode surface are Gram-negatives belonging to the phylum Proteobacteria [62], possessing c-type cytochromes in the outer cell membrane. The connection of this cytochrome with external electron transfer was proved by omcS − mutants (deletion of one of the genes coding for outer membrane cytochromes) showing reduced current production [63]. Pili type IV are relatively big protein filaments (4–5 nm diameter and 20 μm long) and have been recognized as a means of cell-to-cell communication. These pili effectively function in biofilms as distributors and dissipaters of electrons, hence the name ‘nanowires.’ These nanowires enable electrons to be transferred from biofilms as thick as 75 μm to the anode [64].
Indirect electron transfer uses soluble exogenous mediators or electron shuttles that get reduced by the electrigen and diffuses to the anode where reoxidation takes place (Fig. 4). These shuttles are organic compounds which are either produced by the electrigens (e.g., riboflavins [65]) or can in principle be added to the anode culture (e.g., humic acids [66]). However, because of delaying diffusion processes, these shuttles introduce unnecessary energy losses to the system, and thus are not a preferred option for cost-effective MECs [67].
3.3.3 Calculating Efficiencies of the System
The conversion of acetate to H2 is mediated by a voltage over two electrodes, according to (not considering the CO2 formation)
so that two new efficiencies are introduced: (1) coulombic efficiency and (2) cathodic efficiency. The coulombic efficiency can be influenced by, e.g., the presence of microorganisms which can consume the produced hydrogen, such as methanogens. This is often the case when working with undefined microbial consortia and applying too low a voltage to the system [18]. In addition, coulombic losses are also produced by bacteria using part of the electrons for growth and maintenance requirements. Therefore, a balance should be found between bacterial growth and electrode potential for optimal performance of the system.
The hydrogen productivity is proportional to the volumetric current density (I v) and the cathodic hydrogen recovery (r CAT):
and the hydrogen yield is proportional to the current (I):
Because V = R · I, it is obvious that the system becomes more efficient the lower the overall resistance.
The overall energy recovery of the system (η TOT) is estimated as a ratio of the energy content of hydrogen produced (W H2) and the energy added to the system, i.e., energy of the converted substrate (W S) and the energy of the power source (W P):
3.3.4 Factors Affecting Efficiency
Optimal performance of an MEC depends on a combination of parameters: (1) applied voltage, (2) electrode quality and surface area, (3) solution conductivity, (4) microbes, (5) substrate, and (6) cation exchange membrane (CEM). Each of these parameters are briefly discussed below.
-
1.
The minimum voltage necessary is 0.14 V as it is the difference between the two half reactions (9) and (10). However, a voltage of at least 0.22 V is necessary in practice to overcome resistance in the system, but higher voltages up to 0.7 V has been seen to increase the Q H2 [18]. All in all, the potential remains significantly below the value for electrolyzing water (1.6 V). Moreover, it should be noted that too high applied voltages can irreversibly damage biofilms, resulting in declining efficiency of the system [18].
-
2.
Electrodes should have several qualities, including possessing high conductivity and high specific surface area, and should be non-corrosive, non-fouling, inexpensive, easy to manufacture, and scalable [59]. Both carbon and graphite electrodes meet the majority of these requirements, and are especially used as the material for the anode. To increase the surface area, brush-type electrodes are now the regular choice [18]. Graphite-based anodes require heat treatment prior to use as it leads to faster start up. For cathodes usually platinum electrodes are used in lab-scale experiments, but that would make the MEC technology too expensive for scaling up. Fortunately, microbial bio-cathodes have been developed that successfully catalyzed hydrogen production [68]. However, these cathodes have low cathodic hydrogen recovery yields (21%) and maximum hydrogen productivities are in the order of 0.067 mmol H2 L−1 h−1.
-
3.
Increase of the solution conductivity improves hydrogen production, but only to a certain limit as high values are detrimental to microbial activity [69]. Call and Logan [18] showed that increasing the solution conductivity increased the hydrogen production rates but decreased the total energy recovery. Clearly, an optimum should be determined here for each system at hand.
-
4.
The majority of MEC studies mention the use of undefined microbial consortia, usually originating from sediments or wastewater treatment, leaving it up to a selection process as to which bacteria attach to the anode [55]. Thus far, pure culture studies were mainly performed with Geobacter sulfurreducens [56]. Interestingly, both options resulted in similar H2 production rates and recovery yields. So far, no studies have been carried out to select better microorganisms. Most researchers remain with undefined consortia because of several advantages: (1) it improves system robustness, (2) no need to apply aseptic techniques against contaminations, and (3) greater potential for digesting a broader palette of organic compounds. However, care should be taken to avoid methanogen activity as it may remain persistent in the system once it has established itself [70]. To minimize methanogenic activity, several strategies can be followed, such as exposing the reactor to air between feeding cycles or applying polarity reversal at higher applied voltages for a short time [18, 70, 71].
-
5.
Several MEC systems fed with different organics from sugars to fatty acids have been studied and a selection of the results is discussed in a review [56]. From these studies it became clear that acetate is the preferred substrate, as demonstrated by H2 recovery yields >91% [72] compared to recovery yields of 10–28% with wastewater as substrate [73]. Note that for a high productivity the chamber should be well mixed for an optimal substrate flux to the biofilm, reducing diffusional gradients. This might add to energy-demanding mixing devices.
-
6.
The membrane is traditionally applied coming from electrolysis of water, where the production of H2 and O2 should be kept separated. However, experience with H2 production in MECs revealed leakage of H2 at the anode [55], indicating the fallible nature of the membrane. Furthermore, membranes hinder proton diffusion to the cathode, adding resistance to the system. Finally, membranes create a pH gradient across the membrane leading to substantial potential loss [74]. Therefore, removing the membrane altogether might improve operation. Indeed, the first studies with a single chamber MEC revealed a more than doubling of the hydrogen production rates at applied voltages of 0.3–0.8 V (Table 1, [18]), obtaining similar or higher hydrogen recoveries and higher energy recoveries. In addition, placing the electrodes close to each other meant that pH gradients were non-existent, adding to a lower ohmic loss. Thus, simplifying the design of MECs by removing the membrane is a way forward to cost-effective H2 recovery. However, because H2 is mixed with CO2, a gas upgrading step is required.
Finally, performance optimization of MECs needs to take place. The challenge is to fine-tune the system pertaining to the type of substrate and microbial consortia applied.
Thus the overall actual energy requirement of the system (E MEC) can be estimated [57]:
with η A the sum of contributions to the overpotential of the anode, η C the sum of contributions to the overpotential of the cathode, and IR Ω the ohmic loss (R Ω all the resistances in the system).
3.3.5 Conclusions and Challenges
Hydrogen production with MECs has undergone fast development in the last decade and a myriad of studies have demonstrated its potential to become an efficient and reliable technology. The knowledge obtained of MEC technology, including the microbiology and reactor configuration, can soon lead to real applications. Yet two major challenges are to be met in the near future before scaling up, i.e., low-cost cathode material and directions how to increase the Q H2. The essential challenge is to find a solution for the expensive platinum cathode. Fortunately this expensive metal can be replaced by low-cost stainless steel and nickel alloys without loss in performance [75]. New electrodes have become available, consisting of combinations of materials (metals and carbon), although their manufacturing might be too costly for now [76]. The second major challenge, increasing Q H2, can be met by optimizing MECs for high current densities with low overpotentials and low ohmic losses. This can be partly achieved by selecting improved anodic biofilms to enhance microbe–electrode interaction related to electron transfer [77].
Tests with the first MEC pilot scale (1 m3) has revealed a longer initiation time to establish biofilms on the anode (~60 days) and maximum gas production was in the order of 0.32 mmol L−1 h−1, although most of this was methane [78]. Even though this first reactor consisted of up-to-date technology (containing 24 modules, immersing brush anodes, and stainless steel cathodes), it again underlines the pivotal role of troubleshooting. It has been revealed that at a larger scale the operation conditions are crucial, especially at start up to initiate proper development of the microbial population.
Finally, both a thorough LCA and techno-economical evaluation is urgently required to determine the best options of this technology and how to implement it efficiently into other systems.
3.4 Dark Fermentation
About 73% of the research on dark fermentation (DF) has been carried out with mesophilic bacteria [79], whereas thermophilic DF has been researched to a lesser extent. Yet both share common process parameters that similarly affect the fermentation, such as partial hydrogen pressure (P H2 ), pH, substrate concentration, and composition of the feedstock.
The P H2 is a key parameter as high hydrogen concentrations limit its own production because of a thermodynamic constraint (for the thermodynamics of hydrogen formation the reader is referred to reviews [80, 81]. If hydrogen is not removed effectively from the broth it easily accumulates at up to 12–70 times the equilibrium concentration because of liquid-to-gas mass transfer limitations [82]. As a consequence, the intracellular NADH/NAD+ ratios rise, which shifts metabolism toward other reduced end-products rather than H2 (Fig. 5) [24].
Principle of hydrogen metabolism of dark fermentation. A variety of fermentative pathways exist because of facultative (a) and strict anaerobic (b) hydrogen producers. The common route for hexose metabolism is the Embden–Meyerhof pathway (EMP), although several hyperthermophilic archaea and bacteria employ both EMP and the Entner–Doudoroff pathway. (a) Optimal H2 production in facultative anaerobes is via a hydrogenase (H2ase) reoxidizing NADH combined with formate hydrogen lyase (FHL) which includes an [NiFe]-hydrogenase, thus producing CO2 and acetate as by-products. (b) Strict anaerobic mesophiles have several catabolic pathways in common with the thermophiles (black arrows). Both possess pyruvate ferredoxin:oxidoreductase (PFOR) to catalyze the oxidation of pyruvate to acetyl-CoA (AcCoA), thereby delivering the electrons to ferredoxin. H2 is produced via NADH and reduced ferredoxins (Fdred) donating electrons to [FeFe]- and/or [NiFe]-hydrogenases. Thermotoga maritima contains a bifurcating hydrogenase using NADH and Fdred simultaneously [88] (given in red). Strict anaerobic mesophilic hydrogen producers also possess other pathways, leading to less efficient hydrogen production (purple arrows). Among the thermophiles, Thermotoga species and Pyrococcus furiosus can also form and excrete alanine (given in green). Caloramator celer possesses pyruvate formate lyase (PFL) besides PFOR producing formate instead of H2 and CO2 [89] (given in blue). Depending on the microorganism, the alternative pathways to reoxidize NADH occur during conditions that create redox imbalances in the cell (leading to products such as succinate, lactate, ethanol, butanol, butyrate, acetone, alanine, and/or formate). Abbreviations: PEP phosphoenolpyruvate, Pyr pyruvate, Ac-CoA acetyl-CoA, EtOH ethanol
At the industrial scale, removal of H2 using an inert gas (N2) or CO2 is not an option as it dilutes the H2 gas, which drives up gas upgrading costs. In addition, CO2 leads to acidification in the culture because of bicarbonate formation. As a consequence, more caustic agent is required to correct the pH. This unnecessarily increases the osmotic potential, thereby limiting hydrogen production [83]. Even though DF has been observed over a wide pH range [84], a slightly acidic pH (6–7) appears to be optimal for thermophilic H2 production [85].
Increasing the substrate concentration is important for a cost-effective process, as relatively less water is required and it contributes to higher Q H2. However, instead it has been observed that higher sugar concentrations led to decreases in Q H2 which can be because of the limitation of other nutrients, such as iron [86], or critical osmotic potentials [87].
3.4.1 Mesophilic Fermentation
The biggest advantage of mesophilic DF is the capacity to reach very high volumetric hydrogen productivities (100–600 mmol H2 L−1 h−1). Unfortunately, they are accompanied by relatively low Y H2 (<2.5 mol H2 mol glucose−1) (Table 1) [16] because of production of various other reduced byproducts (Fig. 5). For practical reasons, studies on mesophilic DF usually use undefined cultures as inocula originate from wastewater treatment, compost, or soil [79] ( for a list of results see [21]). All these ecosystems contain both facultative and strict anaerobic hydrogen producers, often belonging to enterobacteriaceae and clostridia [84]. Mesophilic dark fermentations are relatively cheap and simple to operate with low or no contamination control, are very robust, and can take broad sources of feedstock [90]. However, undefined inocula contain undesirable metabolic types such as methanogens. Therefore, a pretreatment of these inocula, such as acid shock or heat treatment, is carried out to minimize methanogenic activity. Reducing the HRT is an even better strategy to trim microbial diversity in the culture and clearly has been shown to increase the hydrogen yield [91]. Moreover, besides methanogens, with their low specific growth rates (0.017–0.02 h−1), propionic acid bacteria are also removed, whereas hydrogenic bacteria remain [92, 93].
In the fermentation of sugars there are many by-products formed as NADH reoxidation is easily diverted from hydrogenase to other pathways (Fig. 5). To maximize NADH oxidation via the hydrogenase, the most practical solution is to remove hydrogen effectively from the culture broth. In the case of using pure cultures, knockouts of genes of competing pathways through metabolic engineering is an interesting option, which has indeed been shown to increase hydrogen production [94, 95].
3.4.2 Thermophilic Fermentation
Among thermophilic hydrogen production, three subclasses can be distinguished: moderate thermophiles (50 °C < T opt < 64 °C), extreme thermophiles (65 °C < T opt < 79 °C), and hyperthermophiles (T opt > 80 °C). The interest in thermophilic hydrogen production has increased in the last decade as there are several advantages attached to this process compared to mesophilic fermentation. Many thermophiles have been described to consume a wide range of sugars, including hexoses, pentoses, oligosaccharides, and polysaccharides such as cellulose and pectin (for extensive lists see [81, 96, 97]). According to a techno-economic evaluation, additional heat demand required for thermophilic DF did not incur significantly higher costs compared to mesophilic DF [98]. Instead, the production cost of DF is largely influenced by (1) the cost of media ingredients and (2) low substrate concentrations [98]. Yeast extract and phosphates are the most expensive components in the medium for which cheaper substitutes should be tested such as manure and urine. Alternatively, there are hydrogen producers that do not require a rich medium, as they can synthesize all amino acids and nearly all B vitamins [99]. A solution to using higher sugar concentrations is to apply osmotolerant strains, obtained through genetic engineering or evolutionary adaptation, which can stand higher substrate and product concentrations [100].
For an optimal DF process, an ideal hydrogen producer would be needed possessing superior properties, including (1) generating hydrogen at high Q H2 and Y H2, (2) the ability to degrade a wide variety of biomass, (3) tolerating high sugar concentrations and fermentation products, (4) resisting inhibitors in the feedstock, (5) minimum requirement for a non-complex medium, (6) tolerating oxygen, and (7) easy to engineer genetically. However, so far none of the investigated organisms completely fulfil all these criteria, but thermophilic species of Clostridium, Caldicellulosiruptor, and the order of Thermotogales come very close, and, not surprisingly, are the most studied [81, 85, 101–104] in addition to Cl. thermocellum [105]. Interestingly, the extreme thermophilic Caldicellulosiruptor species can degrade celluloses at the highest temperature so far found [106]. Working at higher temperatures lowers the chance of contamination, enabling one to work with pure cultures, and eliminates cooling of pretreated biomass. Another advantage of thermophilic hydrogen producers is the formation of less different by-products (Fig. 5). Thus, often only acetate is produced together with hydrogen near maximum theoretical yields (4 mol H2 mol hexose−1) under ideal growth conditions. Only under high P H2 or high osmolalities are reduced by-products such as lactate and ethanol produced [107]. Lactate formation is accompanied by less optimal growth and H2 production and might not only be regulated by the redox ratio (NADH/NAD+) but also by the energy status of the cell [108]. As several hydrogenic thermophiles are often isolated from terrestrial hot water springs with lignocellulosics as their primary substrates, they have adapted to low sugar concentrations and low osmolalities in general. Instead, they express a vast array of glycoside hydrolases to grow on (oligo)saccharides released during the rate-limiting breakdown of (hemi)celluloses. For that they adopt one of two strategies: either via secretion of exo hydrolases (e.g., Caldicellulosiruptor and Thermotoga spp.) or via a cellulosome attached to the outer cell surface (Cl. thermocellum) [109, 110].
3.4.3 DF Bioreactors
At lab scale the suspension culture in CSTR with sparging N2 is the preferred system for fundamental research on hydrogen metabolism and factors influencing the Y H2 and Q H2. However, this system has an upper limit for Q H2 of approximately 20 mmol H2 L−1 h−1, but can be increased threefold by increasing the cell density through immobilization [111]. Still, for an economically feasible process the rate should be an order of magnitude higher. The strategy foreseen to improve production yet keeping the operation costs low is based on increasing cell retention, using recirculation fluxes instead of sparging gas, and stirring to improve liquid-to-gas mass transfer rates. For that purpose, other bioreactor systems have been tested such as the up-flow anaerobic reactor (UA) [17], trickling filter [112], packed bed reactor [113], anaerobic sequencing blanket reactor [114], and membrane reactor [115]. These reactor types are further discussed in more detail elsewhere [116]. The most promising results obtained so far were obtained with the thermophile, Thermoanaerobacterium thermosaccharolyticum strain, forming biofilms on granular sludge in a UA reactor [17] (Table 1). However, granular sludge, originating from wastewater treatment, might give rise to contamination from hydrogenotrophic methanogens. This can be avoided by using other appropriate carrier material such as porous glass beads, as recently reported [117].
For optimizing the fermentation process it is important to avoid nutrient limitations, and consequently a feedstock needs to be supplied well-balanced in its elemental composition. Nearly all lignocellulosic-based feedstocks are low in vital elements such as nitrogen, phosphorus, sulfur, and trace elements. Therefore, nutrients need to be added for allowing unlimited growth of the hydrogen producers. In lab-scale experiments yeast extract is often used, but this rich nutrient source is too costly for industrial application [98]. Cheap alternatives, such as manure, urine, and whey, need to be tested in combination with the carbon-rich feedstock.
3.4.4 Conclusions and Challenges
There are two choices of hydrogenic bacteria, either mesophiles or thermophiles. The practical advantage of mesophilic facultative anaerobes is a less stringent application of anaerobic conditions, making the process less expensive than thermophilic DF. Furthermore, if the feedstock is of low-grade organic waste, then it may be better to opt for high Q H2. However, thermophilic DFs operating at ≥70 °C provide ‘pasteurization conditions,’ and thus are less inherent to contaminations (e.g., methanogens) and produce an effluent containing a smaller palette of by-products which complements most appropriately with the second process (either photofermentation or electrohydrogenesis). In addition, thermophilic DF leads to higher yields, which offers a wider choice of feedstocks from cheap waste to more expensive energy crops.
The biggest challenge for thermophilic DF is to increase the Q H2 by an order of magnitude to make it into a cost-effective process. The best way to tackle this might be a combination of several strategies: (1) (artificially) increasing cell densities (biofilm), (2) elevating osmotolerance (evolutionary adaptation), (3) designed co-cultures, and (4) applying an appropriate bioreactor configuration. These reactors should possess a proper manner of H2 removal, thus excluding sparging gases to avoid expensive gas upgrading equipment. High cell densities of osmotolerant strains provide the solution for using high feed concentrations to reduce costs from water consumption and reactor material. Interestingly, applying designed co-cultures of two or more species, instead of pure cultures or undefined consortia, has been shown to create synergies based on complementary substrate utilization [118, 119], O2 scavenging [120], extending optimal process conditions [121], kinship relation [122], and biofilm formation [123]. Finally, the ability to degrade lignocellulosic biomass either untreated, as recently shown for Caldicellulosiruptor species [124, 125], or in defined co-cultures [116], opens up new possibilities to explore whether consolidated bioprocess can be an economical viable replacement for the current proposal of a two-step pretreatment-DF.
Genetic engineering can be of interest to improve hydrogen producers through (1) eliminating pathways leading to undesirable by-product formation (such as lactate (e.g., [126]), (2) implementing new synthetic pathways to raise the Y H2 beyond the theoretical limits [127], and (3) constructing cells that are more robust against inhibitors and stresses (osmolality, inhibitors in hydrolysates) [128]. Most metabolic engineering has been carried out with mesophilic enterobacteria as they are relatively easy to manipulate genetically, but various challenges exist for strict anaerobic mesophilic and thermophilic hydrogen producers, including handling under strict-anaerobic conditions, finding appropriate shuttle vectors and selection markers, and the presence of restriction modification systems preventing uptake of foreign DNA [85, 129]. Through trial and error, a few successes have been accomplished only recently.
4 Integrated Processes
The several possible combinations of BHP processes (Fig. 1b) are discussed below. With integration of these processes, new challenges are added on top of those of each single BHP process. Mostly they are related to the composition of the effluent of the dark fermentation (DFE) not being optimal for the second BHP. In general, all the obstacles related to tuning the two fermentation steps have to be dealt with before any integration can be realized. Few studies had looked beyond mere integration of two fermentation steps. One of the most intensive investigations, including mass, energy and exergy balances, and LCA, has been carried out for the DF-PF integration by the EU-funded project “Hyvolution” [130–133]. The outcome of the process simulations of this project may be similar for other integrated BHP processes. Of course, as a consequence of the simulations being based on experimental data that were available at that time, some conclusions possess limited validity. Nevertheless, it can be concluded that heat integration of effluent recirculation saves on the total required energy input and water demand (can be up to 90%) [132]. The latter is of particular importance when dealing with low substrate concentrations, and it provides a significant reduction in the environment impact of the process [133]. However, recirculation of fermentation effluents have the inherent problem of increasing osmolality. This is mainly because of the continuous correction of the pH with caustic agents (usually sodium or potassium hydroxide) in both fermentation processes. To prevent this, one should investigate the possibility of using other cheaper alternatives such as ammonia which can also be used as a nitrogen source. The outcome of the LCA study revealed that production of each process ingredient (phosphate, caustic agent, etc.) has nearly100% environmental impact, which is in great contrast with the impact of the DF-PF process itself which had a value tenfold lower than that of alternative hydrogen production processes, i.e., reformation of natural gas or the water gas shift reaction [133].
4.1 Integrated DF and PF
Recently an intensification of projects has taken place looking into the possibility of integration of mesophilic or thermophilic dark fermentation and photofermentation [21, 37, 134]. Demonstration of an integrated DF-PF system is currently lacking. Instead, researchers investigated the effect of the DFE on the photofermentation process. The DF was run on either artificial media (glucose or sucrose as substrate) or pretreated biomass (wastewaters, potato starch, algal biomass, beet molasses), which have been reviewed in detail [4]. Use of artificial media and light is a means to determine the potential of the integrated system and how to tune the composition of the medium, considering each fermentation process step. One of the best results was obtained using a PBR with clay carriers and in situ optical fibers in addition to external light sources [135]. With this mesophilic-DF-PF system, maximum Y H2 values of 7.1 mol H2 mol hexose−1 were obtained with a Q H2 of 1.2 mmol H2 L−1 h−1 and nearly 90% carbon conversion. This study showed that elaborate light distribution significantly contributed to overall Y H2 and carbon conversion.
Using realistic feedstocks revealed new bottlenecks such as background color, particles, concentration levels of inhibitors and substrates, and buffering capacity. This may include redesigning the medium composition for the DF to be tuned with the criteria for PF. As an example, the ammonium concentration needs to be within a specific range in the initial feedstock, i.e., the minimum depends on the growth requirements in the DF and the maximum on the threshold value in the DFE that influences nitrogenase expression levels in the PNBS. Hence, it is required to know how much ammonium is consumed in the DF and the ammonium threshold value for the strain(s) used in the PF (average around 2 mM [36]). However, concentration variations are inherent to fermentation processes, and thus for the sake of process robustness it would be safer to remove the ammonium from the DFE by, e.g., electroseparation [136] or pretreatment with clinoptilolite (natural zeolite) [137] even though this brings in additional costs. Alternatively, ammonium concentration does not create any problems by applying ammonia-tolerant PNSB strains [47] which would be the most sophisticated solution.
For large-scale production, mild sterilization of the DFE might be necessary before it is added to the PF [37]. However, this is not required when the DF is thermophilic; although mild sterilization should be necessary for any additional components to the DFE, such as trace elements iron and molybdenum.
4.2 Integrated DF and MEC
Integration of the MEC with DF is an interesting strategy because (1) the MEC functions optimally with compounds that are typically byproducts of the DF, especially acetate [72], (2) both processes are near scaling up, and (3) both possess high Y H2, at least when considering thermophilic DF, and thus complete conversion of sugars can be expected with this combination.
So far, only a few studies have fed DFE to an MEC [138], of which the best performance was seen with a hydrogen-ethanol fermentation reaching 83% conversion to hydrogen and 70% energy recovery, but with a Q H2 of approx. 2.3 mmol H2 L−1 h−1 [139]. An interesting approach was reported by Wang et al. [140] through implementing an MFC in the DF-MEC process that was fed with DFE to produce electricity for driving the MEC. In this way, no external energy source was necessary for the MEC and thus can be a starting point to increase further the energy efficiency of the DF-MEC process.
Before any scaling up is possible, the MEC needs to be improved in performance – as discussed above – plus optimization of the buffer capacity of the feedstock [139].
4.3 Integrated DF and AD
In this case, two different gases are produced and either used separately or mixed, as the latter, called hythane, forms a cleaner fuel (lower production of CO and greenhouse gases) than methane alone when used in combustion engines. In the last decade, the number of studies on the DF-AD process have developed close to a mature state which is ready for scaling up [141]. The process is quite promising as high total energy yields can be reached combined with nearly zero waste. To obtain high total product yields, the best option is to combine thermophilic DF with mesophilic or thermophilic AD. Just to illustrate this fact, several studies are compared with respect to the obtained product yields and productivities (Table 1). It is clear that the thermophilic processes have higher yields for both hydrogen and methane. Various organic acids are produced in the mesophilic DF (e.g., [142]), which require a more complex consortium composition for the methanogenic reactor. Working with pure cultures or designed co-cultures of thermophiles in the DF results mainly in acetate and low quantities of lactate in the DFE [143], which narrows the consortium composition of the AD to mainly acetoclastic methanogens. The study by Kongjan et al. [144], using an undefined consortium, but enriched in hydrogenic thermophiles, lies somewhere in the middle of these two extremes as it produced low quantities of butyrate and propionate.
It can be concluded from these studies that superior performance of the DF-AD process is related to a DF process that produces a DFE containing mainly acetate which simplifies, and thus improves yields of the AD [146]. In addition, the DF-AD process is superior over the single-stage AD process because of higher waste treatment efficiencies [147, 148]. In addition, according to [149] the DF-AD process adds only little production costs to the AD process, although at least 10% more energy is gained. However, the productivities of both the DF and AD remain quite low in the studies (see, e.g., Table 2). One way is to use higher substrate concentrations, but then the microorganisms in both the DF and AD need to be adapted to higher osmolalities, for instance by evolutionary adaptation.
Other challenges are related to adjusting the DFE to the AD. Most important would be the pH, as the DF runs at slightly lower pH (5–6.5) than the AD (pH 7–8), and micronutrients need to be added [143]. Ca2+ is preferred over Na+ for correcting the pH as acetoclastic methanogens are relatively sensitive to the latter [150].
5 Conclusions
Research on BHP processes is a very active area as is reflected in a decade of impressive progress in understanding and genetically improving the metabolism and improving technical cultivation of hydrogen producers. Genomics, genome-wide metabolic models, and molecular technologies have recently matured and are now also entering the field of BHP (e.g., [151, 152]). This is a welcoming asset as BHP has a lot of biological challenges still needing to be tackled and systems biology brings a new approach for finding solutions. On the one hand, undefined consortia, mostly enrichment cultures, can be applied which are related to high Q H2 but low Y H2. Its advantage is that no or little investment has to be made in the control of contamination. On the other hand, there is the possibility of using pure cultures, for which the challenge is to find ways to improve both Q H2 and Y H2. This can be done by genetic engineering and/or evolutionary adaptation. The disadvantage is the high control on contamination prevention. Another strategy lies somewhere in the middle of these two extremes, i.e., by exploiting synergies between two or more species in optimized designed co-cultures, for which genetic engineering might not be required. Which of these three options are to be applied might depend on the costs of the feedstock and should be determined by a careful techno-economical evaluation.
There are also plenty of technological challenges to face before any cost-effective process is possible. The majority of the research has been carried out at lab scale, and several technologies have moved on to – or are on the brink of – scaling up. An important shift has taken place from artificial media to more realistic feedstocks. Likewise, research with integrated BHP systems is increasing as more researchers recognize it as the most suitable way for future biohydrogen production. Important here is the development of operation control of the two processes with all the recirculation flows, heat integration, and gas upgrading included.
The intention of these integrated BHP processes is to convert the biomass-carbon of the feedstocks to CO2, a waste gas which can be applied as aerial fertilizer for greenhouse agriculture or algae ponds, or used in industrial processes based on critical carbon dioxide.
Most attention has been paid to integrative DF-PF processes, revealing there are still many challenges to meet for overall optimization. Further, a major drawback of photofermentation is its dependency on the diffuse nature of solar radiation, dilute streams of organic matter, and limited conversion efficiencies. Consequently, in the current state it requires a huge surface area and material investments [153]. Breakthroughs are needed in smart light distribution if it is to meet a viable industrial BHP process. Instead, the integration of thermophilic DF with MEC might be a better option for the near future, especially as it is concluded here that these two processes are tailor-made for each other.
For the near future, it can be foreseen that more pilot-scale plants of the dark fermentation process, the one most closely resembling a conventional fermentation process, should be operational. An earliest commercial production of such a process would fit best via coupling with existing anaerobic digestion plants for zero-waste production. This leads to a win-win situation as it creates an opportunity to build up essential experience with larger-scale biohydrogen production and to improve the anaerobic digestion process. In addition, decentralized small-scale hydrogen production creates new opportunities, such as jobs at the rural level and new ways of investment for plants and equipment [154].
The complexity of this area lies partly in that each BHP has advantages and disadvantages. One major obstacle related to that is the inverse relationship between Y H2 and Q H2. Thus, improving on yield often directly affects productivity and vice versa. Tackling these challenges requires the work to be done by multidisciplinary teams. Furthermore, in a practical way it depends on the goal of producing H2 and whether to opt for fast or for efficient production. The former process can be carried out in a simpler setup, whereas the latter requires more efficient control. Selection of the appropriate process is further related to the cost of the feedstock and whether the BHP process becomes part of a biorefinery process. In that respect, one of the most fundamental conclusions coming out of all the work is that the BHP process needs to be tailor-made to the specific waste [37].
Scale up and optimized reactor configurations are the next major step necessary to arrive at viable BHP processes. In addition, these activities should be accompanied with rigorous LCA and techno-economical evaluations to enable direct feedback for finding sustainable solutions. High integration, including heat integration and water recirculation, can indeed pay off to make the process more cost- and energy-efficient. However, for the consequential osmolality increase in the system, sustainable solutions need to be found. Preliminary LCA has revealed that integrated processes themselves are highly sustainable, but their high environmental impact is connected to the additional nutrients from non-sustainable origin. Therefore, for lowering the impact of BHP processes, part of the focus should be on finding (cheap) renewable sources for all ingredients required for optimal operation of the fermentations.
Abbreviations
- AcCoA:
-
Acetyl-Coenzyme A
- AD:
-
Anaerobic digestion
- BES:
-
Bio-electrochemical systems
- BHP:
-
Biological hydrogen process
- CBC:
-
Calvin–Benson cycle
- CEF:
-
Cyclic electron flow
- CEM:
-
Cation exchange membrane
- CSTR:
-
Continuous stirred tank reactor
- DF:
-
Dark fermentation
- DFE:
-
Dark fermentation effluent
- DOT:
-
Dissolved oxygen tension
- DW:
-
Dry weight
- E EMF :
-
Electromotive force (V)
- E MEC :
-
Overall actual energy requirement of the system (V)
- EMP:
-
Embden–Meyerhof pathway
- EOC:
-
Excreted organic compounds
- Fd:
-
Ferredoxin
- FHL:
-
Formate hydrogen lyase
- FNR:
-
Ferredoxin:NAD(P)+ oxidoreductase
- H2ase:
-
Hydrogenase
- HE:
-
Hydroelectrogenesis
- HRT:
-
Hydraulic retention time (h)
- I :
-
Current (A)
- I V :
-
Volumetric current density (A m−2)
- LCA:
-
Life cycle assessment
- MEC:
-
Microbial electrolysis cell
- MFC:
-
Microbial fuel cell
- PBR:
-
Photobioreactor
- PF:
-
Photofermentation
- PFL:
-
Pyruvate formate lyase
- PFOR:
-
Pyruvate ferredoxin:oxidoreductase
- P H2 :
-
Partial hydrogen pressure (Pa)
- PHB:
-
Polyhydroxybutyrate
- PS I:
-
Photosystem I
- PS II:
-
Photosystem II
- Q H2 :
-
Volumetric hydrogen productivity (mol H2 L−1 h−1)
- r CAT :
-
Cathodic hydrogen recovery
- RubisCO:
-
Ribulose 1,5-biphosphate carboxylase/oxygenase
- R Ω :
-
All the resistances in the system (Ω)
- UA:
-
Up-flow anaerobic reactor
- W H2 :
-
Energy content of hydrogen produced (J)
- W P :
-
Energy of the power source (J)
- W S :
-
Energy of the converted substrate (J)
- Y H2 :
-
Hydrogen yield (mol H2/mol substrate)
- η A :
-
Sum of contributions to the overpotential of the anode (V)
- η C :
-
Sum of contributions to the overpotential of the cathode (V)
- η TOT :
-
Overall energy recovery of the system
References
Navigant Research (2012) http://www.navigantresearch.com/newsroom/global-biogas-market-to-nearly-double-in-size-to-33-billion-by-2022
MarketsandMarkets (2014) http://www.marketsandmarkets.com/PressReleases/hydrogen.asp
Mansilla C, Avril S, Imbach J, Le Duigou A (2012) CO2-free hydrogen as a substitute to fossil fuels: what are the targets? Prospective assessment of the hydrogen market attractiveness. Int J Hydrog Energy 37:9451–9458
Adessi A, de Philippis R, Hallenbeck PC (2012) Combined systems for maximum substrate conversion. In: Hallenbeck PC (ed) Microbial technologies in advanced biofuel production. Springer, New York, pp 107–126
Guwy AJ, Dinsdale RM, Kim JR, Massanet-Nicolau J, Premier G (2011) Fermentative biohydrogen production systems integration. Bioresour Technol 102:8534–8542
Liu Z, Zhang C, Lu Y, Wu X, Wang L, Wang L, Han B, Xing X-H (2013) States and challenges for high-value biohythane production from waste biomass by dark fermentation technology. Bioresour Technol 135:293–303
Bundhoo MAZ, Mohee R, Hassan MA (2015) Effects of pre-treatment technologies on dark fermentative biohydrogen production: a review. J Environ Manag 157:20–48
Sen U, Shakdwipee M, Banerjee R (2008) Status of biological hydrogen production. J Sci Ind Res 67:980–993
Escapa A, Gómez X, Tartakovsky B, Morán A (2012) Estimating microbial electrolysis cell (MEC) investment costs in wastewater treatment plants: case study. Int J Hydrog Energy 37:18641–18653
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Show K-Y, Lee D-J, Chang J-S (2011) Bioreactor and process design for biohydrogen production. Bioresour Technol 102:8524–8533
Laurinavichene TV, Fedorov AS, Ghirardi ML, Seibert M, Tsyganko AA (2006) Demonstration of sustained hydrogen production by immobilized, sulphur-deprived Chlamydomonas reinhardtii cells. Int J Hydrog Energy 5:659–667
Winkler M, Hemschemeier A, Gotor C, Melis A, Happe T (2002) [Fe]-hydrogenases in green algae: photo-fermentation and hydrogen evolution under sulphur deprivation. Int J Hydrog Energy 27:1431–1439
Sveshnikov DA, Sveshnikova NV, Rao KK, Hall DO (1997) Hydrogen metabolism of mutant forms of Anabaena variabilis in continuous cultures and under nutritional stress. FEMS Microbiol Lett 147:297–301
Li X, Dai Z-Z, Wang Y-H, Zhang S-L (2011) Enhancement of phototrophic hydrogen production by Rhodobacter sphaeroides ZX-5 using fed-batch operation based on ORP level. Int J Hydrog Energy 36:12794–12802
Wu S-Y, Hung C-H, Lin C-N, Chen H-W, Lee A-S (2006) Fermentative hydrogen production and bacterial community structure in high-rate anaerobic bioreactors containing silicone-immobilized and self-flocculated sludge. Biotechnol Bioeng 93:934–946
O-Thong S, Prasertsan P, Karakashev D, Angelidaki I (2008) High-rate continuous hydrogen production by Thermoanaerobacterium thermosaccharolyticum PSU-2 immobilized on heat-pretreated methanogenic granules. Int J Hydrog Energy 33:6498–6508
Call D, Logan BE (2008) Hydrogen production in a single chamber microbial electrolysis cell lacking a membrane. Environ Sci Technol 42:3401–3406
Adessi A, De Philippis R (2014) Photobioreactor design and illumination systems for H2 production with anoxygenic photosynthetic bacteria: a review. Int J Hydrog Energy 39:3127–3141
Adessi A, De Philippis R (2014) Photosynthesis and hydrogen production in purple non sulphur bacteria: fundamental and applied aspects. In: Zannoni D, de Philippis R (eds) Microbial bioenergy: hydrogen production, vol 38, Advances in photosynthesis and respiration. Springer, Dordrecht/Heidelberg/New York/London, pp 269–290
Azwar MY, Hussain MA, Abdul-Wahab AK (2014) Development of biohydrogen production by photobiological, fermentation and electrochemical processes: a review. Renew Sustain Energy Rev 31:158–173
Fernández-Sevilla JM, Acién-Fernández FG, Molina-Grima E (2014) Photobioreactors design for hydrogen production. In: Zannoni D, de Philippis R (eds) Microbial bioenergy: hydrogen production, vol 38, Advances in photosynthesis and respiration. Springer, Dordrecht/Heidelberg/New York/London, pp 291–320
Sakurai H, Masukawa H, Kitashima M, Inoue K (2013) Photobiological hydrogen production: bioenergetics and challenges for its practical application. J Photochem Photobiol C: Photochem Rev 17:1–25
Hallenbeck PC, Benemann JR (2002) Biological hydrogen production: fundamentals and limiting processes. Int J Hydrog Energy 27:1185–1193
Melis A, Zhang LP, Forestier M, Ghirardi ML, Seibert M (2000) Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol 122:127–135
Hemschemeier A, Fouchard S, Cournac L, Peltier G, Happe T (2008) Hydrogen production by Chlamydomonas reinhardtii: an elaborate interplay of electron sources and sinks. Planta 227:397–407
Tolleter D, Ghysels B, Alric J, Petroutsos D, Tolstygina I, Krawietz D, Happe T, Auroy P, Adriano JM, Beyly A, Cuiné S, Plet J, Reiter IM, Genty B, Cournac L, Hippler M, Peltier G (2011) Control of hydrogen photoproduction by the proton gradient generated by cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 23:2619–2630
Melis A (2007) Photosynthetic H2 metabolism in Chlamydomonas reinhardtii (unicellular green algae). Planta 226:1075–1086
Marin-Navarro J, Esquivel MG, Moreno J (2010) Hydrogen production by Chlamydomonas reinhardtii revisited: rubisco as a biotechnological target. World J Microbiol Biotechnol 26:1785–1793
Kruse O, Rupprecht J, Bader KP, Thomas-Hall S, Schenk PM, Finazzi G, Hankamer B (2005) Improved photobiological H2 production in engineered green algal cells. J Biol Chem 280:34170–34177
Ducat DC, Sachdeva G, Silver PA (2011) Rewiring hydrogenase-dependent redox circuits in cyanobacteria. Proc Natl Acad Sci U S A 108:3941–3946
Kontur WS, Noguera DR, Donohue TJ (2012) Maximizing reductant flow into microbial H2 production. Curr Opin Biotechnol 23:382–389
Akkerman I, Janssen M, Rocha JMS, Reith JH, Wijffels RH (2003) Photobiological hydrogen production: photochemical efficiency and bioreactor design. Dutch Biological Hydrogen Foundation, Petten, pp 124–145
Gianelli L, Torzillo G (2012) Hydrogen production with the microalga Chlamydomonas reinhardtii grown in a compact tubular photobioreactor immersed in a scattering light nanoparticle suspension. Int J Hydrog Energy 37:16951–16961
Prince RC, Kheshgi HS (2005) The photobiological production of hydrogen: potential efficiency and effectiveness as a renewable fuel. Crit Rev Microbiol 31:9–31
Wu S, Li X, Yu J, Wang Q (2012) Increased hydrogen production in co-culture of Chlamydomonas reinhardtii and Bradyrhizobium japonicum. Bioresour Technol 123:184–188
Eroglu I, Özgür E, Eroglu E, Yücel M, Gündüz U (2014) Applications of photofermentative hydrogen production. In: Zannoni D, de Philippis R (eds) Microbial bioenergy: hydrogen production, vol 38, Advances in photosynthesis and respiration. Springer, Dordrecht/Heidelberg/New York/London, pp 237–267
Androga DD, Özgür E, Eroglu I, Gündüz U, Yücel M (2011) Significance of carbon to nitrogen ratio on the long-term stability of continuous photofermentative hydrogen production. Int J Hydrog Energy 36:15583–15594
Koku H, Eroglu I, Gündüz U, Yücel M, Türker L (2002) Aspects of the metabolism of hydrogen production by Rhodobacter sphaeroides. Int J Hydrog Energy 27:1315–1329
Harwood CS (2008) Nitrogenase-catalyzed hydrogen production by purple nonsulfur photosynthetic bacteria. In: Wall J, Harwood CS, Demain A (eds) Bioenergy. ASM Press, Washington, pp 259–271
Yilmaz LS, Kontur WS, Sanders AP, Sohmen U, Donohue TJ, Noguera DR (2010) Electron partitioning during light- and nutrient-powered hydrogen production by Rhodobacter sphaeroides. Bioenergy Res 3:55–66
Boran E, Özgür E, Yücel M, Gündüz U, Eroglu I (2012) Biohydrogen production by Rhodobacter capsulatus in solar tubular photobioreactor on thick juice dark fermenter effluent. J Clean Prod 31:150–157
Eroglu E, Eroglu I, Gündüz U, Yücel M (2008) Effect of clay pretreatment on photofermentative hydrogen production from olive mill wastewater. Bioresour Technol 99:6799–6808
McKinlay JB, Harwood CS (2011) Calvin cycle flux, pathway constraints, and substrate oxidation state together determine the H2 biofuel yield in photoheterotrophic bacteria. mBio 2, e00323-10
Kars G, Gündüz U, Rakhely G, Yücel M, Eroglu I, Kovacs KL (2008) Improved hydrogen production by uptake hydrogenase deficient mutant strain of Rhodobacter sphaeroides O.U.001. Int J Hydrog Energy 33:3056–3060
Avcioglu SG, Özgür E, Eroglu I, Yücel M, Gündüz Y (2011) Biohydrogen production in an outdoor panel photobioreactor on dark fermentation effluent of molasses. Int J Hydrog Energy 36:11360–11368
McKinlay JB, Harwood CS (2010) Carbon dioxide fixation as a central redox cofactor recycling mechanism in bacteria. Proc Natl Acad Sci U S A 107:11669–11675
Jeong H-S, Jouanneau Y (2000) Enhanced nitrogenase activities in strains of Rhodobacter capsulatus that overexpress the rnf genes. J Bacteriol 182:1208–1214
Liu T, Li X, Zhou Z (2010) Improvement of hydrogen yield by hupR gene knock-out and nifA gene overexpression in Rhodobacter sphaeroides 6016. Int J Hydrog Energy 35:9603–9610
Boran E, Özgür E, Yücel M, Gündüz U, Eroglu I (2012) Biohydrogen production by Rhodobacter capsulatus Hup- mutant in pilot solar tubular photobioreactor. Int J Hydrog Energy 37:16437–16445
Gebicki J, Modigell M, Schumacher M, van der Burg J, Roebroeck E (2010) Comparison of two reactor concepts for anoxygenic H2 production by Rhodobacter capsulatus. J Clean Prod 18:S36–S42
Özgür E, Afsar N, de Vrije T, Yücel M, Gündüz U, Claassen PAM, Eroglu I (2010) Potential use of thermophilic dark fermentation effluents in photofermentative hydrogen production by Rhodobacter capsulatus. J Clean Prod 18:S23–S28
Stevens P, Vertonghen C, de Vos P, de Ley J (1984) The effect of temperature and light intensity on hydrogen production by different Rhodopseudomonas capsulata strains. Biotechnol Lett 6:277–282
Favinger J, Stadtwald R, Gest H (1989) Rhodospirillum centenum, sp. nov., a thermotolerant cyst-forming anoxygenic photosynthetic bacterium. Ant Leeuwenhoek 55:291–296
Rozendal RA, Hamelers HVM, Euverink GJW, Metz SJ, Buisman CJN (2006) Principle and perspectives of hydrogen production through biocatalyzed electrolysis. Int J Hydrog Energy 31:1632–1640
Wrana N, Sparling R, Cicek N, Levin DB (2010) Hydrogen gas production in a microbial electrolysis cell by electrohydrogenesis. J Clean Prod 18:S105–S111
Logan BE, Hamelers B, Rozendal R, Schroder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192
Clauwaert P, Aelterman P, Pham TH, de Schamphelaire L, Carballa M, Rabaey K, Verstraete W (2008) Minimizing losses in bio-electrochemical systems: the road to applications. Appl Microbiol Technol 79:901–913
Logan BE (2008) Microbial fuel cells. Wiley, Hoboken
Yuan Y, Zhou SG, Tang JH (2013) In situ investigation of cathode and local biofilm microenvironments reveals important roles of OH- and oxygen transport in microbial fuel cells. Environ Sci Technol 47:4911–4917
Torres CI (2014) On the importance of identifying, characterizing, and predicting fundamental phenomena towards microbial electrochemistry applications. Curr Opin Biotechnol 27:107–114
Aelterman P, Rabaey K, De Schamphelaire L, Clauwaert P, Boon N, Verstraete W (2008) Microbial fuel cells as an engineered ecosystem. In: Wall J, Harwood CS, Demain AL (eds) Bioenergy. ASM, Washington, pp 307–320
Holmes DE, Chaudhuri SK, Nevin KP, Mehta T, Methe BA, Liu A, Ward JE, Woodard TL, Webster J, Lovley DR (2006) Microarray and genetic analysis of electron transfer to electrodes in Geobacter sulfureducens. Environ Microbiol 8:1805–1815
Lovley DR (2008) Extracellular electron transfer: wires, capacitors, iron lungs, and more. Geobiology 6:225–231
Marsili E, Baron DB, Shikare ID, Coursolle D, Gralnick JA, Bond DR (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci U S A 105:3968–3973
Sund CJ, McMasters S, Crittenden SR, Harrell LE, Sumner JJ (2007) Effect of electron mediators on current generation and fermentation in a microbial fuel cell. Appl Microbiol Biotechnol 76:561–568
Mahadevan R, Bond DR, Butler JE, Esteve-Nunez A, Coppi MV, Palsson BO, Schilling CH, Lovley DR (2006) Characterization of metabolism in the Fe(III)-reducing organism Geobacter sulfurreducens by constraint-based modeling. Appl Environ Microbiol 72:1558–1568
Rozendal RA, Jeremiasse A, Hamelers H, Buisman C (2008) Hydrogen production with a microbial biocathode. Environ Sci Technol 42:629–634
Liu H, Grot S, Logan BE (2005) Electrochemically assisted microbial production of hydrogen from acetate. Environ Sci Technol 39:4317–4320
Clauwaert P, Verstraete W (2009) Methanogenesis in membraneless microbial electrolysis cells. Appl Microbiol Biotechnol 82:829–836
Tice RC, Kim Y (2014) Methanogenesis control by electric oxygen production in microbial electrolysis cells. Int J Hydrog Energy 39:3079–3086
Cheng S, Logan BE (2007) Sustainable and efficient biohydrogen production via electrohydrogenesis. Proc Natl Acad Sci U S A 104:18871–18873
Wagner RC, Regan JM, Oh SE, Zuo Y, Logan BE (2009) Hydrogen and methane production from swine wastewater using microbial electrolysis cells. Water Res 43:1480–1488
Rozendal RA, Hamelers HVM, Buisman CJN (2006) Effects of membrane cation transport on pH and microbial fuel cell performance. Environ Sci Technol 40:5206–5211
Selembo PA, Merrill MD, Logan BE (2009) The use of stainless steel and nickel alloys as low-cost cathodes in microbial electrolysis cells. J Power Sources 190:271–278
Logan BE (2010) Scaling up microbial fuel cells and other bioelectrochemical systems. Appl Microbiol Biotechnol 85:1665–1671
Yi H, Nevin KP, Kim BC, Franks AE, Klimes A, Tender LM, Lovley DR (2009) Selection of a variant of Geobacter sulfurreducens with enhanced capacity for current production in microbial fuel cells. Biosens Bioelectron 24:3498–3503
Cusick RD, Bryan B, Parker DS, Merrill MD, Mehanna M, Kiely PD, Liu G, Logan BE (2011) Performance of a pilot-scale continuous flow microbial electrolysis cell fed winery wastewater. Appl Microbiol Biotechnol 89:2053–2063
Li CL, Fang HHP (2007) Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Crit Rev Environ Sci Technol 37:1–39
Amend JP, Shock EL (2001) Energetics of overall metabolic reactions of thermophilic and hyperthermophilic archaea and bacteria. FEMS Microbiol Rev 25:175–243
Kengen SWM, Goorissen HP, Verhaart M, Stams AJM, van Niel EWJ, Claassen PAM (2009) Biological hydrogen production by anaerobic microorganisms. In: Soetaert W, Vandamme E (eds) Biofuels. Wiley, Oxford, pp 197–222
Pauss A, Andre G, Perrier M, Guiot SR (1990) Liquid-to-gas mass transfer in anaerobic processes: inevitable transfer limitations of methane and hydrogen in the biomethanation process. Appl Environ Microbiol 56:1636–1644
Willquist K, Claassen PAM, van Niel EWJ (2009) Evaluation of the influence of CO2 as stripping gas on the performance of the hydrogen producer Caldicellulosiruptor saccharolyticus. Int J Hydrog Energy 34:4718–4726
Wang J, Wan W (2009) Factors influencing fermentative hydrogen production: a review. Int J Hydrog Energy 34:799–811
Pawar SS, van Niel EWJ (2013) Thermophilic biohydrogen production: how far are we? Appl Microbiol Biotechnol 97:7999–8009
Van Ginkel S, Logan BE (2005) Inhibition of biohydrogen production by undissociated acetic and butyric acids. Environ Sci Technol 39:9351–9356
Van Niel EWJ, Claassen PAM, Stams AJM (2003) Substrate and product inhibition of the hydrogen production by the extreme thermophile, Caldicellulosiruptor saccharolyticus. Biotechnol Bioeng 81:255–262
Schut GJ, Adams MWW (2009) The iron-hydrogenase of Thermotoga maritima utilizes ferredoxin and NADH synergistically: a new perspective on anaerobic hydrogen production. J Bacteriol 191:4451–4457
Ciranna A, Larjo A, Kivistö A, Santala V, Roos C, Karp M (2013) Draft genome sequence of the hydrogen- and ethanol-producing anaerobic alkalithermophilic bacterium Caloramator celer. Genome Announc 1, e00471-13
Hallenbeck PC, Ghosh D (2009) Advances in fermentative biohydrogen production: the way forward? Trends Biotechnol 27:287–297
Venkata Mohan S (2009) Harnessing of biohydrogen from wastewater treatment using mixed fermentative consortia: process evaluation towards optimization. Int J Hydrog Energy 34:7460–7474
Hussy I, Hawkes FR, Dinsdale R, Hawkes DL (2003) Continuous fermentative hydrogen production from a wheat starch co-product by mixed microflora. Biotechnol Bioeng 84:619–626
Zhang ZP, Show KY, Tay JH, Liang DT, Lee DJ, Jiang WJ (2006) Effect of hydraulic retention time on biohydrogen production and anaerobic microbial community. Process Biochem 41:2118–2123
Abo-Hashesh M, Ghosh D, Tourigny A, Taous A, Hallenbeck PC (2001) Single stage photofermentative hydrogen production from glucose: an attractive alternative to two stage photofermentationor co-culture approaches. Int J Hydrog Energy 36:13889–13895
Oh Y-K, Raj SM, Jung GY, Park S (2011) Current status of the metabolic engineering of microorganisms for biohydrogen production. Bioresour Technol 102:8357–8367
Rittmann S, Herwig C (2012) A comprehensive and quantitative review of dark fermentative hydrogen production. Microb Cell Fact 11:115
Van Niel EWJ, Willquist K, Zeidan AA, de Vrije T, Mars AE, Claassen PAM (2011) Hydrogen production by thermophilic fermentation. In: Azbar N, Levin D (eds) State of the art and progress in production of biohydrogen. Bentham Ebooks, Sharjah, pp 137–159
Ljunggren M, Zacchi G (2010) Techno-economic analysis of a two-step biological process producing hydrogen and methane. Bioresour Technol 101:7780–7788
Willquist K, van Niel EWJ (2012) Growth and hydrogen production characteristics of Caldicellulosiruptor saccharolyticus on chemically-defined minimal media. Int J Hydrog Energy 37:4925–4929
Pawar SS (2014) Caldicellulosiruptor saccharolyticus: an ideal hydrogen producer? PhD thesis, Lund University, Lund
Bielen AAM, Verhaart MRA, van der Oost J, Kengen SWM (2013) Biohydrogen production by the thermophilic bacterium Caldicellulosiruptor saccharolyticus: current status and perspectives. Life 3:52–85
Cappelletti M, Zannoni D, Postec A, Ollivier B (2014) Members of the order Thermotogales: from microbiology to hydrogen production. In: Zannoni D, de Philippis R (eds) Microbial bioenergy: hydrogen production, vol 38, Advances in photosynthesis and respiration. Springer, Dordrecht/Heidelberg/New York/London, pp 197–224
Willquist K, Zeidan AA, van Niel EWJ (2010) Physiological characteristics of the extreme thermophile Caldicellulosiruptor saccharolyticus. Microb Cell Fact 10:11
Zurawski JV, Blumer-Schuette SE, Conway JM, Kelly RM (2014) The extremely thermophilic genus Caldicellulosiruptor: physiological and genomic characteristics for complex carbohydrate conversion to molecular hydrogen. In: Zannoni D, de Philippis R (eds) Microbial bioenergy: hydrogen production, vol 38, Advances in photosynthesis and respiration. Springer, Dordrecht/Heidelberg/New York/London, pp 177–195
Rydzak T, Grigoryan M, Cunningham ZJ, Krokhin OV, Ezzati P, Cicek N, Levin DB, Wilkins JA, Sparling R (2014) Insights into electron flux through manipulation of fermentation conditions and assessment of protein expression profiles in Clostridium thermocellum. Appl Microbiol Biotechnol 98:6497–6510
Ivanova G, Rákhely G, Kovács KL (2008) Hydrogen production from biopolymers by Caldicellulosiruptor saccharolyticus and stabilization of the system by immobilization. Int J Hydrog Energy 33:6953–6961
Ljunggren M, Willquist K, Zacchi G, van Niel EWJ (2011) A kinetic model for quantitative evaluation of the effect of hydrogen and osmolarity on hydrogen production by Caldicellulosiruptor saccharolyticus. Biotechnol Biofuels 4:31
Willquist K, van Niel EWJ (2010) Lactate formation in Caldicellulosiruptor saccharolyticus is regulated by the energy carriers pyrophosphate and ATP. Metab Eng 12:282–290
Blumer-Schuette SE, Kataeva I, Westpheling J, Adams MWW, Kelly RM (2008) Extremely thermophilic microorganisms for biomass conversion: status and prospects. Curr Opin Biotechnol 19:210–217
Blumer-Schuette SE, Brown SD, Sander KB, Bayer EA, Kataeva I, Zurawski JV, Conway JM, Adams MWW, Kelly RM (2013) Thermophilic lignocellulose deconstruction. FEMS Microbiol Rev 38:393–448
Koskinen PEP, Lay C-H, Puhakka JA, Lin P-J, Wu S-Y, Örlyggson J, Lin C-Y (2008) High-efficiency hydrogen production by an anaerobic, thermophilic enrichment culture from an Icelandic hot spring. Biotechnol Bioeng 33:1168–1178
Van Groenestijn JW, Geelhoed JS, Goorissen HP, Meesters KPM, Stams AJM, Claassen PAM (2009) Performance and population analysis of a non-sterile trickle bed reactor inoculated with Caldicellulosiruptor saccharolyticus, a thermophilic hydrogen producer. Biotechnol Bioeng 102:1361–1367
Peintner C, Zeidan AA, Schnitzhofer W (2010) Bioreactor systems for thermophilic fermentative hydrogen production: evaluation and comparison of appropriate systems. J Clean Prod 18:S15–S22
Prasertsan P, O-Thong S, Birkeland N-K (2009) Optimization and microbial community analysis for production of biohydrogen from palm oil mill effluent by thermophilic fermentative process. Int J Hydrog Energy 34:7448–7459
Oh S-E, Iyer P, Bruns MA, Logan BE (2004) Biological hydrogen production using a membrane bioreactor. Biotechnol Bioeng 87:119–127
Ren N, Guo W, Liu B, Cao G, Ding J (2011) Biological hydrogen production by dark fermentation: challenges and prospects towards scaled-up productions. Curr Opin Biotechnol 22:365–370
Ngo TA, Bui HTV (2013) Biohydrogen production using immobilized cells of hyperthermophilic Eubacterium Thermotoga neapolitana on porous glass beads. J Technol Innov Renew Energy 2:231–238
Liu Y, Yu P, Song X, Qu Y (2008) Hydrogen production from cellulose by co-culture of Clostridium thermocellum JN4 and Thermoanaerobacterium thermosaccharolyticum GD17. Int J Hydrog Energy 33:2927–2933
Li Q, Liu C (2012) Co-culture of Clostridium thermocellum and Clostridium thermosaccharolyticum for enhancing hydrogen production via thermophilic fermentation of cornstalk waste. Int J Hydrog Energy 37:10648–10654
Yokoi H, Tokushige T, Hirose J, Hayashi S, Takasaki Y (1998) H2 production from starch by a mixed culture of Clostridium butyricum and Enterobacter aerogenes. Biotechnol Lett 20:143–147
Lu W, Wen J, Chen Y, Sun B, Jia X, Liu M, Caiyin Q (2007) Synergistic effect of Candida maltosa HY-35 and Enterobacter aerogenes W-23 on hydrogen production. Int J Hydrog Energy 32:1059–1066
Zeidan AA, Rådström P, van Niel EWJ (2010) Stable coexistence of two Caldicellulosiruptor species in a de novo constructed hydrogen-producing co-culture. Microb Cell Fact 9:102
Pawar SS, Vongkompean T, Gray C, van Niel EWJ (2015) Biofilm formation by designed co-cultures of Caldicellulosiruptor species as a means to improve hydrogen productivity. Biotechnol Biofuels 8:19
Talluri S, Raj SM, Christopher LP (2013) Consolidated bioprocessing of untreated switchgrass to hydrogen by the extreme thermophile Caldicellulosiruptor saccharolyticus DSM 8903. Bioresour Technol 139:272–279
Kataeva I, Foston MB, Yang S-J, Pattathil S, Biswal AK, Poole FL II, Basen M, Rhaesa AM, Thomas TP, Azadi P, Olman V, Saffold TD, Mohler KE, Lewis DL, Doeppke C, Zeng Y, Tschraplinkski TJ, York WS, Davis M, Mohnen D, Xu Y, Ragauskus AJ, Ding S-Y, Kelly RM, Hahn MG, Adams MWW (2013) Carbohydrate and lignin are simultaneously solubilized from unpretreated switchgrass by microbial action at high temperature. Energy Environ Sci 6:2186–2195
Cha M, Chung D, Elkins JG, Guss AM, Westpheling J (2013) Metabolic engineering of Caldicellulosiruptor bescii yields increased hydrogen production from lignocellulosic biomass. Biotechnol Biofuels 6:85
Chittibabu G, Nath K, Das D (2006) Feasibility studies on the fermentative hydrogen production by recombinant Escherichia coli BL-21. Process Biochem 41:682–688
Nicolaou SA, Gaida SM, Papoutsakis ET (2010) A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: from biofuels and chemicals, to biocatalysis and bioremediation. Metab Eng 12:307–331
Taylor MP, van Zyl L, Tuffin IM, Leak DJ, Cowan DA (2011) Genetic tool development underpins recent advances in thermophilic whole-cell biocatalysis. Microb Biotechnol 4:438–448
Claassen PAM, de Vrije T, Koukios E, van Niel EWJ, Eroglu I, Modigell M, Friedl A, Wukovits W, Ahrer W (2010) Non-thermal production of pure hydrogen from biomass: HYVOLUTION. J Clean Prod 18:S4–S8
Foglia D, Ljunggren M, Wukovits W, Friedl A, Zacchi G, Urbaniec K, Markowski M (2010) Integration studies on a two-stage fermentation process for the production of biohydrogen. J Clean Prod 18:S72–S80
Modarresi A, Wukovits W, Foglia D, Friedl A (2010) Effect of process integration on the exergy balance of a two-stage process for fermentative hydrogen production. J Clean Prod 18:S63–S71
Ochs D, Wukovits W, Ahrer W (2010) Life cycle inventory analysis of biological hydrogen production by thermophilic and photofermentation of potato steam peels (PSP). J Clean Prod 18:S88–S94
Eroglu E, Melis A (2011) Photobiological hydrogen production: recent advances and state of the art. Bioresour Technol 102:8403–8413
Chen C-Y, Yang M-H, Yeh K-L, Liu C-H, Chang J-S (2008) Biohydrogen production using sequential two-stage dark and photo fermentation processes. Int J Hydrog Energy 33:4755–4762
Redwood MD, Macaskie LE (2007) Method and apparatus for biohydrogen production. British patent application no. 0705583.3, UK
Androga DD, Özgür E, Eroglu I, Gündüz U, Yücel M (2012) Amelioration of photofermentative hydrogen production from molasses dark fermenter effluent by zeolite-based remova of ammonium ion. Int J Hydrog Energy 37:16421–16429
Lalaurette E, Thammannagowda S, Mohagheghi A, Maness P-C, Logan BE (2009) Hydrogen production from cellulose in a two-stage process combining fermentation and electrohydrogenesis. Int J Hydrog Energy 34:6201–6210
Liu BF, Ren NQ, Ding FXJ, Zheng GX, Guo WQ, Xu JF, Xie GJ (2009) Hydrogen production by immobilized R. faecalis RLD-53 using soluble metabolites from ethanol fermentation bacteria. Bioresour Technol 100:2719–2723
Wang AJ, Sun D, Cao GL, Wang HY, Ren NQ, Wu WM, Logan BE (2011) Integrated hydrogen production process from cellulose by combining dark fermentation, microbial fuel cells, and a microbial electrolysis cell. Bioresour Technol 102:4137–4143
Ueno Y, Fukui H, Goto M (2007) Operation of a two-stage fermentation process producing hydrogen and methane from organic waste. Environ Sci Technol 41:1413–1419
Giordano A, Cantù C, Spagni A (2011) Monitoring the biochemical hydrogen and methane potential of the two-stage dark-fermentative process. Bioresour Technol 102:4474–4479
Pawar SS, Nkemka VN, Zeidan AA, Murto M, van Niel EWJ (2013) Biohydrogen production from wheat straw hydrolysate using Caldicellulosiruptor saccharolyticus followed by biogas production in a two-step uncoupled process. Int J Hydrog Energy 38:9121–9130
Kongjan P, O-Thong S, Angelidaki I (2011) Performance and microbial community analysis of two-stage process with extreme thermophilic hydrogen and thermophilic methane production from hydrolysate in UASB reactors. Bioresour Technol 102:4028–4035
Van Haandel A, van der Lubbe J (2007) Handbook biological waste water treatment. IWA Publishing, London, pp 8–10
Willquist K, Nkemka V, Svensson H, Pawar SS, Ljunggren M, Hulteberg C, Murto M, van Niel EWJ, Karlsson H, Lidén G (2012) Design of a novel biohythane process with high H2 and CH4 production rates. Int J Hydrog Energy 37:17749–17762
Siddiqui Z, Horan NJ, Salter M (2011) Energy optimisation from co-digested waste using a two-phase process to generate hydrogen and methane. Int J Hydrog Energy 36:4792–4799
Yang ZM, Guo RB, Xu XH, Fan XL, Luo SJ (2011) Hydrogen and methane production from lipid-extracted microalgal biomass residues. Int J Hydrog Energy 36:3465–3470
Lee Y, Chung J (2010) Bioproduction of hydrogen from food waste by pilot-scale combined hydrogen/methane fermentation. Int J Hydrog Energy 35:11746–11755
Kyazze G, Dinsdale R, Hawkes F, Guwy A, Premier G, Donnison I (2008) Direct fermentation of fodder maize, chicory fructans and perennial ryegrass to hydrogen using mixed microflora. Bioresour Technol 99:8833–8839
McKinlay JB (2014) Systems biology of photobiological hydrogen production by purple non-sulfur bacteria. In: Zannoni D, de Philippis R (eds) Microbial bioenergy: hydrogen production, vol 38, Advances in photosynthesis and respiration. Springer, Dordrecht/Heidelberg/New York/London, pp 155–176
Munro SA, Zinder SH, Walker LP (2011) Comparative constraint-based model development for thermophilic hydrogen production. Ind Biotechnol 7:63–82
Ljunggren M, Zacchi G (2009) Techno-economic evaluation of a two-step biological process for hydrogen production. Biotechnol Prog 26:496–504
Zannoni D, Antonioni G, Frascari D, de Philippis R (2014) Hydrogen production and possible impact on global energy demand: open problems and perspectives. In: Zannoni D, de Philippis R (eds) Microbial bioenergy: hydrogen production, vol 38, Advances in photosynthesis and respiration. Springer, Dordrecht/Heidelberg/New York/London, pp 349–356
Acknowledgments
This work was financially supported by the Swedish Energy Agency (Energimyndigheten; project number 31090-2).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
van Niel, E.W.J. (2016). Biological Processes for Hydrogen Production. In: Hatti-Kaul, R., Mamo, G., Mattiasson, B. (eds) Anaerobes in Biotechnology. Advances in Biochemical Engineering/Biotechnology, vol 156. Springer, Cham. https://doi.org/10.1007/10_2016_11
Download citation
DOI: https://doi.org/10.1007/10_2016_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45649-2
Online ISBN: 978-3-319-45651-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)