Abstract
Preeclampsia is a disease of worldwide significance with increasing maternal mortality rate of 20–80 %. Though apoptosis is a normal constituent during pregnancy, there seems to be an altered balance between proliferation and apoptosis of endothelial cell in preeclampsia leading to a placental dysregulation resulting in premature delivery. Molecular chaperones like HSP70 and 90 play a significant role in control of preeclamptic progression and protect the developing fetus. This is governed by alterations in expression of HSF1, HIF1α a nuclear transcription factor and signaling molecule like ERK, JNK1/2, and Bcl-2. Endothelial cell from normotensive and preeclamptic placenta were analyzed for variation in viability and expression of signaling molecules. A significant decrease in viability of endothelial cell (p < 0.05) was noted in preeclamptic samples when compared to normotensive samples. The results indicate that there was an increase in, HSP70 and 90 (p < 0.01), HSF1 (p < 0.01), HIF1α (p < 0.05), ERK (p < 0.05), JNK1/2 (p < 0.05), and Bcl-2 (p < 0.05). Though there is a significant change in the viability of endothelial cell, the live fetal delivery is not predominantly affected during preeclampsia. The interplay between these signaling molecules which alter the apoptotic pathway to sustain endothelial cell viability is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxygen appears to play a critical role in the development of pregnancy complications and is a key mediator of placental development and function. Impaired placental perfusion has been assumed to result in chronic placental ischemia and hypoxia later in gestation, which is detrimental to pregnancy success and may lead to preeclampsia [1]. Up to 15 % of preterm births are a result of preeclampsia [2]. Roberts et al. [3] formally proposed that maternal endothelial cell dysfunction is the key event resulting in the diverse clinical manifestations of preeclampsia. Evidence has since accumulated to support a major role of the endothelium in preeclampsia [4]. The mechanisms involved in the induction of endothelial cell dysfunction are poorly understood. Evidence points to the placenta as a key source of factors that lead to the maternal endothelial cell dysfunction in preeclampsia [5].
The pathogenesis of preeclampsia is related to an imbalance of increased oxidative stress coupled with deficiency of antioxidant protection. Under normal conditions, adequate levels of antioxidants, mainly superoxide dismutase, catalase, glutathione peroxidase, and reductase, trap most of the free radicals and function as a first-line defense mechanism in response to oxidative stress [6]. This delicate balance is lost during preeclampsia due to enormous increase in oxidative stress [7]. This oxidative imbalance results in accumulation of damaged proteins which disturbs the protein homeostasis, ultimately resulting in cell death via apoptosis. The level of oxidative stress and antioxidant in preeclamptic endothelial cell has previously been documented [8, 9]. Monitoring the signaling changes in the placental endothelial cell will aid in identifying the mechanisms underlying the live fetal delivery in spite of the existing complications.
Hypoxia–reoxygenation is a more physiological stimulus than hypoxia alone for generating the placental changes associated with preeclampsia [10], however, the cellular events occurring during hypoxia/reoxygenation are complex because of the rapid rate of reaction of reactive oxygen species with scavengers and other biomolecules. Hypoxia-inducible factor (HIF) is a transcriptional activator that promotes death or survival of cells. HIF1 has attracted the attention of many investigators because of its ability to mediate adaptive cellular responses to a change in oxygen tension [11]. HIF1 is a transcription factor that is composed of two subunits, HIF1α and HIF1β. HIF1α is subject to ubiquitination and proteosomal degradation under normoxic conditions [12]. Thus, the cytosolic and nuclear levels of HIF1α were estimated along with stress proteins during preeclampsia a condition associated with frequent pulse of hypoxia and reoxygenation.
Heat shock proteins (HSPs) have a critical role in the recovery of cells from stress and in cytoprotection, guarding cells from subsequent insults [13]. HSPs interact with multiple key components of signaling pathways that regulate growth and development. The molecular relationships between HSPs, various signaling proteins and partner proteins appear to be critical for the normal function of signal transduction pathways. The relative levels of these proteins may be important, as too little or too much HSP70 or 90 can result in aberrant growth control, developmental malformations and cell death. Thus, overexpression of HSP is an important means of cellular protection during physiological stress. HSP70 and 90 are the predominantly expressed stress-inducible proteins in eukaryotic cell.
HSP70 is an abundant, highly conserved, cellular defense protein, important for normal functioning of cells under stressed and unstressed situations. It functions to refold or eliminate misfolded/denatured proteins playing critical role in many aspects of cellular signaling. HSP70 can function alone to inhibit apoptosis while cooperative interaction with their designated co-chaperone molecules is likely to enhance their anti-apoptotic activities [14]. HSP90 is unique among molecular chaperones. The majority of its known substrates are signaling transduction proteins the classical examples being steroid hormone receptors and signaling kinases [15, 16]. It is a highly conserved, cytosolic, abundant protein of eukaryotic cells necessary for viability under all conditions and also aids in cell proliferation [17]. Heat shock factor (HSF1) is the ubiquitous stress-responsive transcriptional activator essential for the inducible transcription of genes encoding HSPs [18] by binding to regulatory heat shock elements present in the promoter region of all heat shock genes [19].
As metabolically active tissues vital to the maintenance of pregnancy, placental tissue is continuously in the stage of proliferation and apoptosis. HSPs-like HSP90, key HSP involved in cell proliferation and HSP70, an important anti-apoptotic protein were assessed along with the transcriptional HSP regulator HSF, in order to assess its role in preeclamptic endothelial cell.
HSP90 has many client proteins with vital function in cell survival. Extracellular signal regulating kinase (ERK) is one of the client proteins of HSP90 whose expression is greatly regulated by HSP90 [20]. ERK1/2 is a serine/threonine kinase of the mitogen-activated protein kinase (MAPK) superfamily that mediates intracellular signal transduction in response to a variety of stimuli [21], widely expressed protein kinase intracellular signaling molecules. Upon activation, ERKs are involved in functions including the regulation of meiosis, mitosis, and postmitotic functions in differentiated cells [22]. The key nuclear transcription factors implicated as substrates to ERK is ELK. ELK (E-twenty-six [ETS]-like kinase) is an ETS domain transcription factor of the ternary complex factor (TCF) subfamily, is known to be involved in the regulation of immediate-early genes implied in cell proliferation [23]. As an indicator of cell survival and proliferation, the ERK–Elk pathway was analyzed in response to preeclamptic stress in placental endothelial cell.
c-Jun N-terminal kinases (JNK) are members of the MAPK family, playing a pivotal role in the transmission of extracellular signals through the cytosol to the nucleus [24]. The JNK subfamilies are activated by a wide variety of stress. JNK has three isoforms (JNK1, 2, and 3), with slicing variant [25]. Among them, JNK1 and 2 are ubiquitously expressed. It was shown to either induce apoptosis or stimulate cellular proliferation and transformation depending on the cell type and activating stimuli [26]. Thus analysis of changes in this protein in response to the alteration in other signaling proteins aid in analyzing the role of JNK1/2 in preeclamptic placental endothelial cell.
This study thus aims to assess the expression of HSP70 and 90 in modulating the MAPK pathway in placental endothelial cell in response to preeclamptic stress.
Materials and Methods
Selection of Subjects
Patient registered in a public sector Hospital in Chennai were enrolled in this study. Clearance was obtained from Institute Ethical Committee prior to the commencement of study and the informed consent was received from all the subjects. Preeclamptic patients (n = 35) were defined on the basis of: systolic blood pressure ≥140 mmHg and diastolic blood pressure ≥90 mmHg noted on at least two occasions, proteinuria levels >300 mg/dL found in at least two random specimens and the activity of xanthine oxidase (XO) about 2.6 units/mg protein [27]. Similar age matched healthy volunteers who are normotensive (n = 35), similar race, body mass index (BMI), and without maternal and fetal complications during the pregnancy period were selected as control subjects. The clinical characteristics of the preeclamptic patients were tabulated and compared with the normotensive pregnant subjects and the data are presented in Table 1.
Isolation of Endothelial Cell
Endothelial cell was isolated from the normotensive and preeclamptic placenta on the same day of placenta collection. Endothelial cell was isolated by the method described previously [28]. Briefly, placental chorion was excised and thoroughly minced, washed in Hank’s balanced salt solution (HBSS) and passed through a 90 μm sieve. Collagenase type I (Sigma, USA) was added at 1.4 mL/g of placental tissue, and the contents were shaken at 37 °C for 80 min. After several washes with HBSS and centrifugation at 100×g for 5 min, the pellets were placed on ice. After re-suspending and incubating the cell pellet in 0.5 mL trypsin–EDTA/g tissue, the suspension was passed through a 250 μm sieve. The filtrate was centrifuged at 100×g for 5 min. The single cell suspension obtained was treated with Dynabead CD31 (Invitrogen, Canada) and was washed with phosphate-buffered saline (PBS) containing 0.1 % bovine serum albumin. This mixture was incubated at 4 °C for 20 min with tilting and rotation. The Dynabead endothelial cell complex was collected with a magnetic particle concentrator (Invitrogen, Canada). The cells were washed twice with PBS and cultured at 1 million cells per culture flask (125 mm2) in M199 medium containing 10 % fetal calf serum and antibiotic and antimycotic solution (Himedia, Mumbai, India) in a 5 % CO2 atmosphere at 37 °C to obtain confluence. The viability of the cells was assessed by trypan blue dye exclusion test [29]. Cells with viability less than 75 % were excluded from the study.
TEM Analysis of Endothelial Cells
Placental sections were fixed for transmission electron microscopy (TEM) with 3 % glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4, at 4 °C. The tissue remained in this primary fixative for 2 days. After primary fixation, the tissues were washed in sodium cacodylate buffer and postfixed in 1 % osmium tetroxide for 1 h at room temperature in sodium cacodylate buffer. Fixation was followed by dehydration of tissues by ascending series of graded alcohol (10–100 %) and propylene oxide. The tissues were infiltered, embedded in siliconized rubber mold with epoxy resin, and incubated at 60 °C for 48 h, for the preparation of blocks for sectioning. Thick sections (1 µm) were cut through ultramicrotome (Leica ultracut UCT) with glass knife and stained with toluidine blue dye. The sections were then examined by light microscopy to select areas for fine structural study and photomicrography. Ultrathin sections (below 100 nm) were cut through ultramicrotome (Leica) with diamond knife (Diatome). The ultrathin sections were taken on copper grid and stained with 2 % alcoholic uranyl acetate and Reynold’s lead citrate solution. The samples were viewed at 80 kV with an electron microscope (201C; Philips Electronic Instruments, Inc., Mahwah, NJ).
ELISA of HSP70 and 90
The inducible form of HSP70 in the placental endothelial cells was quantified using HSP70 (EKS-700B, Stressgen, Canada) and HSP90 (EKS-895, Stressgen, Canada) ELISA kit according to the manufacturer’s instruction.
Coimmunoblot Analysis of Signaling Molecules
The placental endothelial cell protein aliquots containing 50 μg proteins were ran on 10 % SDS-polyacrylamide gels simultaneously. The gels were then blotted on to PVDF membranes (BioTrace PVDF 0.4 µm, Pall Corporation, Germany) according to the method of Towbin et al. [30]. The antibodies used were anti HSP70 (SPA810), anti HSP90 (SPA-835), anti JNK1/2 [KAP-SA011], anti ERK1/2 [KAP-MA001], anti Elk1 [KAP-MA035], anti HSF1 [SPA 901] antibody, and anti β-actin (CSA-400), and followed by goat antimouse IgG secondary antibody treatment and color development was done using BCIP–NBT substrate system. The band intensities were scanned with the Hp Scan Imager and quantified using the TotalLab software, gels, USA. The results were confirmed by individually performing the blotting studies of the signaling proteins.
Immunohistochemical Expression of Bcl-2 and HIF1α
Placental sections were fixed in 10 % phosphate-buffered formaldehyde solution and embedded in paraffin. These sections were cut into specimens of 7-µm thick, mounted onto poly-l-Lysine-coated slides and stored under dry conditions until histologic analysis. The sections were deparaffinized in xylene, rehydrated in ethanol and incubated in 3 % H2O2 in absolute methanol for 5 min in order to inhibit endogenous peroxidase activity. It was then rinsed in 0.05 M Tris-buffered saline (TBS), pH 7.6, for 5 min. Antigen retrieval was performed by heat treating sections in 0.01 mol/L citrate buffer (pH 6.0) at 95 °C in a microwave oven for 5 min (three cycles). To reduce non specific binding, slides were incubated in 10 % normal goat serum for 10 min at room temperature before 1 h incubation with antibodies against Bcl-2 oncoprotein (AM287-5M) and HIF1α (AD1-OSA-602-E) in a humidified chamber at 4 °C. After rinsing with TBS, the procedure for the detection of these proteins includes sequential application of biotinylated secondary link antibodies and streptavidin–peroxidase conjugate and incubation at 4 °C for 30 min in each of them. Peroxidase activity was detected using 0.1 % H2O2 in 3,3′-diaminobenzidine (DAB) solution applied to the tissue sections for 5 min. It was then counterstained with hematoxylin for 5 s before rinsing, dehydrating and mounting with coverslips using xylene and DPX mountant. The immunohistochemical images were acquired with Magnus Pro microscopes (Magnus pro MLXiTR, Olympus, US).
Statistical Analysis
All results were expressed as mean ± standard deviation. Each experiment was performed thrice and data were analyzed by two-way ANOVA test. The differences were considered significant at p < 0.05, 0.01, 0.001.
Results
Selection of Subjects
The clinical characteristics of the patients recorded (Table 1) showed a significant variance in few parameters like pregnancy blood pressure, parity, XO, proteinuria, gestational age, baby weight as reported earlier [9].
Viability of Endothelial Cell
The viability of endothelial cells was monitored immediately after isolation and also after growing the cells to confluence. There was a significant decrease (p < 0.05, 15.7 %) in the viability of endothelial cells during preeclampsia. There was a negligible difference between the cell viability immediately after isolation and after growth (Fig. 1).
Morphological Changes in Endothelial Cell
The morphological changes in the normotensive and preeclamptic endothelial cells as observed in TEM. The low power objective analysis revealed that the fenestra in the endothelial cell is damaged during preeclampsia (Fig. 2 panel a, b). Further observation showed an altered structural morphology of the preeclamptic endothelial cell associated with a change in the cell size (Fig. 2 panel c, d). Analysis of endothelial cell mitochondria showed that the mitochondrial membrane remained intact (Fig. 2 panel e, f). Also, the number of mitochondria was increased in preeclamptic endothelial cell.
Transmission electron microscopy of placenta from normotensive women (panel a, c, e) preeclamptic women (panel b, d, f). The fenestra of endothelial cell from normotensive and preeclampsia subjects are shown in the panel a, b (1.5 K). Panel c, d (10 K) represents altered cell morphology and cell size. Panel e, f (45 K) shows the mitochondria in the normotensive and preeclamptic endothelial cell
Quantification of HSP70 and 90
In the preeclamptic endothelial cell, a highly significant increase in the expression of HSP70 (p < 0.01) and HSP90 (p < 0.01) was observed when compared with the normotensive endothelial cell (Table 2).
Immunohistochemical Studies
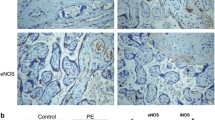
The changes noted in Bcl-2 through histochemical analysis revealed an increasing cytosolic distribution. HIF1α analysis demonstrated an increasing cytosolic and nuclear expression and the results are represented in Fig. 3a–d. The intensities of the expression as quantified by the magnus pro image analysis software also showed a significant increase (p < 0.05) in the expression of Bcl-2 and HIF1α in preeclamptic endothelial cell when compared with normotensive endothelial cell.
Immunohistochemical staining of Bcl-2 (a, b) and HIF1α (c, d) in the placenta from normotensive women and preeclamptic women, Arrow heads indicate the expression of Bcl-2 and HIF1α protein. The preeclamptic placental sections show more intense staining for both the Bcl-2 and HIF1α indicating the increased expression of the protein during preeclampsia. Scale 5 μm
Blotting Analysis of Signaling Molecules
Blotting analysis showed a highly significant increase in HSP70 and 90 in preeclamptic endothelial cell in line with the results obtained by quantification (Fig. 4a, b). Assessment of other signaling proteins showed a constant increase in all the proteins at varying significance range; JNK1 (p < 0.05), ERK (p < 0.01), Elk (p < 0.05), HSF1 (p < 0.01). However, the increase in JNK2 noted was not in a significant range. The representative blots for all the proteins are given in Fig. 4a–f.
Discussion
Placenta is a metabolically active tissue which remains in a dynamic state due to the repetitive proliferation and apoptosis noted in these cells at various stages of gestation. The presence of stress like preeclampsia shifts the delicate balance between proliferation and apoptosis in placenta thereby making the placenta more vulnerable to further complications. Placental endothelial cells are major cells whose dysfunction results in the primary symptomatic manifestation of preeclampsia [3]. In spite of the significant decrease in the viability of preeclamptic endothelial cell, the morphological changes were not completely apoptotic. The fenestra of endothelial cell during preeclampsia was compromised demonstrating an altered cell–cell interaction. Preeclampsia also showed an altered cell shape and nuclear morphology, however, the cells remained intact. This suggests the presence of a protective anti-apoptotic mechanism in placental endothelial cell during preeclampsia.
Diminished or poor placental function in preeclampsia also increases the chances of apoptosis by enhancing the state of oxidative stress [27]. In response to oxidative stress, the expression of HIF1α was increased in preeclamptic endothelial cell. HIF1α is a cytoprotective protein with vital function in angiogenesis, energy metabolism and cell survival. This protein acts as an oxygen sensor that gets stimulated in response to hypoxia. Reoxygenation is found to promote HIF1α degradation [12]. In spite of the repetitive hypoxia/reoxygenation demonstrated in preeclampsia [10], the level of HIF1α increased both in the cytosol and nucleus during preeclampsia. This suggests the involvement of proteins that help in stabilizing these proteins in the cytosol and transport of this protein to the nucleus for activation. HIF1α will bind to HIF1β in the nucleus to form a stable compound which can bind to the hypoxia response element present in the target genes of HIF1 [31], thus inducing its expression.
Simultaneously, an increase in the expression of HSF1, HSP70, and HSP90 was also observed. Previous reports suggest that HSF1, a nuclear transcription factor of HSP synthesis, is one of the target genes containing the hypoxia responsive element [32]. The increased HSF1 would aid in increasing the expression of HSP70 and 90 which protects the cells from damaging stimuli. Both the proteins HSP70 and 90 have anti-apoptotic function [33, 34].
It has also been reported that transgenic gene knockout mice lacking ERK1 have major defects in early development [35]. Upon mitogenic stimulation, ERK translocates from the cytoplasm to the nucleus, where it phosphorylates the ternary complex factors Elk-1 [36, 37]. Once phosphorylated, Elk-1 can stimulate transcription of important regulators of cell survival and gene expression [38]. Hence, the activation of ERK–Elk pathway noted in preeclamptic endothelial cell finally has a role in proliferation [39], development, differentiation [40], and cellular survival [21, 41]. Thus increase in the ERK and Elk proteins during preeclampsia might be a probable reason for sustained endothelial cell survival. HSP90 will aid in the activation of this survival protein, which is one of its client protein [20].
JNK protein showed a differential expression in preeclamptic endothelial cell. There was a significant increase in JNK1 and an insignificant increase in JNK2. This could suggest that the differential function of JNK1/2 in both cell survival and apoptosis [42] might be due to the change in the JNK1/2 ratio JNK1 has been found to stimulate HSP70/90 expression by stabilizing HSF1 activation [43], while JNK2 in contrast to JNK1, is a negative regulator of cellular proliferation in multiple cell types [44]. It has been found to block excessive activation of HSP70. Similarly, JNK1 has also been found to regulate HIF1α stability in the cytosol [45]. This suggests the probable role of JNK1 in promoting endothelial cell survival during preeclampsia.
An increase in Bcl-2 and the stability of mitochondria in preeclampsia suggest that JNK2 level is not sufficient enough to stimulate apoptosis. However, JNK1 mediated increase in Bcl-2 also contributes to the protection of cell from apoptosis [46].
Preeclampsia is not always associated with live fetal delivery, however, under conditions when live fetal delivery occur during preeclampsia, there exists a defensive strategy to assist in the successful progression of the fetal delivery. Thus analysis of signaling changes from placental endothelial cell isolated from preeclamptic subjects who gave birth to a live fetus can suggest the probable mechanism. Our results show that HSP70 and 90 functions in a coordinated manner in controlling the death and survival signals of the MAPK pathway (Fig. 5) in preeclamptic endothelial cell thereby providing a degree of protection to these cells in spite of the existing complication. This might be the probable reason for the live fetal delivery associated with preeclampsia.
Schematic representation of the signal transduction mechanism in the preeclampsia endothelial cell. This represents that the stress built by preeclampsia stimulates the expression of transcription factor like HIF in endothelial cell which favors the synthesis of essential cytoprotective protein HSP70 and 90 through the activation of HSF1. HSP70 and 90 establishes its cytoprotective mechanism by altering the expression of JNK1, JNK2, and ERK. JNK1-activated Bcl-2 and promotes cell survival by maintaining the mitochondrial membrane integrity. ERK activates the expression of another nuclear transcription factor Elk which promotes cell survival. JNK2 on the other hand is a pro-apoptotic protein whose expression is relatively down-regulated in this condition
Abbreviations
- HSP:
-
Heat shock protein
- HSF:
-
Heat shock factor
- HIF:
-
Hypoxia inducible factor
- ERK:
-
Extracellular signal regulating kinase
- JNK:
-
c-jun N-terminal kinase
- Bcl-2:
-
B cell CLL/lymphoma like protein-2
- Elk:
-
ETS (E-twenty-six)-like kinase
- TCF:
-
Ternary complex factor
- MAPK:
-
Mitogen activate protein kinase
- XO:
-
Xanthine oxidase
- BMI:
-
Body mass index
- EDTA:
-
Ethylene diamine tetra acetic acid
- CD31:
-
Cluster of differentiation 31
- PBS:
-
Phosphate-buffered saline
- TEM:
-
Transmission electron microscopy
- ELISA:
-
Enzyme linked immunosorbent assay
- PVDF:
-
Polyvinylidene di-flouride
- SDS:
-
Sodium dodecyl sulfate
- BCIP–NBT:
-
Bromo chloro indolyl phenol–nitro blue tetrazolium
- TBS:
-
Tris-buffered saline
- DAB:
-
Diaminobenzedine
- ANOVA:
-
Analysis of variance
References
Chaddha, V., Viero, S., Huppertz, B., & Kingdom, J. (2004). Developmental biology of the placenta and the origins of placental insufficiency. Seminars in Fetal and Neonatal Medicine, 9, 357–369.
Meis, P., Goldenberg, R., & Mercer, B. (1998). The preterm prediction study: Risk factors for indicated preterm births. Maternal-Fetal Medicine Units Network of the National Institute of Child Health and Human Development. American Journal of Obstetrics and Gynecology, 179, 562–567.
Roberts, J. M., Taylor, R. N., Musci, T. J., Rodgers, G. M., Hubel, C. A., & McLaughlin, M. K. (1990). Preeclampsia: An endothelial cell disorder. American Journal of Obstetrics and Gynecology, 163, 1365–1366.
Roberts, J. M. (1998). Endothelial dysfunction in preeclampsia. Seminars in Reproductive Endocrinology, 16, 5–15.
Redman, C. W. (1991). Current topic: Preeclampsia and the placenta. Placenta, 12, 301–308.
Redman, C. W. G., & Sargent, I. L. (2000). Placental debris, oxidative stress and preeclampsia. Placenta, 21(7), 597–602.
Ilhan, N., Ilhan, N., & Simsek, M. (2002). The changes of trace elements, malondialdehyde levels and superoxide dismutase activities in pregnancy with or without preeclampsia. Clinical Biochemistry, 35(5), 393–397.
Padmini, E., Usha Rani, M., & Lavanya, S. (2008). Effect of mint and tea infusions on the antioxidant capacity of preeclamptic endothelial cells. Asian Journal of Microbiology, Biotechnology and Environmental Science, 10(4), 903–909.
Padmini, E., Lavanya, S., & Uthra, V. (2009). Preeclamptic placental stress and mitochondrial HSP70 over expression. Clinical Chemistry and Laboratory Medicine, 47(9), 1073–1080.
Cindrova-Davies, T., Spasic-Boskovic, O., Jauniaux, E., Charnock-Jones, D. S., & Burton, G. J. (2007). Nuclear factor-κB, p38, and stress-activated protein kinase, mitogen-activated protein kinase signaling pathways regulate proinflammatory cytokines and apoptosis in human placental explants in response to oxidative stress. American Journal of Pathology, 170(5), 1511–1520.
Semenza, G. L., Roth, P. H., Fang, H. M., & Wang, G. L. (1994). Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. Journal of Biological Chemistry, 269, 23757–23763.
Salceda, S., & Caro, J. (1997). Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin–proteasome system under normoxic conditions: Its stabilization by hypoxia depends on redox-induced changes. Journal of Biological Chemistry, 272, 22642–22647.
Bukau, B., & Horwich, A. L. (1998). The HSP70 and HSP60 chaperone machines. Cell, 92(3), 351–366.
Beere, H. M., Wolf, B. B., Cain, K., Mosser, D. D., Mahboubi, A., Kuwana, T., et al. (2000). Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nature Cell Biology, 2, 469–475.
Picard, D., Khursheed, B., Garabedian, M. J., Fortin, M. G., Lindquist, S., & Yamamoto, K. R. (1990). Reduced levels of HSP90 compromise steroid receptor action in vivo. Nature, 348, 166–168.
Xu, Y., & Lindquist, S. (1993). Heat-shock protein HSP90 governs the activity of pp 60v-src kinase. Proceedings of the National Academy of Sciences of the United States of America, 90, 7074–7078.
Cs, Soti, Nagy, E., Giricz, Z., Vigh, L., Csermely, P., & Ferdinandy, P. (2005). Heat shock proteins as emerging therapeutic targets. British Journal of Pharmacology, 146, 769–780.
Wu, C. (1995). Heat shock transcription factors: Structure and regulation. Annual Review of Cell and Developmental Biology, 11, 441–469.
Jaattela, M. (1999). Escaping cell death: Survival proteins in cancer. Experimental Cell Research, 248, 30–43.
Koga, F., Xu, W., Karpova, T. S., McNally, J. G., Baro, R., & Neckers, L. (2006). HSP90 inhibition transiently activates Src kinase and promotes Src-dependent Akt and Erk activation. Proceedings of the National Academy of Sciences of the United States of America, 103, 11318–11322.
Dou, F., Yuan, L. D., & Zhu, J. J. (2005). Heat shock protein 90 indirectly regulates ERK activity by affecting Raf protein metabolism. Acta Biochimica et Biophysica Sinica, 37(7), 501–505.
Davie, J. R., & Spencer, V. A. (2001). Signal transduction pathways and the modification of chromatin structure. Progress in Nucleic Acid Research and Molecular Biology, 65, 299–340.
Demir, O., & Kurnaz, I. A. (2008). Wildtype Elk-1, but not a SUMOylation mutant, represses egr-1 expression in SH-SY5Y neuroblastomas. Neuroscience Letters, 437(1), 20–24.
Hibi, M. A., Lin, T., Smeal, A., Minden, A., & Karin, M. (1993). Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes & Development, 7, 2135–2148.
Davis, R. J. (2000). Signal transduction by the JNK group of MAP kinases. Cell, 103, 239–252.
Weston, R. C., & Davis, R. J. (2002). The JNK signal transduction pathway. Current Opinion in Genetics & Development, 12, 14–21.
Brown, M. A., Lindheimer, M. D., de Swiet, M., Van Assche, A., & Moutquin, J. M. (2001). The classification and diagnosis of the hypertensive disorders if pregnancy: Statement form the International Society for the study of hypertension in Pregnancy (ISSHP). Hypertension Pregnancy, 20, 9–14.
Padmini, E., & Lavanya, S. (2011). HSP70 mediated control of endothelial cell apoptosis during pre-eclampsia. European Journal of Obstetrics & Gynecology Reproductive Biology, 156, 158–164.
Strober, W. (2001). Trypan blue exclusion test of cell viability. Current Protocols in Immunology, Appendix 3, Appendix 3B.
Towbin, H., Staehelin, T., & Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proceedings of the National Academy of Sciences of the United States of America, 76, 4350–4354.
Wang, G. L., Jiang, B. H., Rue, E. A., & Semenza, G. L. (1995). Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proceedings of the National Academy of Sciences of the United States of America, 92, 5510–5514.
Baird, N. A., Turnbull, D. W., & Johnson, E. A. (2006). Induction of the heat shock pathway during hypoxia requires regulation of heat shock factor by hypoxia-inducible factor-1. Journal of Biological Chemistry, 281(50), 38675–38681.
Pelham, H. R. (1982). A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell, 30, 517–528.
Orosz, A., Wisniewski, J., & Wu, C. (1996). Regulation of Drosophila heat shock factor trimerization: Global sequence requirements and independence of nuclear localization. Molecular and Cellular Biology, 16, 7018–7030.
Yao, Y., Li, W., Wu, J., et al. (2003). Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proceedings of the National Academy of Sciences of the United States of America, 100(22), 12759–12764.
Chen, R. H., Sarnecki, C., & Blenis, J. (1992). Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Molecular and Cellular Biology, 12, 915–927.
Gille, H., Strahl, T., & Shaw, P. E. (1995). Activation of ternary complex factor Elk-1 by stress-activated protein kinases. Current Biology, 5, 1191–1200.
Pearson, G. F., Robinson, T., Beers Gibson, B., Xu, M., Karandikar, K., & CobbMH, Berman. (2001). Mitogen-activated protein (map) kinase pathways: Regulation and physiological functions. Endocrine Reviews, 22, 153–183.
Pages, G., Lenormand, P., L’Allemain, G., Chambard, J. C., Meloche, S., & Pouyssegur, J. (1993). Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proceedings of the National Academy of Sciences of the United States of America, 90, 8319–8323.
Buchkovich, K. J., & Ziff, E. B. (1994). Nerve growth factor regulates the expression and activity of p33cdk2 and p34cdc2 kinases in PC12 pheochromocytoma cells. Molecular Biology of the Cell, 5, 1225–1241.
Cowan, K. J., & Storey, K. B. (2003). Mitogen-activated protein kinases: New signaling pathways functioning in cellular responses to environmental stress. Journal of Experimental Biology, 206, 1107–1115.
Liu, J., & Lin, A. (2005). Role of JNK in apoptosis: A double edged sword. Cell Research, 15(1), 36–42.
Sreedhar, A. S., & Csermely, P. (2004). Heat shock proteins in the regulation of apoptosis: New strategies in tumor therapy. A comprehensive review. Pharmacology Therapy, 101, 227–257.
Hochedlinger, K., Wagner, E. F., & Sabapathy, K. (2002). Differential expression of JNK1 and JNK2 on signal specific induction of apoptosis. Oncogene, 21, 2441–2445.
Zhang, D., Li, J., Costa, M., Gao, J., & Huang, C. (2010). JNK1 mediates degradation of HIF-1α by a VHL-independent mechanism that involves the chaperones Hsp90/Hsp70. Cancer Research, 70, 813–823.
Maundrell, K., Antonsson, B., Magnenat, E., et al. (1997). Bcl-2 undergoes phosphorylation by c-Jun N-terminal kinase/stress-activated protein kinases in the presence of the constitutively active GTP-binding protein Rac1. Journal of Biological Chemistry, 272, 25238–25242.
Acknowledgments
The project funded by National Tea Research Foundation, Tea Board of India is acknowledged. Project referral number—NTRF: 115/07. V. Uthra and S. Lavanya thank Indian council for Medical Research for providing financial assistance in the form of senior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Padmini, E., Uthra, V. & Lavanya, S. Effect of HSP70 and 90 in Modulation of JNK, ERK Expression in Preeclamptic Placental Endothelial Cell. Cell Biochem Biophys 64, 187–195 (2012). https://doi.org/10.1007/s12013-012-9371-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-012-9371-0