Abstract
Purpose
Blended cements use waste products to replace Portland cement, the main contributor to CO2 emissions in concrete manufacture. Using blended cements reduces the embodied greenhouse gas emissions; however, little attention has been paid to the reduction in CO2 capture (carbonation) and durability. The aim of this study is to determine if the reduction in production emissions of blended cements compensates for the reduced durability and CO2 capture.
Methods
This study evaluates CO2 emissions and CO2 capture for a reinforced concrete column during its service life and after demolition and reuse as gravel filling material. Concrete depletion, due to carbonation and the unavoidable steel embedded corrosion, is studied, as this process consequently ends the concrete service life. Carbonation deepens progressively during service life and captures CO2 even after demolition due to the greater exposed surface area. In this study, results are presented as a function of cement replaced by fly ash (FA) and blast furnace slag (BFS).
Results and discussion
Concrete made with Portland cement, FA (35 % FA), and BFS blended cements (80 % BFS) captures 47, 41, and 20 % of CO2 emissions, respectively. The service life of blended cements with high amounts of cement replacement, like CEM III/A (50 % BFS), CEM III/B (80 % BFS), and CEM II/B-V (35 % FA), was about 10 % shorter, given the higher carbonation rate coefficient. Compared to Portland cement and despite the reduced CO2 capture and service life, CEM III/B emitted 20 % less CO2 per year.
Conclusions
To obtain reliable results in a life cycle assessment, it is crucial to consider carbonation during use and after demolition. Replacing Portland cement with FA, instead of BFS, leads to a lower material emission factor, since FA needs less processing after being collected, and transport distances are usually shorter. However, greater reductions were achieved using BFS, since a larger amount of cement can be replaced. Blended cements emit less CO2 per year during the life cycle of a structure, although a high cement replacement reduces the service life notably. If the demolished concrete is crushed and recycled as gravel filling material, carbonation can cut CO2 emissions by half. A case study is presented in this paper demonstrating how the results may be utilized.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Over the recent decades, there has been a growing concern about the need to protect the environment and keep our planet safe. While the embodied greenhouse gas (GHG) emissions were limited by the Kyoto Protocol, in 2001, the Spanish construction industry produced 1.76 m3 of concrete per capita per year; Spain has the highest consumption rate in Europe, more than twice the average of other countries (ECO-SERVE 2004). The construction industry is second to the food industry in raw materials consumption (Berge 2000); in fact, building construction consumes 40 % of the world’s raw materials. Furthermore, it generates 40–50 % of all global GHG emissions and agents of acid rain (CIWMB 2000). Concrete is the most heavily consumed material in the construction sector (ISO 2005; Weil et al. 2006). The manufacture of Portland cement contributes large amounts of CO2 to the total emissions from concrete construction given the high energy demands during manufacture and limestone calcination, totaling to 5 % of the world’s GHG emissions (Worrell et al. 2001).
Now, more than ever, engineers should choose environmentally friendly materials and cross-section dimensions to minimize the impact of their projects as well as the consumption of natural resources. Optimization is a suitable tool to minimize costs and, hence, reduce construction material consumption. Although optimization of construction costs is proportional to that of the CO2 emissions, the diversity of materials used in concrete production requires more detailed analysis. Several recent studies have used multiobjective optimization algorithms to compare CO2 efficiency and the cost design applied to reinforced concrete (RC) structures (Paya et al. 2008; Payá-Zaforteza et al. 2009; Yepes et al. 2012; Martínez-Martín et al. 2012; Camp and Huq 2013). These studies have only examined emissions at the different stages of production and placement; therefore, this research aims to analyze the cradle to grave life cycle of concrete, especially blended cement mixes. Thus, and in line with Jönsson et al. (1988), the life cycle of alternative construction materials can be assessed before the type of material is chosen for a particular construction project.
The current sustainability debate has seemingly overlooked the question of how concrete reabsorbs CO2 from the atmosphere during its service life and in its secondary life following recycling. Leber and Blakey (1956) used chemical analysis to estimate the effects of CO2 on mortars and concrete, assuming that all absorbed CO2 reacts with the lime to form calcium carbonate. Aïtcin (2000) later affirmed the importance of not just the cost of 1 m3 of concrete but rather the cost of 1 MPa or 1 year of the life cycle of a structure. Collins (2010) studied the inclusion of carbonation during the life cycle of built and recycled concrete, assuming a primary life of 100 years plus a 30-year secondary life. In contrast, this research sets out to first assess durability as determined by the lifetime of a structure and then compare the emissions per year of blended cements. As durability allows for the recovery of emissions from previous stages, scientists have searched for ways to extend concrete durability. However, the RC durability can be substantially reduced by degradation processes of environmental or functional origins (Angst et al. 2009; Guzmán et al. 2011). In fact, durability is also fundamental for good conceptual design, quality management in construction, and a good maintenance plan.
From the point of view of cement manufacturers, the carbonation process can be identified as recarbonation because the final product, calcium carbonate, is chemically the same as one of cement’s primary raw ingredients. This process has been assessed using three different models: theoretical models (Papadakis et al. 1991), experimental models (Jiang et al. 2000), and models based on the diffusion theory and practical testing (Houst and Wittmann 2002). Concrete carbonation can completely dissolve the protective layer inducing corrosion, which may lead to a shortened service life. The rate of ingress of the aggressive agents depends on the quality of the porosity, the permeability of the concrete cover, and wetting and drying conditions (Bertolini et al. 2004). Nevertheless, concrete carbonation captures CO2 and compensates for the emissions from other stages of the life cycle. If carbon capture is ignored (Flower and Sanjayan 2007), emission rates may be overestimated by as much as 13–48 %, depending on the type of cement and the use of recycled concrete during its secondary life (Collins 2010). About two thirds of the calcination emissions can be reabsorbed, if the crushed concrete is exposed for 30 years (Dodoo et al. 2009). In fact, 70 % of the CO2 released in cement production would be recaptured by the hardened concrete within 100 years (Börjesson and Gustavsson 2000). However, this effect is largely dependent on the recycling practices in each country (Pade and Guimaraes 2007).
The development of blended cements has resulted in CO2 savings, since Portland cement is replaced with either blast furnace slag (BFS), a by-product of the steel industry, or fly ash (FA), a by-product of burning coal. It is known that the mix design can be adjusted in order to avoid changes in the properties of blended cement concrete. The variation in the mass of raw materials in the mix design can lead to differences in GHG emissions of less than 0.2 %, and there is only a negligible effect on embodied water (O’Brien et al. 2009), hence this study will assume the same mix design (Table 1).
The purpose of this study is, therefore, to quantify GHG emissions as a function of the cement used during the concrete life cycle and to determine if the reduction in production emissions of blended cements compensates for the reduced durability and CO2 capture. The paper is organized following the different stages of concrete life, incorporating durability and CO2 capture during the use stage and after demolition. A case study provides the life cycle assessment (LCA) of a RC column.
2 Materials and methods
Although several tools have been developed to measure sustainability, none are more complete than the LCA, as it evaluates the inputs–outputs as well as the potential environmental impacts of a product system throughout its life cycle. The LCA is divided into four phases: the goal and scope definition phase, the inventory analysis phase, the impact assessment phase, and the interpretation phase (ISO 2006). In the construction industry, only the inventory analysis and interpretation are common and are known as a life cycle inventory study. The LCA covers every product process, from obtaining the raw materials and processing them, to demolition and recycling; hence, in this study, the LCA is useful to compare the environmental impact of different concrete mixes.
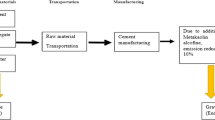
Several complications arise when a construction system is assessed. The cradle to grave life cycle of concrete is longer than other industrial systems, and numerous product processes are often entwined. Figure 1 illustrates the four stages within a construction system. The production stage involves obtaining raw materials and processing them, transport to the concrete plant, and the mixing. This stage also takes into account steel production of bars and transport to the building site. The second stage is construction, that is, when the structure is actually built. The next stage, and the most long-lasting one, is the use stage. Finally, once the structure’s life has ended, it is pulled down, and the demolished concrete is crushed and recycled as gravel filling material.
2.1 Production stage
The concrete production stage is the main contributor to CO2 emissions, specifically those derived from quarrying, crushing, and cement manufacture. The high CO2 emissions are due to the energy demands during manufacture and limestone calcination. Hence, cement is commonly replaced with cementitious materials like FA and BFS. It is considered that these materials do not emit CO2, except for post-process and transport emissions, since they are waste products from other materials production. Besides, FA is collected at power stations for air quality reasons, even if it is not to be used in concrete production (O’Brien et al. 2009). Additionally, FA needs little processing after being collected, so it emits less CO2 than BFS.
As presented in Table 2, the environmental data analyzed in this study include the production emissions of all materials and their transport distances, transport emissions by lorry, concrete plant emissions and their transport distances, and concrete transport emissions by the mixer. Production emissions were taken from European associations, whose reports are corroborated and used by many companies. The source of energy has a strong influence on primary energy use and net CO2 balance. Data on industrial energy use can vary depending on how the said data were obtained. Since industrial technologies differ in each country, in this study, data were selected for Spanish industrial energy (Institute for Diversification and Energy 2010). As this study assumed a manufacturing process after considering recycling, data emissions for reinforcing steel bars were taken from Yiwei el al. (2011). It is worth noting that recycling rates in steel construction average about 85 %, and CO2 emissions of steel made from ore total around 1.8 kg CO2 per kilogram of steel, while scrap using the basic oxygen furnace emit about 0.4 kg CO2 per kilogram of steel (World Steel Association 2010). Transport distances vary depending on the locations; nevertheless, a standard building in central Valencia was assumed, and distances were doubled to account for the return trip. All concrete components were transported from the nearest factory to the concrete plant; the reinforcing steel bars were transported directly to the building site. Once the concrete was made, a mixer transported it from the concrete plant to the building site. Transport emissions were based on the transport vehicle specifications given by national contractors and subcontractors, and energy emission data were taken from the Institute for Diversification and Energy Saving (2010).
2.2 Construction stage
During the construction stage, no differences were noted between the two blended cement mixes. In addition, the emissions during construction were almost negligible compared to those during production. So, it was not an outstanding stage, and therefore, only pumping and vibration for the construction activities were studied. Table 2 shows the total construction emissions. Like transport emissions, construction emissions were obtained from a survey of national contractors and subcontractors, and energy emission data were taken from the Institute for Diversification and Energy Saving (2010).
2.3 Use stage
Structure life is influenced by atmospheric agents, which limit RC durability. According to the EHE code (M. Fomento 2008), there are two main types of concrete depletion: carbonation and chloride penetration (Saassouh and Lounis 2012). This study focuses on carbonation not only because it leads to the depletion of concrete alkalinity and thus steel embedded corrosion, but also because CO2 is captured, and the emissions from other stages can be subtracted.
Carbonation is the main cause of RC decay. The degree of carbonation depends on three decisive factors:
-
The w/b is decisive as far as porosity is concerned. Porosity can speed up carbonation. Cement replacement increases carbonation rates, as the pozzolanic reaction reduces the alkaline reserve (Zornoza et al. 2009).
-
Decisive as well is the concentration of CO2 in the surrounding air and the specific climatic conditions. The carbonation rate is higher in concrete protected against rain, as the pores are partially blocked by the rain (Galán 2011). If we compare the differences between protected and unprotected concrete, it is clear that humidity is one of the most influential factors in concrete carbonation (Galán et al. 2010).
-
The depth of the embedded steel is also decisive in terms of RC decay. The greater the depth of the embedded steel, the longer it takes for CO2 to carbonate the steel protection.
This study evaluates durability according to the EHE code (M. Fomento 2008), which is based on the Tuutti (1982) model. The service life of RC structures is divided in two distinct phases. The first phase is initiation of corrosion; carbonation penetrates in the concrete cover and leads to the loss of reinforcement passivity. The second phase involves the propagation of corrosion that begins when the steel is depassivated and ends when a limiting state is reached, beyond which consequences of corrosion can no longer be tolerated. The service life is therefore calculated as the sum of the two phases:
Where t are the years of service life, d is concrete cover (in millimeter), k is the carbonation rate coefficient, ∅ is the bar diameter (in millimeter), and v c is the corrosion speed (in micrometers per year). The carbonation rate coefficient varies with rain-protected and unprotected concretes, the percentage of occluded air, and the cement type. The rate of corrosion is dependent on the type of exposure of the structural element. Table 5 indicates the values of k and v c as obtained from the EHE code (M. Fomento 2008).
Service life defines the moment when concrete deterioration reaches an unacceptable limit. The use stage is considered finalized when the service life ends. Thus, there is no need for maintenance during this stage. However, we can lengthen the technical lifetime of a RC structure, but this will involve high costs for maintenance and repair. It is crucial to consider service life to calculate annual emissions, and therefore, a more reliable benchmark.
Once the life of the structure is known, CO2 capture can be assessed during the service life. Pade and Guimaraes (2007), Dodoo et al. (2009), and Collins (2010) used Eq. (2) to estimate CO2 capture based on the predictive models of Fick’s first law of diffusion and the study by Lagerblad (2005). CO2 capture can be calculated here as the product of the carbonation rate coefficient k, which was calculated previously in the durability assessment, the structure service life t, the quantity of Portland cement per cubic meter of concrete c, the amount of CaO content in Portland cement CaO (assumed to be 0.65), the proportion of calcium oxide that can be carbonated r (assumed to be 0.75, Lagerblad 2005), the exposed surface area of concrete A, and the chemical molar fraction M (CO2/CaO is 0.79.) It can be said that the reduction in CO2 capture is proportional to the reduction in Portland cement in blended cements:
2.4 Demolition stage
There is a greater degree of carbonation at the end of concrete’s structural life, when it is typically crushed for reuse as an aggregate. This results from the significant increase in surface area of the aggregate, which helps to speed up CO2 absorption, even when used in ground works. Nowadays, crushed concrete is increasingly reused as an aggregate in the production of new concrete, although the mechanical and durability properties are worse in a concrete with recycled aggregates. To obtain the same compressive strength and the same workability as unrecycled concrete, it is possible to increase the amount of cement (about 5 %) (Marinkovic et al. 2010); however, this will increase CO2 emissions once more. Nevertheless, limiting the additional amount of cement used for recycled concrete to about 10 % keeps the impact in a comparable range (Knoeri et al. 2013).
In this study, the crushed concrete was employed as gravel-filling material, which will expose new surfaces to air for a significant period of time with a fresh paste, and a new cycle of CO2 uptake will start. Even though a filling material submerged in water or buried in soil carbonates more slowly, with time, all concrete structures will indeed carbonate (Lagerblad 2005). Recycling or recovering concrete materials conserves natural landfill materials and eliminates the associated environmental costs of exploitation and transportation. These additional savings in emissions were not taken into account in this study. Table 2 summarizes the CO2 emissions arising from the fuel consumed during demolition and crushing. The data are based on a survey of national contractors and subcontractors, and energy emission data were published by the Institute for Diversification and Energy Saving (2010).
3 Results
As the objective of this study was to analyze carbonation as a function of concrete mix, results are presented here from the LCA of a RC building column made with FA and BFS blended cements. The column is 3 m high, and the cross-section is 30 × 30 cm2. Steel reinforcement consisted of four 20-mm diameter steel bars. The concrete cover is 30 mm. The concrete mix, specified in Table 1, provides a compressive strength value of 25 MPa. In this study, the functional unit is the RC column during the lifetime of the structure.
To obtain the production emissions, the amount of concrete mix design and steel were multiplied by the emissions associated with their materials production and transport by lorry. The concrete manufacture and transport by mobile agitator were also considered. Construction and demolition emissions are the product of the emission factors, shown in Table 2, multiplied by the concrete volume.
3.1 Emissions from blended cement production
Table 3 shows data for the CO2 balances from cement production and transport to the concrete plant for Portland cement and three types of BFS blended cement (CEN 2011). Table 4 illustrates the results for Portland cement and two types of FA blended cement (CEN 2011).
The overall CO2 balance is the sum of the emissions from production and transport of cement. Greater reductions were achieved using BFS, since the amount of Portland cement that can be replaced by FA is less than BFS. The greatest effect was observed in CEM III/B, about a 70 % reduction compared to CEM Portland. Concerning the FA blended cement, CEM II/B-V achieved a 36 % reduction compared to Portland. If we examine two blended cements with the same replacement quantities, like CEM II/B-S and CEM II/B-V, the results reveal that the FA replacement is more environmentally friendly, since this material has fewer product emissions and shorter transport distances.
3.2 Service life
Durability, according to the EHE code (M. Fomento 2008), depends on the cement used. The column was considered to be protected against rain, and the percentage of occluded air was less than 4.5 %. Table 5 presents the results for the durability of blended cements. Results for CEM Portland, CEM II/B-S, and CEM II/A-V were the same. The quantity of cement replacement is so low that the code uses the same carbonation rate coefficient as for Portland. Similar results were obtained for CEM III/A, CEM III/B, and CEM II/B-V, which all had a higher carbonation rate coefficient and therefore a 10 % shorter service life.
3.3 Carbonation during use stage and after demolition
During the use stage, CO2 capture depends on the type and quantity of cement and the service life; hence, there is less carbonation in blended cement than in Portland cement. Carbonation of CEM III/B during the use stage was 22 % than that of Portland cement, reflecting a 10 % reduction in service life with an 80 % BFS replacement for Portland cement. CEM II/B-V with 35 % FA and 65 % Portland cement showed the same reduction in service life along with a CO2 capture equal to 71 % of Portland cement capture. The calcium oxide that did not carbonate during the use stage can be carbonated after demolition (Table 6). Unsurprisingly, blended cements with higher carbonation rate coefficients captured more CO2 during the use stage than after demolition.
We assumed that during the post-use stage, all concrete will be carbonated. Lagerblad (2005) reported the carbonation rate coefficients according to the environmental factors, since concrete submerged in water or buried in soil carbonates but not as fast. Based on these carbonation rate relations, the crushed concrete aggregate (20-mm diameter) takes 44.44, 6.25, 177.78 or 100.00 years to carbonate, depending on whether it is exposed to rain (k = 1.5 mm/year0.5), protected (k = 4 mm/year0.5), wet (k = 0.75 mm/year0.5), or buried (k = 1 mm/year0.5), respectively. The results show the importance of the exposure environment.
3.4 Emissions summary
Table 7 presents the results of life cycle emissions. These results are not surprising, if we remember that the cement production stage contributes most CO2 emissions. Figure 2 shows CO2 emissions rising through every stage of concrete life, using FA and BFS blended cements. There is a huge difference between Portland cement and blended cement, when CO2 emissions are taken into account up to the construction stage. CO2 emissions for Portland cement and blended cement after the use stage and primarily after demolition were not significant, since there was less carbonation in blended cement than in Portland cement.
Concerning the concrete mix design with Portland cement, CO2 capture during the use stage represents 22 % of the total CO2 emissions. If the demolished concrete is crushed and recycled as gravel-filling material, concrete made with Portland cement, FA (35 % FA), and BFS blended cements (80 % BFS) captures 47, 41, and 20 % of the CO2 emissions, respectively. It is essential to guarantee that the gravel is exposed to atmospheric conditions after being recycled to assure complete carbonation. These results confirmed Collins’ conclusions (2010). Besides, half of all the CO2 emissions in the cement production stage come from the calcination of limestone (Dodoo et al. 2009). The results from our study also show that 66 % of the calcination emissions are reabsorbed by Portland carbonation during the post-use stage. It is worth noting that if we assess the carbon implications of an entire building, the carbonation uptake will not be so significant (Dodoo et al. 2009).
As durability depends on the cement, it was necessary to assess the ratio of CO2 emissions per year (see Table 8). The data obtained indicate that the reduction in CO2 per year was not as remarkable as that of the total emissions. CEM III/B reduced CO2 emissions by 70 % due to its production, and in terms of CO2 emissions per year, the reduction dropped to 20 %. As for CEM II/B-V, emissions fell from 36 to 7 %. Besides, the loss of durability of the blended cements with high rates of replacement resulted in higher values for CO2 emissions per year compared to the lower replacement rates.
4 Conclusions
This study aimed to evaluate the CO2 emissions of a RC building column made with BFS and FA blended cements. The emissions are analyzed during the life cycle of the column, whose lifetime finishes when the service life is exhausted and, after that, the column is demolished and used as gravel-filling material. Concrete carbonation is assessed during the service life and after demolition. The total emission, after subtracting the CO2 captured, is divided throughout the service life to obtain the emissions per year. Under the assumptions adopted in this specific case study, the following conclusions may be drawn:
-
Replacing Portland cement with FA rather than BFS lowers the material emission factor, since FA needs less processing after being collected and more coal-fired power stations mean shorter FA transport distances. However, greater reductions are achieved using BFS, since the amount of cement that can be replaced is higher.
-
Blended cements with high rates of cement replacement, like CEM III/A (50 % BFS), CEM III/B (80 % BFS), and CEM II/B-V (35 % FA), decreases service life about 10 % due to the higher carbonation rate coefficients. The loss of durability of these blended cements leads to more CO2 emissions per year compared to lower replacement rates.
-
The reduction in CO2 capture is proportional to the replacement of Portland cement in blended cements. For instance, during the use stage, CEM III/B (80 % BFS) captures only 22 % of the CO2 captured by Portland cement, and considering demolition and reuse as gravel in land filling, it captures 20 % of the CO2 captured by Portland cement. In this case, CEM III/B carbonation is higher during the use stage than after demolition.
-
To obtain reliable results in a LCA, it is crucial to consider carbonation during the use stage and after demolition. Concerning the concrete mix design with Portland cement, the CO2 capture during the use stage represents 22 % of the total CO2 emissions. If the demolished concrete is crushed and recycled as gravel-filling material, the reduction in CO2 emissions reaches 47 %. Concrete made with FA (35 % FA) and BFS blended cements (80 % BFS) capture 41 and 20 % of CO2 emissions, respectively. Thus, it is essential to guarantee the gravel atmospheric exposure after recycling to assure complete carbonation. If the gravel is buried, the carbonation rate may drop, but over time, it too will carbonate.
-
Blended cement production for CEM III/B (80 % BFS) emits 70 % less GHG than Portland cement, although considering concrete life cycle with CEM III/B, there are only 20 % fewer emissions per year than with Portland cement. Regarding CEM II/B-V, the emissions drop from 36 to 7 %. Therefore, the slight reduction in production emissions of blended cements compensates the reduced durability and CO2 capture.
References
Aïtcin PC (2000) Cements of yesterday and today: concrete of tomorrow. Cem Concr Res 30(9):1349–1359
Angst U, Elsener B, Larsen C (2009) Critical chloride content in reinforced concrete—a review. Cement Concr Res 39(12):1122–1138
Berge B (2000) The ecology of building materials. Architectural Press, Oxford
Bertolini L, Elsener B, Pedeferri P, Polder R (2004) Corrosion of Steel in Concrete—Prevention Diagnosis. Repair, Wiley-VCH, Weinheim
Börjesson P, Gustavsson L (2000) Greenhouse gas balances in building construction: wood versus concrete from life cycle and forest land-use perspectives. Energy Policy 28(9):575–588
Camp CV, Huq F (2013) CO2 and cost optimization of reinforced concrete frames using a big bang-crunch algorithm. Eng Struct 48:363–372
CEN (2011) EN 197–1: Cement. Part 1: Composition, specifications and conformity criteria for common cements. European Committee for Standardization, Brussels
CIWMB (2000) Designing with vision: a technical manual for materials choices in sustainable construction. California Integrated Waste Management Board, Sacramento
Collins F (2010) Inclusion of carbonation during the life cycle of built and recycled concrete: influence on their carbon footprint. Int J Life Cycle Assess 15(6):549–556
Database BEDEC (2012) Institute of Construction Technology of Catalonia. Barcelona, Spain
Dodoo A, Gustavsson L, Sathre R (2009) Carbon implications of end-of-life management of building materials. Resour Conserv Recy 53(5):276–286
ECO-SERVE Network Cluster 3 (2004) Baseline Report for the Aggregate and Concrete Industries in Europe. European Commission, Hellerup: http://www.eco-serve.net/uploads/479998_baseline_report_final.pdf, accessed 10 September 2012
European Federation of Concrete Admixtures Associations (2006) Environmental Product Declaration (EPD) for Normal Plasticizing admixtures. Environmental Consultant, Sittard: http://www.efca.info/downloads/324%20ETG%20Plasticiser%20EPD.pdf, accessed 13 October 2012
Galán I (2011) Carbonatación del hormigón: combinación de CO2. Dissertation, Universidad Complutense de Madrid, Spain
Galán I, Andrade C, Mora P, Sanjuan MA (2010) Sequestration of CO2 by concrete carbonation. Environ Sci Technol 44(8):3181–3186
Flower DJM, Sanjayan JG (2007) Greenhouse gas emissions due to concrete manufacture. Int J Life Cycle Assess 12(5):282–288
Guzmán S, Gálvez JC, Sancho JM (2011) Cover cracking of reinforced concrete due to rebar corrosion induced by chloride penetration. Cement Concr Res 41(8):893–902
Houst YF, Wittmann FH (2002) Depth profiles of carbonates formed during natural carbonation. C Cement Concr Res 32(12):1923–1930
Institute for Diversification and Energy Saving (2010) Conversion factors of primary energy and CO2 emissions of 2010. M. Industria, Energía y Turismo, Madrid, Spain: http://www.idae.es/index.php/mod.documentos/mem.descarga?file=/documentos_Factores_Conversion_Energia_y_CO2_2010_0a9cb734.pdf, accessed 10 September 2012
ISO (2005) ISO/TC 71—Business plan. Concrete, reinforced concrete and prestressed concrete. International Organization for Standardization (ISO), Geneva, Switzerland
ISO (2006) ISO 14040: Environmental management—life-cycle assessment—principles and framework. International Organization for Standardization, Geneva, Switzerland
Jiang L, Lin B, Cai Y (2000) A model for predicting carbonation of high-volume fly ash concrete. Cement Concr Res 30(5):699–702
Jönsson A, Björklund T, Tillman AM (1988) LCA of concrete and steel building frames. Int J Life Cycle Assess 3(4):216–224
Knoeri C, Sanyé-Mengual E, Althaus HJ (2013) Comparative LCA of recycled and conventional concrete for structural applications. Int J Life Cycle Assess 18(5):909–918
Lagerblad B (2005) Carbon dioxide uptake during concrete life-cycle: State of the art. Swedish Cement and Concrete Research Institute, Stockholm
Leber I, Blakey FA (1956) Some effects of carbon dioxide on mortars and concrete. J Am Concr Inst 53:295–308
Fomento M (2008) EHE-08; Code of Structural Concrete. M. Fomento, Madrid, Spain
Marinkovic S, Radonjanin V, Malešev M, Ignjatovic I (2010) Comparative environmental assessment of natural and recycled aggregate concrete. Waste Manag 30(11):2255–2264
Martinez-Martin FJ, Gonzalez-Vidosa F, Hospitaler A, Yepes V (2012) Multi-objective optimization design of bridge piers with hybrid heuristic algorithms. J Zhejiang Univ-SCI A 13(6):420–432
O’Brien KR, Ménaché J, O’Moore LM (2009) Impact of fly ash content and fly ash transportation distance on embodied greenhouse gas emissions and water consumption in concrete. Int J Life-cycle Assess 14(7):621–629
Pade C, Guimaraes M (2007) The CO2 uptake of concrete in a 100-year perspective. Cem Concr Res 37(9):1384–1356
Papadakis VG, Vayenas CG, Fardis MN (1991) Fundamental modeling and experimental investigation of concrete carbonation. ACI Mater J 88(4):363–373
Payá I, Yepes V, González-Vidosa F, Hospitaler A (2008) Multiobjective optimization of reinforced concrete building by simulated annealing. Comput-Aided Civ Inf 23(8):596–610
Payá-Zaforteza I, Yepes V, Hospitaler A, González-Vidosa F (2009) CO2-efficient design of reinforced concrete building frames. Eng Struct 31(7):1501–1508
Saassouh B, Lounis Z (2012) Probabilistic modeling of chloride-induced corrosion in concrete structures using first- and second-order reliability methods. Cement Concrete Comp 34(9):1082–1093
The Concrete Centre (2009) The Concrete Industry Sustainability Performance Report. The Concrete Center, Camberley: http://www.admixtures.org.uk/downloads/Concrete%20Industry%20Sustainable%20Performance%20Report%202009.pdf, accessed 9 September 2012
Tuutti K (1982) Corrosion of steel in Concrete. CBI Forskning Research Report, Swedish Cem Concr Res Inst. Stockholm, Sweden
Weil M, Jeske U, Schebek L (2006) Closed-loop recycling of construction and demolition waste in Germany in view of stricter environmental threshold values. Waste Manage Res 24(3):197–206
World Steel Association (2010) Fact sheet: the three Rs of sustainable Steel. World Steel Association, Brussels: http://www.steel.org/Sustainability/~/media/Files/SMDI/Sustainability/3rs.ashx, accessed 15 September 2012
Worrell E, Price L, Martin N, Hendriks C, Meida LO (2001) Carbon dioxide emissions from the global cement industry. Annu Rev Energy Environ 26:303–329
Yepes V, González-Vidosa F, Alcalá J, Villalba P (2012) CO2-optimization design of reinforced concrete retaining walls based on a VNS-threshold acceptance strategy. J Comput Civ Eng 26(3):378–386
Yiwei T, Qun Z, Jian G (2011) Study on the Life-cycle Carbon Emission and Energy-efficiency Management of the Large-scale Public Buildings in Hangzho. China. International Conference on Computer and Management, Wuhan, pp 546–552
Zornoza E, Payá J, Monzó J, Borrachero MV, Garcés P (2009) The carbonation of OPC mortars partially substituted with spent fluid catalytic catalyst (FC3R) and its influence on their mechanical properties. Const Build Mater 23(3):1323–1328
Acknowledgments
This research was financially supported by the Spanish Ministry of Science and Innovation (research project BIA2011-23602). The authors thank the anonymous reviewers for their constructive comments and useful suggestions. The authors are also grateful for the thorough revision of the manuscript by Dr. Debra Westall.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Matthias Finkbeiner
Rights and permissions
About this article
Cite this article
García-Segura, T., Yepes, V. & Alcalá, J. Life cycle greenhouse gas emissions of blended cement concrete including carbonation and durability. Int J Life Cycle Assess 19, 3–12 (2014). https://doi.org/10.1007/s11367-013-0614-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-013-0614-0