Abstract
Purpose
Perforated diverticulitis with advanced generalized peritonitis is a life-threatening condition requiring emergency operation. To reduce the rate of colostomy formation, a new treatment algorithm with damage control operation, lavage, limited closure of perforation, abdominal vacuum-assisted closure (VAC; V.A.C.®), and second look to restore intestinal continuity was developed.
Methods
This algorithm allowed for three surgical procedures: primary anastomosis ± VAC in stable patients (group I), but damage control with lavage, limited resection of the diseased colonic segment, VAC and second-look operation with delayed anastomosis in patients with advanced peritonitis or septic shock (group II), and Hartmann procedure was done for social reasons in stable patients (group III)
Results
All 27 consecutive patients (16 women; median age 68 years) requiring emergency laparotomy for perforated diverticulitis (Hinchey III/IV) between October 2006 and September 2008 were prospectively enrolled in the study. No major complications were observed in group I (n = 6). Nine patients in group II (n = 15) had intestinal continuity restored during a second-look operation, of whom one patient developed anastomotic leakage. The median length of stay at intensive care unit was 5 days. Considering an overall mortality rate of 26% (n = 7), the rate of anastomosis in surviving patients was 70%.
Conclusions
Damage control with lavage, limited bowel resection, VAC, and scheduled second-look operation represents a feasible strategy in patients with perforated diverticulitis (Hinchey III and IV) to enhance sepsis control and improve rate of anastomosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perforated diverticulitis with generalized peritonitis is a life-threatening condition with a mortality rate of up to 13% for Hinchey score III and 43% for Hinchey score IV [1–5, 14]. The surgical intervention should realize immediate source control, efficient clearance of the abdomen, and preferably restoration of the intestinal continuity with prevention of recurrence [19]. Up to now, for perforated diverticulitis with generalized peritonitis, the Hartmann procedure is considered as the surgical gold standard by many surgeons [5, 6, 13, 20]. However, Hartmann reversal with restoration of the bowel continuity is associated with significant morbidity: Anastomotic leak rates of up to 30% and mortality rates of up to 15% have been reported [7]. Furthermore, colostomy complications often require additional surgical procedures with increased morbidity. Many patients (20% to 50%) even may never have their colostomy reversed because of serious perioperative risks [8–11].
Therefore a lot of alternative treatment strategies for perforated diverticulitis have been presented in the last years to avoid colostomy and reduce morbidity: Essentially, primary anastomosis has become a promising alternative with acceptable mortality and a low risk of anastomotic leakage [8, 10, 15–18]. However, studies that compare Hartmann procedure and resection with primary anastomosis are hampered by an important selection bias: In fact, primary anastomosis is often reserved for patients with no or little co-morbidity and a lesser degree of peritoneal contamination [5, 8, 12]. Moreover at the moment of the emergency operation, the further evolution of the patient can hardly be estimated but should be taken into account for the decision to make an anastomosis. Recently abdominal vacuum-assisted closure (VAC; V.A.C.® Abdominal Dressing System, V.A.C.® ATS®, KCI, Austria) has been proposed as a new treatment option in patients with severe abdominal trauma, abdominal compartment syndrome, or complex septic intraabdominal complications when primary closure is not possible and repeat abdominal entries are necessary [21–25].
Due to our favorable experience with the VAC system in various intraabdominal complications, we developed the later-described algorithm for the surgical treatment of patients with advanced generalized peritonitis due to perforated diverticulitis. This algorithm was applied in all patients either presenting severe peritonitis not allowing a safe anastomosis or patients in septic shock not tolerating a long operating procedure. With the aid of VAC, we tried to accelerate control of sepsis and clearance of the abdominal cavity, to reduce the rate of colostomy formation in a second-look operation in an elective setting. The aim of this analysis of the first 2 years is to evaluate the feasibility of this surgical strategy and analyze its impact on morbidity and mortality.
Materials and methods
Study design and surgical technique
All consecutive patients operated on for perforated sigmoid diverticulitis with purulent or fecal peritonitis (Hinchey III and IV) between October 2006 and September 2008 were included in this prospective study (informed consent). Diverticulitis was staged according to Hinchey’s classification [26]. The Mannheim peritonitis index (MPI) was used to calculate the severity of peritonitis (Table 1) [27]. After urgent laparotomy and identification of the perforation site, the surgeon had to decide on the treatment modality according to an algorithm that includes three different treatment options (Table 2). The decision on which strategy to choose depended on the local situation (extent of peritonitis and bowel inflammation) and the clinical presentation of the patient (ASA score, pre- or intraoperative deterioration, and need for catecholamines). In any case, all quadrants of the peritoneal cavity were generously irrigated until the irrigation liquid became clear. Whenever an anastomosis was done, resection was performed accordingly to eliminate the complete high pressure zone with mobilization of the left colonic flexure.
Strategy I (Sigmoid resection with primary anastomosis; group I) was executed in patients with acceptable intraoperative conditions tolerating a longer operating time (no septic shock and no organ failure) and a local situation allowing a safe bowel anastomosis. The use of VAC therapy was additionally indicated in cases of severe peritonitis with gross contamination or massive bowel edema to improve clearance of the peritoneal cavity or to permit a reevaluation of the anastomosis in a second-look operation.
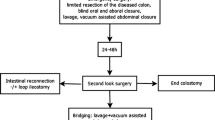
Strategy II (Sigmoid resection with blind colonic ends, VAC, and second look; group II) was indicated in patients with poor pre- and intraoperative conditions (septic shock and organ failure) and a significant need for catecholamines due to perioperative deterioration requiring rapid source control. After adequate lavage, a limited resection of the diseased colonic segment (blind ending colonic stumps) or even only closure of the perforation site by single sutures was performed. In order not to open clean spaces, a mobilization of the colon was avoided, and the procedure was completed as fast as possible. The decision to restore continuity (IIa) or create a colostomy (IIb) was postponed to a second-look operation (24–48 h after emergency laparotomy) in an elective setting supervised by a colorectal surgeon. Further continuation of VAC therapy was done to improve clearance of the abdominal cavity or reevaluate a critical anastomosis according to the surgeons’ discretion.
Strategy III (Hartmann procedure; group III) corresponded to the classical Hartmann procedure and was strictly reserved for bedridden patients in permanent need of nursing, for patients with fecal incontinence, and for patients with limited perspective of life, but in stable conditions tolerating the operative procedure.
Application of VAC system
Before application of the intraabdominal VAC system, care was taken to cover the small bowel and any sutures with omentum. After the positioning of the intraabdominal part of the VAC system in the peritoneal cavity, four capillary drains were placed along the parietal peritoneum above the polyvinyl layer to all four quadrants to enhance the suction effect (Fig. 1).To prevent retraction of the abdominal wall by musculofascial tension, the fascia was approximated using four vessel loop sutures (Fig. 2). Then the abdomen was closed with subcutaneously placed black polyurethane foam (V.A.C.® GranuFoam®, KCI, Austria) and an adhesive film as described by the manufacturer. Negative pressure was adjusted to 125 mm Hg. All subsequent surgical interventions such as change of the VAC system, closure of the abdominal wall, anastomosis, or colostomy creation were performed as scheduled operations.
Results
Patients
Between October 2006 and September 2008, 27 consecutive patients with perforated diverticulitis and generalized peritonitis Hinchey score III or IV requiring emergency laparotomy (Fig. 3) were treated according to the above mentioned algorithm. Clinical data are shown in Table 3. Sixteen (59%) patients were women, and the median age was 68 years (range 35 to 89). The median BMI was calculated to be 24 kg/m2 (range 17.3–32.5). Thirteen patients (48%) were already hospitalized at other departments for different serious medical conditions (subdural hematoma, cerebral or spinal metastases, glioblastoma multiforme, fractured spine or femur, implantation of a hip prosthesis, myocardial infarction, pneumonia, adnexitis, adnexectomy, and myasthenia gravis). Preoperative signs of sepsis with acute renal, pulmonary, and cardiac dysfunction were observed in ten patients. All but five patients had at least one clinical relevant coexistent disease: A cardiovascular disease was diagnosed in eight patients (coronary heart disease, myocardial infarction, heart insufficiency, pulmonary embolism, and stroke), chronic renal failure in seven patients, COPD in five patients, and diabetes mellitus in three patients. Three patients suffered from malignancy, and eight patients (30%) had a continuous therapy with steroids or immunosuppressive agents (arthritis, COPD, cerebral edema, kidney transplantation, and lung transplantation). Calculation of the Mannheim peritonitis index yielded a median (range) score of 24 (6–39) points.
Surgical management and complications
The operative management of the patients is depicted in Table 3 and Fig. 3. Three patients underwent resection of the sigmoid colon with primary anastomosis and instant closure of the abdominal wall without VAC, and three patients had resection of the sigmoid colon with primary anastomosis and VAC (group I). A primary Hartmann procedure was performed in six patients (group III). Groups I and III included 22% of study patients, each. The majority of patients (n = 15; 56%) was treated according to strategy II with limited resection of the diseased segment or simple closure of the perforation site and VAC at emergency laparotomy. A total of 17 patients required catecholamines before or during the first surgical procedure, and three more patients required catecholamines postoperatively. One patient developed cardiac arrest during the initiation of anesthesia and was successfully resuscitated. The operation time of the first surgical procedure ranged from 60 to 210 min with a median value of 170, 100, and 140 min for groups I, II, and III, respectively. After 24 to 36 h, all patients with VAC underwent a scheduled second look to decide on the further surgical management: Nine patients were considered for primary anastomosis (strategy IIa); in six cases (strategy IIb), a Hartmann procedure was necessary due to the lack of clinical improvement, further deterioration, or persisting peritonitis. The total number of operations adds up to 58 interventions including six unplanned procedures for complications in three patients due to anastomotic leakage (patient 15) and dehiscence of the abdominal wall (patient 18 and 21). Definitive closure of the abdominal wall could be achieved in all surviving patients without prosthetic grafts. The maximal time interval from emergency laparotomy to VAC device displacement was 7 days with a maximum of four operative procedures. Postoperatively all patients were treated on the surgical intensive care unit (ICU) between 1 and 30 days (median 5 days). Four patients died in the ICU (1, 7, 8, and 30 days after emergency laparotomy), and five patients were transferred to their referring department because of their pre-existing diseases. Six patients were transferred to other hospitals for rehabilitation, and 12 patients were discharged home after a total hospital stay between 7 and 37 days (median 20). Analysis of postoperative morbidity revealed a total number of 59 complications (17, 33, and 9 in groups I, II, and III, respectively). Surgical complications occurred in three patients (11%): One patient after lung transplantation suffered from anastomotic leakage (group II), and two patients presented with abdominal wall dehiscence (17 and 35 days after emergency laparotomy, group II). Infectious complications were observed in 14 patients (52%) including seven catheter-related infections, six superficial wound infections, six urinary tract infections, three intraabdominal abscesses (managed by CT-guided drainage), and two pneumonias. Fifteen patients (56%) developed a significant postoperative organ dysfunction with lethal multiorgan dysfunction syndrome in four patients. Thus, hospital mortality was 15% (three patients in group II and one patient in group III). Two further patients (groups II and III), who were transferred back to the primary hospital, died from their cancer 1 and 6 months after emergency laparotomy, and one patient (group II) with a history of lung transplantation died due to pneumonia 3 months following surgery before scheduled ostomy reversal. Thirteen patients (48%) ended with a colostomy: Seven of these patients died without possibility of ostomy reversal, two patients had their colostomy reversed 6 months after emergency laparotomy, and four patients live with a permanent colostomy with no desire for reversal. Thus, the overall rate of anastomosis mounts to 52% (14/27), but 70% (14/20) for the surviving patients.
Discussion
This study presents a new surgical approach to treat patients with perforated diverticulitis and advanced generalized peritonitis (Hinchey score III and IV) combining emergency laparotomy with the application of abdominal VAC in order to achieve a rapid source control and to reduce the rate of colostomies. The Hartmann procedure certainly represents a convincing or even sometimes unavoidable solution for bedridden persons in permanent need of nursing as well as for patients with pre-existing fecal incontinence. Furthermore, it is not reasonable to go for a primary anastomosis at any rate in patients with pre-existing life-threatening co-morbidities. These considerations justified to treat 22% of patients according to strategy III with a primary Hartmann procedure. Favorable clinical presentation of 22% of patients allowed to follow strategy I performing a primary anastomosis (mean MPI score 21.7). As in this group of patients, no anastomotic leak occurred, and the clinical judgment of the responsible surgeon obviously had been correct. Doubtlessly, the most sophisticated patients were those in group II with septic condition or advanced peritonitis, not allowing a safe anastomosis. In these patients, the aim was to achieve a rapid damage control without obligation to make a premature decision on the definitive surgical management. Thus, the extent of tissue dissection and organ mobilization during the primary procedure was kept as low as possible to prevent propagation of the inflammatory process and augmentation of toxemia. A limited resection by stapling off the diseased colonic segment leaving two blind ending colonic stumps or even only a sutured closure of the perforation site offered a quick and technically easy elimination of the inflammatory focus, thereby avoiding any contamination of the retroperitoneal space. The surgeon was not forced to make a decision toward performing an anastomosis or a colostomy. After application of an abdominal VAC system, the scheduled second-look procedure allowed a decision-making under convenient conditions with the possibility to consider the patients clinical decourse (deterioration or improvement), patient co-morbidities (malignant disease, and prognosis), and to reevaluate the local situation (extent of peritonitis, tissue quality, and bowel edema). The second-look operation was done or observed by an experienced colorectal surgeon. This is an important fact, because it has already been shown that mortality and morbidity of colorectal surgery is significantly higher, when surgical interventions are performed during night duty or outside regular working hours [28, 29]. In addition, the colostomies, which are performed in an emergency setting, often are misplaced and cause subsequent problems of care. In our study, no ostomy-related complications were observed. The simplicity of strategy II as an emergency procedure makes our algorithm feasible and practicable for surgeons with limited experience in the field of colorectal surgery or during night duties. The VAC dressing is an ideal tool when a second look after initial control of a bowel perforation is planned. In contrast to a temporary closure of the abdominal wall with subsequent relaparotomy, the VAC device offers remarkable advantages: reduction of operation time, prevention of a compartment syndrome, clearance of the peritoneal cavity by aspiration of intraabdominal liquid, and conservation of fascial edges [23]. Resolution of peritonitis in our study was obviously adequate since none of the patients had to be reoperated on for a deep surgical site infection. Moreover in contrast to open abdomen treatment, VAC therapy allows to extubate and even mobilize the patient out of bed. Furthermore, we have not observed any fistula formation following the VAC therapy. Dynamic fascial closure and early removal of the VAC system allowed for primary closure of the abdominal wall in all patients. Leaving blind stapled colonic ends up to 48 h in the abdomen may at first glance be considered an unusual approach; however, we did not observe any leakage at the staple lines. This may be explained by the fact that there is no relevant peristalsis in severe peritonitis during the first 2 days. The second-look operation in group II resulted in a bowel anastomosis in nine of 15 patients (60%). Before implementation of this new concept, all of these patients would have been treated by a Hartmann procedure at our hospital. Unfortunately, one patient suffered from anastomotic leakage and had to undergo surgical revision with externalization of the colon. This patient had a previous bilateral lung transplantation and was taking high-dose corticosteroids. Good intraabdominal clearance misled the responsible surgeon to perform an anastomosis, underestimating the risk of long-time application of steroids for healing of the anastomosis. All nonsurvivors in group II (five patients) ended up with a colostomy during the second-look operation. This fact could be interpreted as confirmation of a good patient assessment, since none of these patients would have benefited from a bowel anastomosis. Furthermore, both survivors in group IIb have meanwhile undergone reversal of the colostomy without any complications. In summary, all patients with a long-time survival had a high rate of primary bowel anastomosis (70%). Only four patients live with an intended definitive colostomy. The overall mortality rate in our study amounted to 26% (seven patients), which is acceptable regarding the high percentage of patients with severe co-morbidities and underlying life-threatening diseases. Of these seven patients, four deaths were directly related to the perforated sigmoid diverticulitis (15%). Three patients deceased during long-term follow-up due to other co-morbidities. Thus, the mortality rate in our study is acceptable when compared to other studies [5, 30]. The median length of stay on the intensive care unit was 5 days, showing that all patients improved rather fast after surgery. Obviously, the value of this study is limited by the lack of a comparative group and the small number of patients, but has the advantage that all consecutive patients in a 2-year period were integrated, and therefore, the results approve the feasibility of this new algorithm. Unfortunately there are few experimental data on the specific effect of negative pressure therapy on peritonitis and sepsis available, despite the spreading use of this technique in septic abdomen. Randomized controlled trials with adequate numbers of patients are needed to evaluate the advantages and disadvantages of this concept compared to the current standard procedures.
Conclusion
Surgical treatment of perforated diverticulitis with advanced generalized peritonitis (Hinchey score III and IV) should be individually tailored according to the clinical presentation of the patient and pre-existing co-morbidities. For critically ill patients with advanced peritonitis, a limited bowel resection, or a simple closure of the perforation site and application of an abdominal VAC system as a first step or damage control procedure represent a quick and easy surgical intervention. The decision to perform an anastomosis is postponed to a second-look operation under elective conditions. This strategy allows a high rate of restoration of the intestinal continuity.
References
Chautems RC, Ambrosetti P, Ludwig A, Mermillod B, Morel P, Soravia C (2002) Long-term follow-up after first acute episode of sigmoid diverticulitis: is surgery mandatory?: a prospective study of 118 patients. Dis Colon Rectum 45:962–966
Chapman J, Davies M, Wolff B, Dozois E, Tessier D, Harrington J, Larson D (2005) Complicated diverticulitis: is it time to rethink the rules? Ann Surg 242:576–581
Nagorney DM, Adson MA, Pemberton JH (1985) Sigmoid diverticulitis with perforation and generalized peritonitis. Dis Colon Rectum 28:71–75
Salem L, Anaya DA, Roberts KE, Flum DR (2005) Hartmann’s colectomy and reversal in diverticulitis: a population-level assessment. Dis Colon Rectum 48:988–995
Jacobs DO (2007) Clinical practice. Diverticulitis. N Engl J Med 357:2057–2066
Janes SE, Meagher A, Frizelle FA (2006) Management of diverticulitis. BMJ 332:271–275
Krukowski ZH, Matheson NA (1984) Emergency surgery for diverticular disease complicated by generalized and faecal peritonitis: a review. Br J Surg 71:921–927
Constantinides VA, Tekkis PP, Athanasiou T, Aziz O, Purkayastha S, Remzi FH, Fazio VW, Aydin N, Darzi A, Senapati A (2006) Primary resection with anastomosis vs. Hartmann’s procedure in nonelective surgery for acute colonic diverticulitis: a systematic review. Dis Colon Rectum 49:966–981
Ferzoco LB, Raptopoulos V, Silen W (1998) Acute diverticulitis. N Engl J Med 338:1521–1526
Salem L, Flum DR (2004) Primary anastomosis or Hartmann’s procedure for patients with diverticular peritonitis? A systematic review. Dis Colon Rectum 47:1953–1964
Seetharam S, Paige J, Horgan PG (2003) Impact of socioeconomic deprivation and primary pathology on rate of reversal of Hartmann’s procedure. Am J Surg 186:154–157
Bordeianou L, Hodin R (2007) Controversies in the surgical management of sigmoid diverticulitis. J Gastrointest Surg 11:542–548
Constantinides VA, Heriot A, Remzi F, Darzi A, Senapati A, Fazio VW, Tekkis PP (2007) Operative strategies for diverticular peritonitis: a decision analysis between primary resection and anastomosis versus Hartmann’s procedures. Ann Surg 245:94–103
Vermeulen J, Akkersdijk GP, Gosselink MP, Hop WC, Mannaerts GH, van der HE C, PP WWF, Lange JF (2007) Outcome after emergency surgery for acute perforated diverticulitis in 200 cases. Dig Surg 24:361–366
Chouillard E, Maggiori L, Ata T, Jarbaoui S, Rivkine E, Benhaim L, Ghiles E, Etienne JC, Fingerhut A (2007) Laparoscopic two-stage left colonic resection for patients with peritonitis caused by acute diverticulitis. Dis Colon Rectum 50:1157–1163
Taylor CJ, Layani L, Ghusn MA, White SI (2006) Perforated diverticulitis managed by laparoscopic lavage. ANZ J Surg 76:962–965
Favuzza J, Friel JC, Kelly JJ, Perugini R, Counihan TC (2009) Benefits of peritoneal lavage for complicated sigmoid diverticulitis. Int J Colorectal Dis 24:797–801
Franklin ME Jr, Dorman JP, Jacobs M, Plasencia G (1997) Is laparoscopic surgery applicable to complicated colonic diverticular disease? Surg Endosc 11:1021–1025
Frattini J, Longo WE (2006) Diagnosis and treatment of chronic and recurrent diverticulitis. J Clin Gastroenterol 40:S145–S149
Kohler L, Sauerland S, Neugebauer E (1999) Diagnosis and treatment of diverticular disease: results of a consensus development conference. The Scientific Committee of the European Association for Endoscopic Surgery. Surg Endosc 13:430–436
Barker DE, Kaufman HJ, Smith LA, Ciraulo DL, Richart CL, Burns RP (2000) Vacuum pack technique of temporary abdominal closure: a 7-year experience with 112 patients. J Trauma 48:201–206
Perez D, Wildi S, Demartines N, Bramkamp M, Koehler C, Clavien PA (2007) Prospective evaluation of vacuum-assisted closure in abdominal compartment syndrome and severe abdominal sepsis. J Am Coll Surg 205:586–592
Miller PR, Meredith JW, Johnson JC, Chang MC (2004) Prospective evaluation of vacuum-assisted fascial closure after open abdomen: planned ventral hernia rate is substantially reduced. Ann Surg 239:608–614
Cipolla J, Stawicki SP, Hoff WS, McQuay N, Hoey BA, Wainwright G, Grossman MD (2005) A proposed algorithm for managing the open abdomen. Am Surg 71:202–207
Teixeira PG, Salim A, Inaba K, Brown C, Browder T, Margulies D, Demetriades D (2008) A prospective look at the current state of open abdomens. Am Surg 74:891–897
Hinchey EJ, Schaal PG, Richards GK (1978) Treatment of perforated diverticular disease of the colon. Adv Surg 12:85–109
Linder MM, Wacha H, Feldmann U, Wesch G, Streifensand RA, Gundlach E (1987) The Mannheim peritonitis index. An instrument for the intraoperative prognosis of peritonitis. Chirurg 58:84–92
Ansari MZ, Collopy BT, Hart WG, Carson NJ, Chandraraj EJ (2000) In-hospital mortality and associated complications after bowel surgery in Victorian public hospitals. Aust N Z J Surg 70:6–10
Komen N, Dijk JW, Lalmahomed Z, Klop K, Hop W, Kleinrensink GJ, Jeekel H, Schouten WR, Lange JF (2009) After-hours colorectal surgery: a risk factor for anastomotic leakage. Int J Colorectal Dis 24:789–795
Schwesinger WH, Page CP, Gaskill HV III, Steward RM, Chopra S, Strodel WE, Sirinek KR (2000) Operative management of diverticular emergencies: strategies and outcomes. Arch Surg 135:558–562
Author information
Authors and Affiliations
Corresponding author
Additional information
Conception, acquisition of data, analysis, and interpretation of data were carried out by Alexander Perathoner, Alexander Klaus, and Reinhold Kafka-Ritsch. Drafting and revision of the article were made by Alexander Perathoner, Alexander Klaus, Gilbert Mühlmann, Michael Oberwalder, Raimund Margreiter, and Reinhold Kafka-Ritsch.
Rights and permissions
About this article
Cite this article
Perathoner, A., Klaus, A., Mühlmann, G. et al. Damage control with abdominal vacuum therapy (VAC) to manage perforated diverticulitis with advanced generalized peritonitis—a proof of concept. Int J Colorectal Dis 25, 767–774 (2010). https://doi.org/10.1007/s00384-010-0887-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-010-0887-8